Local Treatment of Peritoneal Metastasis from Renal Cell Carcinoma: Case Report
- 1. Urology B Department, Avicenne Hospital, University Mohammed V, Rabat, Morocco
Abstract
There has been much discussion on the role of metastasectomy in metastatic renal cell cancer (mRCC). The majority of clinical data on metastasectomy has come from small retrospective studies with a variety of patient types. It is unclear in what setting patients may confer the most clinical benefit. Furthermore, a large amount of clinical data supporting the added advantage of metastasectomy originates from an earlier period than the present.
We report the case of a patient who presented renal carcinoma with a single peritoneal location treated by extended total nephrectomy and metastasectomy
Keywords
• Renal cell carcinoma
• Cytoreductive nephrectomy
• Peritoneal metastasis
• Metastasectomy
CITATION
Ilyass Z, Jaafar F, Youssef M, Amine S, Tariq K, et al. (2024) Local Treatment of Peritoneal Metastasis from Renal Cell Carcinoma: Case Report. J Urol Res 11(2): 1152.
ABBREVIATIONS
RCC: Renal Cell Carcinoma; mRCC: metastatic Renal Cell Carcinoma; CN: Cytoreductive Nephrectomy; PM: Peritoneal Metastases; IMDC score: International Metastatic RCC Database Consortium; IL-2: Interleukin-2; IFNa: Interferon alpha; RT: Radiation Therapy; OS: Overall survival
INTRODUCTION
Renal cell carcinoma (RCC) accounted for 78,000 newly diagnosed cases in the United States in 2018; it is the ninth most prevalent cancer in women and the sixth most common cancer in men. Among genitourinary malignancies, RCC represents the third largest cause of death. For large and locally advanced RCC, radical nephrectomy is currently the gold standard [1]. While nephron sparing surgery is preferred for smaller localized tumors, with percutaneous tumor ablation acknowledged as a safe option [2,3]. The management of metastatic RCC (mRCC) is more difficult, with reported results that are inconsistent. In fact, there is a 0–20% 5-year survival rate for mRCC [4,5]. While some retrospective investigations have presented compelling evidence supporting cytoreductive nephrectomy (CN) [6,7]. Recent years have seen a paradigm shift in the way patients with mRCC are managed [8].
Rarely do metastatic lesions interest the peritoneum. Synchronous excision of the primary RCC with its peritoneal metastases is not frequently recorded, if metachronous resections are rare at all.
We present the case of a peritoneal metastasis discovered in per operative total nephrectomy, treated by a metastasectomy.
CASE REPORT
We report the case of a 56 years old woman, with unremarkable past medical history, who presented increasing lower abdominal pain over three weeks without associated symptoms.
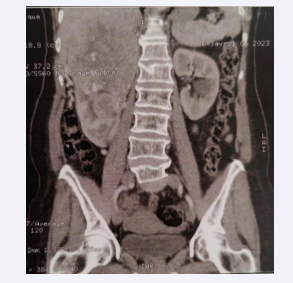
With radiological appearance of mid-renal and upper polar oval tumor mass with heterogeneously enhanced calcifications measuring 135*105*170mm arriving in contact with the Gerota fascia (Figure 1), with renal vena and inferior vena cava thrombosis visible on a Doppler ultrasound.
Figure 1: Right upper renal mid and polar tumor process scanner 131*108*145mm.
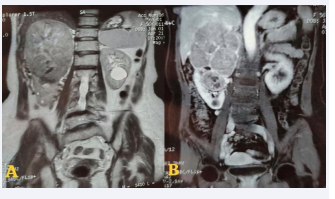
An MRI carried out to better for staging the tumor, classified the tumor as T3aN0M0 (Figure 2).
Figure 2: A. T2: Heterogeneous right upper and middle renal mass measuring 145*124*112mm restricting diffusion, contains a necrotic center. B.T1 FS Gado+: enhancement after Gadolinium injection.
we decide to perform an open enlarged total nephrectomy given the size of the tumor and the presence of a vena cava thrombus.
Explorative surgery showed a peritoneal nodule measuring 3,5*2,5cm.
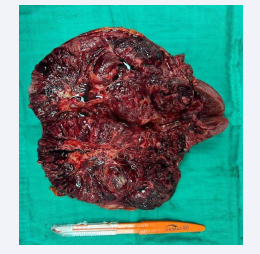
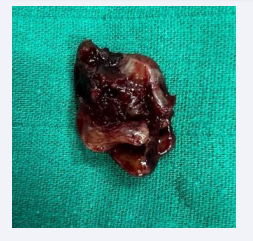
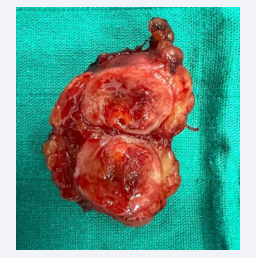
An extended nephrectomy together (Figure 3) with a vena cava thrombectomy (Figure 4) and a resection of the peritoneal nodule (Figure 5) were performed.
