Comparative Assessment of Edwardsiella ictaluri Live Attenuated Vaccine and Wild type Strains-induced Phagocytic Vesicle Formation and Apoptosis in Catfish B cells
- 1. Department of Comparative Biomedical Sciences, Mississippi State University, USA
Abstract
In this study, we applied comparative morphological assessment of autophagic changes in catfish anterior kidney (AK) B cells after exposure to Edwardsiella ictaluri live attenuated vaccines (LAVs; EiΔevpB and ESC-NDKL1) and wild-type (WT) strains. Bacterial uptake followed by induced phagocytic vesicle formation and late apoptotic changes were analyzed by applying light compound microscopy and blind cell counting to the slides with fixed Giemsa stained highly enriched IgM+ AK B cells. We confirmed and extended our previous report on the ability of catfish AK B cells to engulf and eliminate E. ictaluri WT and LAV strains. Importantly, in this study we documented significant decreases in the percentage of B cells with internalized bacterial strains combined with significant increases of cells with phagolysosome vesicles alone after exposure to the EiΔevpB strain compared to the repressed vesicle formation by the ESC-NDKL1 and WT strains. This observation suggested the higher intensity of catfish B cells in killing the EiΔevpB LAV strain compared to the ESC-NDKL1 and WT strains after initial short-term bacterial exposure. Furthermore, we showed that the WT strain had a more profound proapoptotic effect on catfish B cells compared to the EiΔevpB and ESC-NDKL1 counterparts. Despite the initial advantage in vesicle formation activity against EiΔevpB strain, the subsequent increase in the killing ability of ESC-NDKL1 previously reported in catfish B cells suggests an advantage of the strain to be specifically recognized as inducing protective immunity against enteric septicemia of catfish (ESC). Moreover, losing the pathogenicity of ESC-NDKL1 by significantly repressing apoptosis could be used as a tool in vaccine design. Our data suggest that catfish B cells possess important phagocytic and microbicidal ability in initiation of protective innate and adaptive immune responses against ESC.
Keywords
• Channel catfish
• B cells
• Phagosome/phagolysosome
• Bacterial killing
• Edwardsiella ictaluri
• Live attenuated vaccines
CITATION
Kordon AO, Rowe AE, Lawrence ML, Abdelhamed A, Pinchuk LM (2023) Comparative Assessment of Edwardsiella ictaluri Live Attenuated Vaccine and Wild type Strains-induced Phagocytic Vesicle Formation and Apoptosis in Catfish B cells. J Vet Med Res 10(5): 1256.
INTRODUCTION
Autophagy is an evolutionary conserved cellular response that functions to protect cells from multiple stressors in physiological and pathological contexts. Autophagic mechanisms are involved with the normal function of innate and adaptive immunity at almost every level [1]. Cargo with bacterial pathogens is directed to a double membrane vacuole that fuses with lysosome to form autophagosomes for direct pathogen degradation/elimination followed by antigen processing and presentation [2]. Pathogenic bacteria have developed strategies to sabotage, subvert or exploit these selective intracellular degradation mechanisms [3-5].
Edwardsiella ictaluri (E. ictaluri) is a Gram-negative facultative intracellular pathogen that causes an economically significant disease in farm raised catfish called enteric septicemia of catfish (ESC). E. ictaluri can survive and proliferate inside channel catfish professional phagocytes, such as macrophage and neutrophils [6-9]. In addition, E. ictaluri can survive and replicate in catfish peritoneal and the AK-derived macrophages [10]. E. ictaluri has also been reported to survive in catfish neutrophils for a short period of time [11].
Commercial ESC vaccine Aquavac-ESC (RE-33) was developed by Klesius and Shoemaker, and this vaccine can efficiently protect juvenile catfish [12]. In addition, immersion studies with this vaccine demonstrated that protective immunity was stimulated in catfishfry, fingerlings, and eyed catfish eggs from E. ictaluri infections [13-16]. Another safe and effective live attenuated E. ictaluri isolate (S97-773) was generated by Wise, and strong protection in catfish fingerlings was provided by oral administration when added in feed [17].
