An Appropriate Cast for the Rabbit Achilles tendon Full Transection Pre-Clinical Model
- 1. Division of Plastic Surgery and Hand Surgery, University Hospital Zurich, Switzerland
- 2. Center for Preclinical Development, University Hospital Zurich, Switzerland
Abstract
Two major problems might occur after a tendon rupture. On the one hand side, the (fibrotic) scar tissue may lead to re-ruptures due to inferior mechanical properties. On the other hand, adhesion formation may occur between the healing tendon and the surrounding tissue, ending up in a reduced range of motion.
In order to study improvement of tendon rupture repair by novel methods, we have established a full transection rabbit Achilles tendon model in the past, where the two tendon stumps have been sutured together. To prevent such sutured tendons from immediate re-rupture after the operation, a cast was used to immobilize the tendon in a fully relaxed position, i.e. with an angle at the ankle of 180°. The method of how such a cast has to be applied so the rabbits feel comfortable, do not bite the cast material and still the healing process is not compromised, has been iteratively improved over the last 15 years.
We provide a detailed description of the casting method for the purpose of immobilizing the rabbits’ hind legs and support a proper Achilles tendon healing. Particularly, we provide a series of photos that show every step and action needed during casting the hind leg. Such protocols may be interesting for researchers intending to apply a full transection model in rabbit Achilles tendons in their preclinical studies, and who are looking for a detailed protocol with hints on what may be particularly important (dos and don’ts) during the application of a cast.
Keywords
• Achilles tendon; Cast; Rabbit; Tenotomy; Suture; Immobilization
Citation
Bäuerle V, Nicholls F, Giovanoli P, Calcagni M, Buschmann J (2025) An Appropriate Cast for the Rabbit Achilles tendon Full Transection Pre-Clinical Model. J Vet Med Res 12(2): 1284
ABBREVIATIONS
3Rs: Reduction, Refinement, Replacement
INTRODUCTION
In order to improve tendon rupture repair, many different in vitro [1,2], and preclinical in vivo models [3,4], have been developed. Among the in vivo models, the rabbit Achilles tendon full transection model [5], has been shown to mimic hand flexor tendons with respect to biomechanical strength and has been shown to be ideal to study the cellular response towards implant materials [6,7]. Furthermore this model allowed our research team to test novel implant materials in form of tubular anti-adhesive sleeves with hyaluronic acid [8], or bioactive electrospun tubes with a sustained release of PDGF-BB to enhance the strength of the healing tendons at early time points such as three weeks post-operation, enabling a faster return to daily loading activities [9].
To prevent initial re-rupture of freshly sutured tendons in animal models where overload is a widespread model and even multi-strand sutures may not be strong enough to withstand jumping or running of rodents, a proper plaster cast immobilization is necessary. During the last 15 years, our research team optimized iteratively the casting protocol for hind legs of rabbits after full transection of the Achilles tendon and conventional suture. In order to avoid any stress on the freshly sutured tendons, the angle at the ankle should be 180°, so that the foot is fully stretched and in line with the lower leg. Also, as a peculiar finding over the years, we determined that the colour of the cast is an indication for whether the rabbits are prone to bite into the cast and nibble the material or tolerate it like it is [10].
We here provide a detailed description of the casting method, demonstrate our procedure with multiple photo series taken in the operation theatre after the transection and suture, including particular recommendations of what has been proven to be good and tolerated well by the experimental New Zealand White rabbits and what on the other hand side should be avoided not to cause any burden to the animal in the sense of the 3Rs [11], and also not compromising the experimental outcome. So far, we did not find any methodological paper that had the same aim and we therefore believe that this methodological contribution may help researchers using the full transection rabbit Achilles tendon model in the future.