Figure 3: Enlarged total nephrectomy specimen showing the tumor mass.
Figure 4: Vena cava thrombosis.
Figure 5: Peritoneal nodule completely resected 3cm*2.5cm.
Peri-operative hemoglobin loss was 2.8 g/dL. Total operative time was 130 min. No post-operative complications were recorded and patient was discharged in 7th post-operative day.
Histopathological examination showed a clear cell carcinoma, measuring 170mm in major axis classified pT3b RCC, grade III ISUP, with peritoneal metastasis with negative surgical margins.
9 months follow up of the patient using Thoraco-abdomino- pelvic CT showed no evidence of disease recurrence.
DISCUSSION
Renal cell carcinoma (RCC) is acknowledged as a highly aggressive tumor that requires early diagnosis to maximize the chances of cure [9]. RCCs have the potential to metastasize to lymph nodes, lungs, liver, contralateral kidney, adrenal glands, brain, and bone [10].
Peritoneal metastases (PM) have been reported in approximately 1% of patients with metastatic Renal Cell Carcinoma, Outcome data are limited due to the rarity of this metastatic site [11].
Risk factors and pathological features of RCC that are associated with peritoneal metastasis is unknown, but Intraperitoneal spread of RCC may occur by one of two mechanisms: (a) the renal neoplasm may break through the renal capsule, the anterior renal fascia, and the closely apposed posterior parietal peritoneum to spread along the peritoneal surfaces. Involvement of peritoneal surfaces may induce ascites which facilitates further intraperitoneal spread by deposition of malignant cells onto peritoneal reflections were ascites collects, and (b) alternatively, embolic hematogenous metastases may reach the omentum, mesentery, or other peritoneal organs with subsequent intraperitoneal spread.
Despite the importance of imaging in the detection of metastases, surgical exploration remains interesting to eliminate the presence of visceral metastases.
The discovery of a metastasis during surgery can disrupt the therapeutic decision, moving from the chapter of localized and locally advanced renal tumor to the chapter of metastatic renal tumor with a defensive strategy which is mainly based on systemic treatment as a first step.
On the other hand, in our case, the patient had a good prognostic IMDC score, with a single metastatic location allowing the performance of a cytoreductive nephrectomy with local treatment of metastases [12,13], the main prognostic factor of which is to have a negative excision margin.
The efficacy of metastasectomy in metastatic renal cell carcinoma(mRCC) has been a topic ofextensivedebate. Much of the clinical data on metastasectomy comes from small retrospective studies with diverse patient populations. It remains unclear under what circumstances patients derive the greatest clinical benefit from this procedure. Additionally, a significant portion of the evidence supporting the added benefits of metastasectomy comes from a previous era when interleukin-2, interferon-alpha, and a limited number of tyrosine kinase inhibitors were the main treatment options alongside surgery [14].
To address these uncertainties, we review the current literature on metastasectomy in mRCC based on specific anatomical locations, examine the clinicopathological factors linked to metastasectomy outcomes, and explore the potential role of metastasectomy in conjunction with targeted therapy and immunotherapy [15].
Treatment options for metastatic renal cell carcinoma (mRCC) are still up for debate, particularly when it comes to metastases in atypical locales, about which there isn’t as much published research [16]. Chemotherapy resistance and only modest susceptibility to radiation therapy are common in mRCC. Immunotherapies such as interleukin-2 (IL-2) or interferon alpha (IFNa) are frequently employed as first-line treatments in the past due to their immunogenic nature [17]. Additionally, preclinical research has demonstrated a favorable synergy between immunotherapy (IL-2) and radiation therapy (RT) [18,19].
Although the majority of metastases are found in the initial years following surgery, late metastases can still occur even after what appears to be a protracted period of remission. As a result, continuous surveillance and monitoring are essential for identifying and treating any possible metastasis or recurrence. Nevertheless, the best surveillance plan for long-term follow-up has not yet been established, and more investigation is required to find the best monitoring strategy for these patients.
CONCLUSION
Metastasectomy appears to be a viable treatment that improves the likelihood of OS in some patients with mRCC, despite the dubious logic behind local treatment and the fact that systemic treatments remain the norm for most patients in metastatic situations. A rising body of evidence in the literature also points to metastasectomy as the best course of action for survival in situations involving total resection, especially in cases where a metastasis is found during surgery.
REFERENCES
- Crocerossa F, Carbonara U, Cantiello F, Marchioni M, Ditonno P, Mir M.C, et al. Robot-assisted Radical Nephrectomy: A Systematic Review and Meta-analysis of Comparative Studies. Eur Urol. 2021; 80: 428- 439.