More recently, two promising live attenuated vaccine (LAV) strains (named as EiΔevpB and ESC-NDKL1) were developed in our laboratory by the deletion of one or more genes of E. ictaluri genome. The EiΔevpB strain has in-frame deletion of the evpB gene in the type VI secretion system (T6SS) operon [18]. The ESC-NDKL1 constructed by in-frame deletion of genes encoding glycine dehydrogenase (gcvP), succinate dehydrogenase (sdhC), and fumarate reductase (frdA) in E. ictaluri strain 93-146 [19].
Vaccination with the EiΔevpB strain resulted in 100% survival in fingerlings and 80% survival in catfish fry after WT E. ictaluri challenge [18]. Vaccination of catfish fingerlings with ESC NDKL1 showed survival rates similar to the EiΔevpB LAV strain; however, challenge of fry with ESC-NKDL1 showed moderately elevated 3-4% mortality rates [19]. Recently, we documented active phagocytosis of the E. ictaluri WT and two LAVs strains in channel catfish AK B cells by flow cytometry. Furthermore, we observed the E. ictaluri strains induced phagosome and/or phagolysosome formation in the cytoplasm of catfish B cells. On the note, we demonstrated that the ability of catfish AK B cells to efficiently destroy the internalized E. ictaluri WT and LAV strains by phagocytosis. Finally the E. ictaluri WT and LAV strains-induced early and late apoptotic and necrotic changes have been described in catfish AK B cells [20].
In addition to the role of B cells in adaptive immunity, such as secreting antibodies and maintaining an immunological memory, mammalian B cells were able to perform critically important antibody- independent functions, namely, cytokine production, antigen capturing, antigen presentation, and microbial killing characterized B cells as powerful professional antigen presenting cells [21,22]. B cells in teleost fish similar to mammalian B-1 cells, expressed well pronounced characteristics of potent antigen presenting cells [23,24]. The phagocytic and bacterial killing properties have been well described previously in teleost fish, such as zebrafish, rainbow trout, Atlantic salmon and Atlantic cod [25-28]. However, in contrast to mammalian lymphocytes, teleost fish phagocytic B cells account for 60% of all B cells and are found in every systemic compartment, including blood, spleen, and anterior kidney [23,29]. Finally large numbers of phagocytic B cells have been found in catfish blood [23,30].
Considering that B cells are powerful antigen presenting cells contributing to both innate and adaptive immune responses, there is need to delineate the mechanistic framework of the role of B cells in ESC. The purpose of this study was to investigate and extend our knowledge of the differential ability of AK B cells to endocytose E. ictaluri LAV and WT strains, to form strain-related autophagic vesicles, and to undergo late apoptotic changes.
MATERIAL AND METHODS
Animals
Selection and maintenance of fish was done as described previously [20,31]. Briefly, specific pathogen free (SPF) channel catfish (150-200 g) were obtained from the fish hatchery at the College of Veterinary Medicine, Mississippi State University. All fish experiments were conducted according to a protocol approved by the Mississippi State University Institutional Animal Care and Use Committee (IACUC). Fish were maintained at 25 28oC throughout the experiments. Tricaine methanesulfonate (MS-222, Western, Chemical, Inc.) was used for the catfish sedation and euthanizing.
Bacterial strains and growth conditions
Bacterial strains used in this study are listed in [Table 1]. E. ictaluri 93–146 wild-type (WT) and two LAVs strains were grown in brain heart infusion (BHI) agar or broth (Difco, Sparks, MD, United States), and incubated at 30°C for overnight with constant shaking of 180 rpm.