MATERIALS AND METHODS
Animals
Female New Zealand White rabbits with an age between 12 and 16 weeks were used for this study. The specific pathogen free rabbits were obtained from Charles River, Research Models and Services, Germany. In groups of 4 animals, the rabbits were held in four interconnected cages, where each of the cages had a bottom area of 70 cm x 70 cm = 4900 cm2 and a height of 62 cm and with an elevated platform of 30 cm height, allowing the rabbits to jump up to these platforms (Indulab, Switzerland). In the room, there were controlled conditions: temperature 22 ± 1°C, 45% relative humidity, 15 air changes per hour and a light/dark rhythm of 12 hours. The rabbits were always given free access to water (automatic water supply), sterilized hay and straw ad libitum and to standard pellet diet (Kliba Nafag, Nr. 3410, Provimi Kliba AG, Switzerland). The veterinary office of Zurich, Switzerland, gave its ethical approval for our experiments (reference number ZH080/2021). The animal welfare committee of the veterinary office of Zurich approved these experiments. All experiments were carried out according to the relevant guidelines and regulations given by the veterinary office of Zurich and furthermore in compliance with the ARRIVE guidelines. All animals were acclimatized to their environment for 14 days before starting the experiments by surgery.
Achilles tendon repair
Premedication with 65 mg/kgbodyweight ketamine and 4 mg/kgbodyweight xylazine was given to the rabbits. Into the marginal ear vein, a venous catheter was laid. The rabbits were then intubated with Propofol i.v. 0.6 mg/ kg –1.3 mg/kg. Anaesthesia was kept at 1–2 % isoflurane. To be sure to maintain systemic analgesia while performing the surgery, 0.2 –0.3 mg/kgbodyweight butorphanol (Dr. E. Graeub AG, Berne, Switzerland) was applied pre- operatively. Two local anesthetics were in addition injected in the tissue surrounding the incision before the surgery started: Bupivacaine in a concentration of 0.36 mg/kgbodyweight, corresponding to 0.07 mL/kgbodyweight, and Lidocaine with 1.42 mg/kgbodyweight, corresponding to 0.07ml/kgbodyweight. To prevent infection, the hind legs were purged with iodine. The AT then was exposed by a para-tendineal incision of cutis, subcutis and fascia, like reported previously [12]. A perpendicular section to the length of the tendon was subsequently made through the medial and lateral M. gastrocnemius of the AT complex around 2.0 cm above the calcaneus. The two tendon stumps were sutured by a 4-strand Becker suture with a USP 4-0 polypropylene thread. After that, the wound was shut with a running suture (using a USP 6.0 polypropylene thread) on the fascia as well as the inter- rupted skin. Immediately after that, a Durogesic Matrix patch (Janssen-Cilag AG, Switzerland) was applied for analgesia, containing 4.2 mg Fentanyl per patch, ensuring no pain for the next approximately 72 hours due to 25 µg/h Fentanyl. At the endpoint, euthanasia of the rabbits in deep anaesthesia caused by 100 mg/ kgbodyweight ketamine and 4mg/ kgbodyweight xylazine was conducted, with 80 mg/ kgbodyweight pentobarbital (Esconarkon ad us. vet., Switzerland).
Re-casting option – the ankle angle matters
Depending on the planned endpoint of the rabbit Achilles tendon experiments, one cast may be enough; however, for longer periods, it is recommended to re-cast the hind leg, meaning removing the first cast and replace it by a new one. During our experiments in the past, we had different times until the endpoint, covering 3 [8], 6 [13], and 12 weeks [7], respectively. If a 3-week experiment is planned, one cast is considered to be enough during these three weeks. As for a 6-week experiment, it is recommended to change the cast after three weeks and apply a new one, because the muscle volume is reduced over time and the casts may get loose. There are two options for the second cast; either it has the same angle at the ankle as the first cast, i.e. 180 °, which we refer to as an adhesion-promoting model of casting [5]; or the second cast has a slightly lower angle at the ankle with 150°, referred to as the adhesion-inhibiting model of casting. We found that this slight change in ankle angle results in prominently lower adhesion formation, because initial fibrotic (weak) adhesions evolving during the first three weeks post-operation may break upon angle change during re-casting. Nevertheless, this immobilization technique still allows adhesion formation, just on a lower level compared to the adhesion provoking model [5].