- Pandolfo SD, Beksac AT, Derweesh IH, Celia A, Schiavina R, Bianchi L, et al. Percutaneous Ablation vs Robot-Assisted Partial Nephrectomy for Completely Endophytic Renal Masses: A Multicenter Trifecta Analysis with a Minimum 3-Year Follow-Up. J Endourol. 2022; 37: 279-285.
- Piasentin A, Claps F, Silvestri T, Rebez G, Traunero F, Mir MC, et al. Assessing Trifecta Achievement after Percutaneous Cryoablation of Small Renal Masses: Results from a Multi-Institutional Collaboration. Medicina (Kaunas). 2022; 58: 1041.
- Heng DY, Xie W, Regan MM, Warren MA, Golshayan AR, Sahi C, et al. Prognostic Factors for Overall Survival in Patients with Metastatic Renal Cell Carcinoma Treated with Vascular Endothelial Growth Factor–Targeted Agents: Results From a Large, Multicenter Study. J Clin Oncol. 2009; 27: 5794-5799.
- Napolitano L, Orecchia L, Giulioni C, Carbonara U, Tavella G, Lizzio L, et al. The Role of miRNA in the Management of Localized and Advanced Renal Masses, a Narrative Review of the Literature. Appl Sci. 2022; 13: 275.
- Bhindi B, Abel EJ, Albiges L, Bensalah K, Boorjian SA, Daneshmand S, et al. Systematic Review of the Role of Cytoreductive Nephrectomy in the Targeted Therapy Era and Beyond: An Individualized Approach to Metastatic Renal Cell Carcinoma. Eur Urol. 2019; 75: 111-128.
- Pal SK, Nelson RA, Vogelzang N. Disease-Specific Survival in De Novo Metastatic Renal Cell Carcinoma in the Cytokine and Targeted Therapy Era. PLoS One. 2013; 8: e63341.
- Napolitano L, Manfredi C, Cirillo L, Fusco GM, Passaro F, Abate M, et al. Cytoreductive Nephrectomy and Metastatic Renal Cell Carcinoma: State of the Art and Future Perspectives. Medicina (Kaunas). 2023; 59: 767.
- Novick AC. Walsh PC, Retik AB, Vaughan ED, Wein AJ, editors. Surgery of the kidney. Campbell’s urology. 2002. Philadelphia: WB Saunders; 3602-3613.
- Ather MH, Masood N, Siddiqui T. Current management of advanced and metastatic renal cell carcinoma. Urol J. 2010; 7: 1-9.
- Stellato M, Buti S, Maruzzo M, Fotia G, Sorarù M, Bassanelli M, et al. Real world analysis of peritoneal metastasis from renal cell carcinoma: Meet-Uro 27. Clin Genitourin Cancer. 2024; 22: 102078.
- Jacob J. Adashek, Ahmet Murat Aydin, Patricia Kim, Philippe E. Spiess. The role of metastasectomy in the treatment of metastatic renal cell carcinoma. Ann Hepatobiliary Pancreat Surg. 2019; 23: 91-95.
- Flanigan RC, Mickisch G, Sylvester R, Tangen C, Van Pop-pel H, Crawford ED. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol. 2004; 171:1071-1076.
- Méjean A, Ravaud A, Thezenas S, Colas S, Beauval JB,Bensalah K, et al. Sunitinib alone or after nephrectomyin metastatic renal-cell carcinoma. N Engl J Med. 2018; 379: 417-427.
- Méjean A, Ravaud A, Thezenas S, Chevreau C, BensalahK, Geoffrois L, et al. Sunitinib alone or after nephrectomy for patients with metastatic renal cell carcinoma: is there still a role for Cytoreductive Nephrectomy?. N Engl J Med. 2018; 379: 417-427.
- Antonelli A , Arrighi N, Corti S, Legramanti S, Zanotelli T, Cozzoli A, et al. Surgical treatment of atypical metastasis from renal cell carcinoma (RCC). BJU Int. 2012; 110: E559-E563.
- Kerst JM, Bex A, Mallo H, Dewit L, Haanen JB, Boogerd W, et al. Prolonged low dose IL-2 and thalidomide in progressive metastatic renal cell carcinoma with concurrent radiotherapy to bone and/or soft tissue metastasis: a phase II study. Cancer Immunol Immunother. 2005; 54: 926-931.
- Younes E, Haas GP, Dezso B, Ali E, Maughan RL, Kukuruga MA, et al. Local tumor irradiation augments the response to IL-2 therapy in a murine renal adenocarcinoma. Cell Immunol. 1995; 165: 243-251.
- Dezso B, Haas GP, Hamzavi F, Kim S, Montecillo EJ, Benson PD, et al. The mechanism of local tumor irradiation combined with interleukin 2 therapy in murine renal carcinoma: histological evaluation of pulmonary metastases. Clin Cancer Res. 1996; 2: 1543-1552.