Phagocytosis and Cytospins
Generation of highly enriched IgM+ B cells and challenges of B cells with LAVs and WT bacterial strains were done as described previously [20]. Briefly, AKs were dissected from 5 catfish, tissues were pooled, and a single cell suspension was obtained by using sterile forceps and dissociation sieves (Sigma). Harvested cell suspensions were overlaid on Histopaque 1077 (Sigma) and centrifuged at 400 g for 30 min to extract white mononuclear cells (WMCs). Following centrifugation, WMCs were recovered from the interface, washed three times with cold phosphate buffered saline (PBS) and resuspended in L-15 medium (Thermo Fisher Scientific). Monoclonal antibodies (mAbs, clone 9E1) specific to catfish IgM+ B cell-specific marker were incubated with WMCs on ice for 30 min. Highly enriched catfish AK IgM+ B cells were positively selected by magnetic sorting as described [20]. In brief, cells were washed and resuspended in MACS buffer (Miltenyi Biotec), and anti-mouse IgG (H + L)-magnetic microbeads (Miltenyi Biotec) were added to cell suspensions and incubated at 4°C for 15 min in the dark. Next, cell suspensions were washed and transported onto an LS separation column (Miltenyi Biotec), according to manufacturer instructions. Positively selected B cells were resuspended in L-15 medium and incubated for overnight.
Following overnight incubation at 28°C, IgM+ B cells were collected and followed by Histopaque 1077 separation. Then, cells were counted and assessed for viability by using a hemocytometer and trypan blue exclusion. AK IgM+ B cells were resuspended in L-15 medium and 2 x 106 cells per well were transferred into 6-well plates (FisherScientific, Pittsburgh, PA, United States). The bacterial strains (EiΔevpB, ESC-NDKL1, and WT) were applied to B cells for 30 min at multiplicity of infection (MOI) of 1:50 (cell to bacteria ratio) as described [20]. Non infected cells were included as a negative control. Following the incubation, cells were collected and washed in cold PBS. Next, the cytospins were centrifuged at 500 rpm for 1 min by using a Cyto Tek centrifuge machine. All samples were fixed on the slides, and Giemsa staining procedure (May-Grunwald Procedure) was applied to observe the morphology of IgM+ B cell population.
Cell Count and Light Microscopy
E. ictaluri LAVs and WT strains-induced vesicle formation and late apoptotic changes were analyzed by applying light compound microscopy and blind count of the total cell numbers on the slides with fixed Giemsa stained highly enriched IgM+ B cells (Table 2). Samples were analyzed with an Olympus BX60 microscope (OlympusU-TV1 X) and photographed by using Infinity software (Lumenera Corporation).
Statistical Analysis
The Chi-Square option with the PROC FREQ procedure in SAS for Windows 9.4 (SAS Institute, Inc., Cary, NC) was used to describe the differences in the proportions of cells with vesicles only, vesicle with bacteria, and apoptotic changes for the experimental and control groups. The Bonferroni Test was used to evaluate differences among the bacterial challenges with LAVs and WT E. ictaluri strains. The level of significance for all tests was set at P < 0.0083.
RESULTS AND DISCUSSION
Following the uptake of microbial into phagosomes, phagosomes and lysosomes are fused, and phagolysosomes with acidic and oxidizing environment were formed in phagocytic cells, such as macrophages and neutrophils [323-35]. Degradation and elimination of the ingested pathogens took place by the activation of enzymes and generation of superoxidase and other reactive oxygen species in phagolysosomes [36,37].
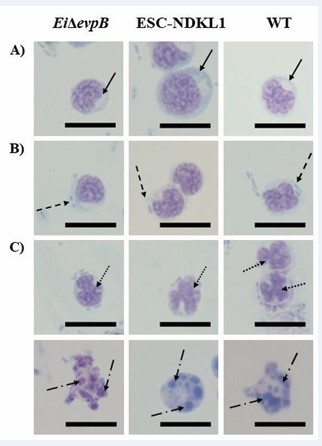
Multiple vesicle formation was observed in catfish AK B cells exposed to the LAVs and WT strains of E. ictaluri (Figure 1A).