Finally, for a 12-week experiment, after the two casts (the first for 0-3 weeks and the second for 4-6 weeks), no further cast is recommended as the healing rabbit Achilles tendons proved to be stable enough at that time point and the rabbits were able to move and hopple normally in their interconnected cages until week 12, without any re-rupture observed.
Photos
All photos have been taken by Christoph Stulz, the photographer at the University Hospital Zurich, Switzerland. They were taken just right after the operation during the casting of one rabbit. The protocol of casting, however, was the same for all rabbits.
RESULTS AND DISCUSSION
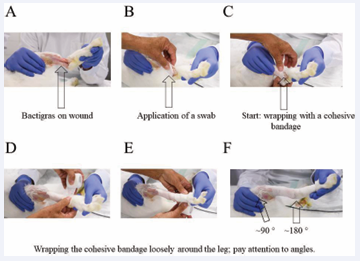
The animals were still under anaesthesia during the whole casting procedure. Post- operative treatment started with the application of Bactigras directly on the closed wound in order to avoid bacterial infection (Figure 1). Then, a small swab consisting of a sterile gauze was deposited, before a cohesive bandage was loosely wrapped around the leg. Already at this point, the angles have to be examined carefully and approximately 180 ° should be reached at the ankle angle and 90 ° at the knee. It should be paid attention not to wrap it too tight because otherwise the healing will be impaired as the circulation may be hampered. Particular attention should be paid to the (free) toes; they should not be constricted nor cramped.
Figure 1: Initial steps for the cast. Bactigras (A), swab, a sterile gauze (B) and cohesive bandage application (C-F)
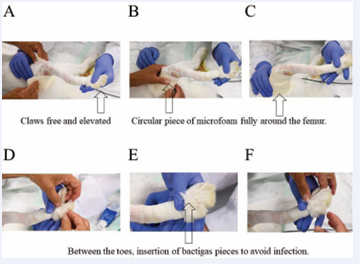
After that, a piece of Microfoam was put tightly around the femur, so that the femur is fully surrounded by this soft foam pad. This is important because later during casting the hard plaster cast should not notch and harm the very sensitive skin tissue at the femur (Figure 2). The Microfoam should stick directly on the skin/fur at the upper rim. In order to prevent bacterial infection, pieces of Bactigras applied folded as two layers are put between the toes of the treated hind leg. They should be inserted between the toes with some pressure to avoid that they fall out again when the rabbits move in their cages later on. These Bactigras pieces should stay in place.
Figure 2: Padding the area around the femur with a piece of Microfoam (A-C) and insertion of Bactigras between the toes (D-F).
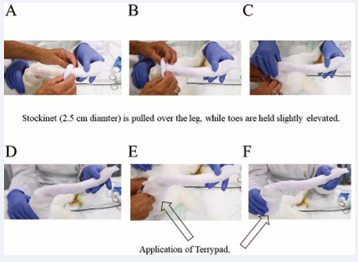
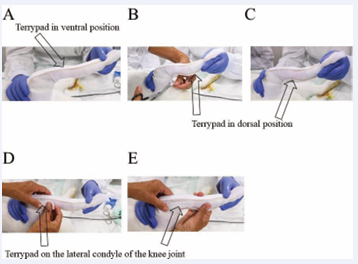
As a next step, a Stockinet with a diameter of 2.5 cm was applied, starting with an initially rolled up stocking as received by the provider (3M™ Synthetic Cast Stockinet), rolling it gently over the toes and up the leg. After that, a piece of Terrypad (LOMED Terry Pad Polster) was used to have a well-padded area around the femur (Figure 3). Then two long pieces of Terrypad were applied on the ventral and dorsal surface of the leg and a third short piece of Terrypad was applied to cover the lateral condyle of the knee joint (Figure 4). Also the medial condyle of the knee joint received a short piece of Terrypad, with the same length as the lateral side. Moreover, if there were any other bony protrusions, they should also be covered with a piece of Terrypad to ensure a good and well-padded area inside the final (hard) cast.