Figure 1: Vesicle formation and late apoptotic changes in catfish B cells exposed to the E. ictaluri strains by light compound microscopy. (A) Autophagic vesicle formation indicated by solid arrows in the cytoplasm of B cells with no bacteria. (B) Engulfed bacteria shown by arrows with dash in the vesicles of B cells. (C) Late apoptotic changes in B cells induced by both LAVs and WT strains. Late apoptotic nuclei fragmentation (upper row-arrows with dots) and disintegration (lower row-arrows with long dash dot) in B cells exposed to the LAVs and WT E. ictaluri strains. Magnification 100 X, scale bar 20 micrometers
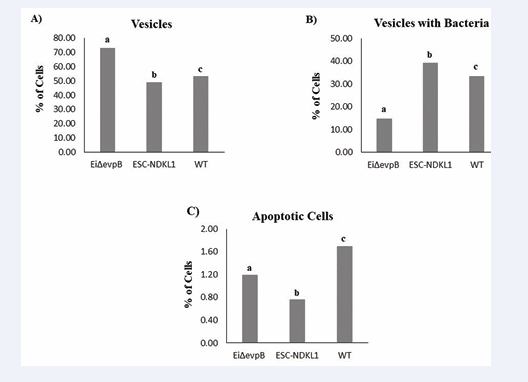
The EiΔevpB LAV strain promoted a significant increase in vesicle formation in catfish AK B cells compared to the ESC-NDKL1 LAV and WT strains (Figure 2A).
Figure 2: The statistical assessment of vesicle formation and late apoptotic changes in catfish AK B cells exposed to the E. ictaluri LAVs and WT strains. Percentages of B cells with vesicles only (A), vesicles with bacteria (B), and late apoptotic changes (nuclei fragmentation and disintegration) (C). Letters (a, b, and c) show significant differences between the treatments (P < 0.0083).
At the same time, the numbers of WT E. ictaluri strain-induced vesicles were significantly higher compared to the ESC-NDKL1 LAV strain (Figure 2A). There was no vesicle formation detected in the control non-infected cells AK B cells (data not shown). These data are in agreement with our previous observation on the enhanced bacterial uptake of AK B cells exposed to the EiΔevpB LAV strain compared to its counterparts, the E. ictaluri WT and ESC-NDKL1 LAV strains [20].
Numerous bacterial cells were detected in the vesicles of catfish IgM+ AK B cells (Figure 1B). Significantly elevated numbers of cells with vesicles containing bacteria were detected in catfish B cells treated with the ESC-NDKL1 LAV strain compared to the EiΔevpB LAV and WT strains of E. ictaluri (Figure 2B). At the same time, the effect of vesicle formation repression was evident after exposure to the ESC-NDKL1 LAV strain compared to the EiΔevpB LAV and WT strains (Figure 2A). As expected, bacteria were not found in the control catfish B cells (data not shown). An increase in the number of cells with vesicles containing significantly lower amounts of bacterial cells followed by 30 min exposure to the EiΔevpB LAV strain suggest significantly increased bactericidal properties of catfish B cells challenged with the EiΔevpB strain. This observation agrees with our previous report that the intensity of destroying the WT and EiΔevpB strains at initial time of exposure (up to 36 h) in catfish AK B cells was significantly higher compared to the B cells challenged with ESC-NDKL1 LAV and negative control groups by measuring bacterial luminescence [20]. Importantly, our previous research demonstrated that catfish B cells were capable of phagocytosing and efficiently killing of E. ictaluri WT and both LAVs strains. We documented significant time-dependent decreases in bacterial luminescence for both LAVs and WT strain of E. ictaluri; however, more than 50% of the initial ESC-NDKL1 LAV uptake was eliminated at 3 h post exposure while the EiΔevpB LAV uptake was eliminated at 4 h and the WT E. ictaluri uptake was eliminated at 5 h post exposure [20]. We conclude that decreased numbers of cells with vesicles and higher percentage of intracellular bacteria suggested lower bactericidal activity of catfish B cells against ESC-NDKL1 LAV strain at the initial early time of exposure, which agreed with our previous data [20]. However, despite the initial advantage in vesicle formation activity against EiΔevpB LAV, the subsequent increase in the killing ability of ESC-NDKL1 previously reported in catfish B cells suggests an advantage of the strain to be specifically recognized inducing protective immunity against ESC [20]. Our recent data reported that sustainable production of IgM in catfish serum induced by exposure of fish to the EiΔevpB and ESC-NDKL1 strains, suggesting both the development of primary immune responses and the possibly of generation of the E. ictaluri WT and LAV strains-specific memory B cells against ESC [38]. Interestingly, the antibody levels in the sera of catfish in response to the EiΔevpB strain significantly increased at 7 days post exposure whereas the ESC-NDKL1 strain significantly elevated the antibody production at 28 days post exposure [38].