Figure 3: Application of a 2.5 cm Stockinet (A-C) and of a piece of Terrypad around the femur (D-F). It should be particularly well padded around the upper rim.
Figure 4: Application a piece of Terrypad in the ventral position (A) and in the dorsal position (B, C) as well as a small piece of Terrypad in over the lateral condyle of the knee joint (D-E).
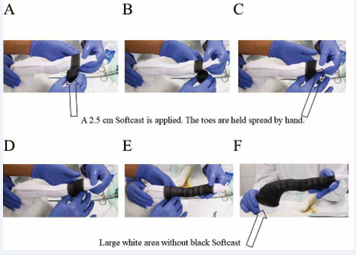
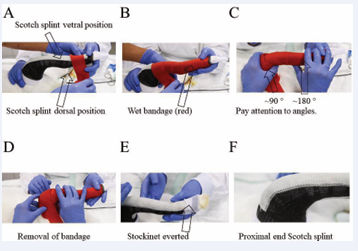
After that, a ground coat of 2.5 cm Softcast was disposed, starting from the toes (Figure 5). It was gently and circularly applied up to the femur, with half-half overlapping. At the beginning, near the toes, there should absolutely no pressure be applied, however, further up to the femur, a little tension can be applied during Softcast application. After that, a double-layered Scotch cast was positioned ventrally and laterally and it was fixated with a wet bandage by holding it for two minutes to get hard; in the shown case the bandage was red (Figure 6).
Figure 5: A 2.5 cm Softcast is applied, starting at the toes, no pressure (A-C) and moving up the leg (D), slightly increasing the pressure a bit (E) and ending at the femur, but not until to the rim, leaving a large white area left where no Stockinet is applied (F).
Figure 6: A bi-layered Scotch splint is applied in ventral and dorsal position, and a wet bandage is circularly rolled to fix the splints (A-C) and is removed after two minutes (D) the Stockinet is everted so that the toes appear in a relaxed position (E), proximal end of Scotch splint in the ventral position (F).
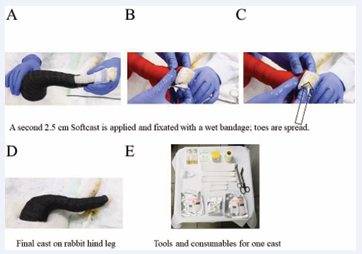
Finally, a second 2.5 cm Softcast was applied over the Scotch splints; this time starting from the femur. This Softcast was fixated with a wet bandage. Particular attention was paid to the toe region where the toes were spread (Figure 7). To sum up what is needed to fabricate on cast as described, the consumables used are shown in Figure 7E: Upper row: Bactigras° 5 cm x 5 cm, containing 0.5 % chlorhexidine acetate (left); swab, sterile gauze, sterile Gazin® compresse, Lohman & Rauscher International GmbH & Co, Rengsdorf, Germany (right from Bactigras°); DermaPlast COFIX 3M Microfoam with corresponding box and unpacked (left). Middle row: One 2.5 cm diameter Stockinet (left); 5 pieces of Terrypad, cut into the according sizes needed for the well-padded cast (middle); and the scissors (right). Lower row: two Softcasts packages Nemoa™ Flex 2.5 cm x 1.8 m Polyester black 90 and one Scotch splint 3M Scotchcast™ Plus 2.5 cm (in the middle of the two Softcast packages).
Figure 7: A second Softcast is applied, starting from the femur and is fixated with a wet bandage (A-C). Final cast on the right hindleg of the rabbit (D). Summary of necessary consumabless and tools to make one cast (E).