Apoptosis, or programmed cell death, is distinguished by multiple distinct patterns, including cell shrinkage, nuclear condensation, DNA breakage, and protein cleavage [39,40]. Apoptosis is a critical component of immunological responses to a wide range of physiological and pathological stimuli [41,42]. We performed an assessment of late apoptotic changes in catfish B cells exposed to E. ictaluri WT and LAV strains. Both LAVs and WT E. ictaluri strains induced late apoptosis-dependent morphological patterns, such as B cells nuclei fragmentation and disintegration (Figure 1C). The number of E. ictaluri WT induced late apoptotic B cells was significantly higher than the numbers in both LAV strains (Figure 2C), which is consistent with our previous data on E. ictaluri WT and LAV strains-induced apoptotic changes assessed by flow cytometry [20]. We did not document any late apoptotic changes within control B cells (data not shown). Importantly, E. ictaluri ESC-NDKL1 LAV strain had significantly weaker effect on the apoptosis-dependent morphological changes in catfish B cells compared to EiΔevpB LAV and WT strains (Figure 2C).
In this study, we confirmed and extended our previous observations on the differential ability of catfish AK B cells to phagocytose and inactivate the E. ictaluri LAVs and WT strains, and also resist E. ictaluri LAVs and WT strains-induced apoptosis. We applied a similar bacterial challenge protocol (1:50 cell to bacteria ratio and 30 min exposure) as previously described using phagocytosis and flow cytometry studies [20]. Unlike in the previously published report, here we were able to show significant strain-related differences in the numbers of phagosomes/phagolysosome vesicles induced, as well as bacteria endocytosed in catfish AK B cells. Furthermore, we also were able to demonstrate significant LAV strain-related differences in the induction of late apoptotic changes in the AK B cells.
Increased bactericidal activity of catfish B cells against ESC-NDKL1 LAV in combination with significant repression of proapoptotic effects compared to the EiΔevpB LAV and WT strains suggested the weakening of the pathogenic effects of the ESC-NDKL1 LAV strain as an additional novel mechanism of the E. ictaluri LAVs pathogenesis. Although assessment of the role of the E. ictaluri type III secretion system was beyond the scope of this study, further research should address the role of this system and other molecular mechanisms used by E. ictaluri to resist selective intracellular degradation and subsequent specific antigen presentation in catfish AK B cells, which is important for initiating a protective immune response against ESC in channel catfish.
AUTHOR CONTRIBUTIONS
LP and AOK conceived and designed the experiments. LP provided the original idea of the study, and also contributed reagents, materials, and tools. ML and LP mentored and advised the veterinary student AER. AOK and AER performed the experiments and wrote the first draft of manuscript. LP, AOK, ML and HA were involved in critical interpretation of the data. All authors were involved in manuscript revisions, and final version approval.
ACKNOWLEDGEMENTS
We thank Dr. Robert Wills for directing the statistical analysis. We also acknowledge the assistance of Dr. Sinan Kordon in preparing the figures and Maryana Pinchuk in editing the manuscript.
Institutional Review Board Statement
All fish experiments were carried out based on a protocol approved by the Mississippi State University Institutional Animal Care and Use Committee (IACUC#17-288).
Funding
NIH 5T35OD10432-19 Summer Research Experience for Veterinary Students.