CONCLUSION
We provide a detailed description for the immobilization of an operated rabbit hind leg; demonstrated for full Achilles tenotomy that was sutured and immobilized by a cast.
ACKNOWLEDGEMENTS
This research was funded by the Swiss National Science Foundation SNSF, grant number 310030_197578. We thank Christoph Stulz for taking the photos.
REFERENCES
- Stauber T, Wolleb M, Snedeker JG. Engineering tendon assembloids to probe cellular crosstalk in disease and repair. J Vis Exp. 2024; 205.
- Gautieri A, Passini FS, Silván U, Guizar-Sicairos M, Carimati G, Volpi P, et al. Advanced glycation end-products: Mechanics of aged collagen from molecule to tissue. Matrix Biol. 2017; 59: 95-108.
- Diaz A, Sang L, Garcia S, Wague A, Davies M, Youn A, et al. Age- dependent decline of B3AR agonist-mediated activation of FAP UCP- 1 expression in murine models of chronic rotator cuff repair. J Orthop Res. 2024; 42: 2307-2317.
- Shen C, Sun X, Li Z, Zhang R, Huang J, Tang K, et al. Panda rope bridge technique promoted achilles tendon regeneration in a novel rat tendon defect model. Knee Surg Sports Traumatol Arthrosc. 2025; 33: 1531-1543.
- Meier Bürgisser G, Calcagni M, Bachmann E, Fessel G, Snedeker JG, Giovanoli P, et al. Rabbit Achilles tendon full transection model - wound healing, adhesion formation and biomechanics at 3, 6 and 12 weeks post-surgery. Biol Open. 2016; 5: 1324-1333.
- Buschmann J, Calcagni M, Bürgisser GM, Bonavoglia E, Neuenschwander P, Milleret V, et al. Synthesis, characterization and histomorphometric analysis of cellular response to a new elastic DegraPol® polymer for rabbit Achilles tendon rupture repair. J Tissue Eng Regen Med. 2015; 9: 584-594.
- Bürgisser GM, Evrova O, Heuberger DM, Wolint P, Rieber J, Miescher I, et al. Electrospun tube reduces adhesion in rabbit Achilles tendon 12 weeks post-surgery without PAR-2 overexpression. Sci Rep. 2021; 11: 23293.
- Miescher I, Schaffner N, Rieber J, Bürgisser GM, Ongini E, Yang Y, et al. Hyaluronic acid/PEO electrospun tube reduces tendon adhesion to levels comparable to native tendons - An in vitro and in vivo study. Int J Biol Macromol. 2024; 273: 133193.
- Evrova O, Bürgisser GM, Ebnöther C, Adathala A, Calcagni M, Bachmann E, et al. Elastic and surgeon friendly electrospun tubes delivering PDGF-BB positively impact tendon rupture healing in a rabbit Achilles tendon model. Biomaterials. 2020; 232: 119722.
- F. Nicholls; V. Bäuerle; J. Buschmann. Casting hind legs of rabbits after full transections of Achilles tendons - impact of cast’s color and composition. J Vet Clin Practice and Petcare. 2017; 2: 1-8.
- Rusche B. The 3Rs and animal welfare - conflict or the way forward? ALTEX. 2003; 20: 63-76.
- Buschmann J, Meier-Bürgisser G, Bonavoglia E, Neuenschwander P, Milleret V, Giovanoli P, et al. Cellular response of healing tissue to DegraPol tube implantation in rabbit Achilles tendon rupture repair: an in vivo histomorphometric study. J Tissue Eng Regen Med. 2013; 7: 413-420.
- Meier Bürgisser G, Calcagni M, Müller A, Bonavoglia E, Fessel G, Snedeker JG, et al. Prevention of peritendinous adhesions using an electrospun DegraPol polymer tube: A histological, ultrasonographic, and biomechanical study in rabbits. Biomed Res Int. 2014; 2014: 656240.