Comparative effects of dietary organic and inorganic copper on growth, tissue mineral concentration, and antioxidant enzyme activities of juvenile Nile tilapia, Oreochromis niloticus
- 1. Laboratory of Fish and Shellfish Nutrition, School of Marine Science, Ningbo University, Ningbo 315211, China
Abstract
This study was conducted to compute dietary organic (OC) and inorganic copper (IC) requirements in juvenile Nile tilapia (Oreochromis niloticus) (NT); copper sulfate (CuSO4 ) and Availa-Cu100 were added to a basal diet. Nine tanks of NT with an average weight of 4.30 ± 0.02 g (mean ± SD) were fed semi-purified diets including AvilaCu0, AvilaCu20, AvilaCu40, AvilaCu80, and AvilaCu120; and nine tanks were fed CuSO4 -IC containing 7.86, 15.72, 31.43 and 47.15 mg kg-1 in triplicate groups for 8 weeks. No significant differences were found in survival, hepatosomatic indexes (HSI), visceral indexes (VSI), or condition factors (CF) among all treatments (P> 0.05). The NT fed diets containing OC8 and IC4 had significantly higher weight gains (WG), specific growth rates (SGR), and protein efficiency ratios (PER), with significantly lower feed conversion ratios (FCR) than NT fed AvilaCu0 control diet. The NT feed that contained OC8 and IC12 showed significantly increased magnesium (Mg) levels in muscle and calcium (Ca) content in bone. NT fed diets containing OC12 and IC12 showed significantly higher Cu content in liver levels; while significantly lower in NT fed diets containing OC8, IC2, IC4 and IC8. NT treated with inorganic copper had higher activities of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx); and lower malondialdehyde (MDA) levels than the control fish. Whereas, no significant differences were found in SOD and CAT activities for all test groups of NT fed OC diets. According to the results of this study, the copper amino acid complex (Availa®Cu100) and copper sulphate (CuSO4 ) optimal dietary requirements for NT, Oreochromis niloticus, are Availa®Cu80 and 15.72 mg CuSO4 kg-1, respectively.
Citation
Timothée Andriamialinirina HJ, Taj S, Irm M (2021) Comparative effects of dietary organic and inorganic copper on growth, tissue mineral concentration, and antioxidant enzyme activities of juvenile Nile tilapia, Oreochromis niloticus. J Vet Med Res 8(1): 1205.
Keywords
• Organic copper
• Inorganic copper
• Amino acid complex
• Growth performance
• Oreochromis niloticus
ABBREVIATIONS
Availa®Cu-100: Copper Amino Acid Complex; CAT: Catalase; CF: Condition Factors; CuSO4: Copper Sulphate; FBW: Final Body Weight; FCR: Feed Conversion Ratio; FE: Feed Efficiency; FI: Feed Intake; GPx: Glutathione Peroxidase; HSI: Hepatosomatic Indexes; IBW: Initial Body Weight; IC: Inorganic Copper; MDA: Malondialdehyde; NADPH: Nicotinamide Adenine Dinucleotide Phosphate; NBT: Nitroblue Tetrazolium; NT: Nile Tilapia; OC: Organic Copper; PER: Protein Efficiency Ratio; SGR: Specific Growth Rate; SOD: Superoxide Dismutase; TBARS: Thiobarbituric-Acid-Reacting Substances; VSI: Visceral Indexes; WG: Weight Gain
INTRODUCTION
The development of fish nutrition has come a long way in recent years, but more research is needed for information about micronutrients. Copper (Cu) is a trace element present at the level of the earth’s crust and is necessary for life’s development. It has a crucial role in the production of many enzymes in animal organisms and participates in iron absorption. Cu is also involved in the body’s immune defenses and the fight against free radicals; therefore, it is a very important mineral for feeding animals including fish. For at least over 6000 years, Cu has been to human’s body. Recently, copper compounds have become a significant additive used in aquaculture, fish farming, and fish nutrition. Dietary Cu supplementation have been studied in several species such as blunt snout bream (Megalobrama amblycephala) [1], Korean bullhead (Pseudobagrus fulvidraco) [2], common carp (Cyprinus carpio) [3,4], Nile tilapia (Oreochromis niloticus) [5, 6]. Cu may often be excessively added into fish feed and significant Cu accumulation in specific tissues were observed by dietary Cu exposure [2]. It is an essential trace element for vertebrates, as well as fish. Cu is a necessary mineral for a huge number of biological developments, principally as a cofactor for enzymes such as superoxide dismutase, catalase, glutathione peroxidase and lipid peroxidation [7]. It is also needed for blood formation such as hemoglobin and haemocyanin which are proteins that carry oxygen in the bodies of some invertebrates and shellfish. The absence of dietary copper decreases the concentration of iron in the liver of Nile tilapia [8]. These tests consider that Cu concentrations that surpass 20 µg g-1 could be lethal [9]. With the use of Cu to kill fungi, mollusks and algae show the effects of highly toxicity in aquaculture [10,11] reports that Cu is recorded as the most toxic metals for ecosystems and aquatic organisms, including fish. It effects various blood parameters, growth performance, behavior, reproduction, enzyme activities [12, 13], and increased mortality [14]. The Cu element may be detrimental to fish metabolism, even if they are existent in minor amounts in the ecosystems [15]. Excess Cu in the environment as well as dietary Cu concentrations of ≥ 500 mg Cu kg-1 may be toxic and adversely effect the physiological functions of fish [16, 17]. Exposure to waterborne Cu does not increase tissue Cu accumulation as much as feeding elevated levels of dietary Cu [18, 19].
The Nile tilapia (Oreochromis niloticus) are native to Africa and is one of the first fish raised by humanity. The Egyptians raised Nile tilapia for ornamental purposes as evidenced by a low relief discovered on a tomb dated 4000 years. But more appreciated and efficient is that the breeding really took its momentum between the years 60 and 80 with Nile tilapia’s introduction in Japan, Thailand (1965), Philippines, Brazil (1971), USA (1974) and China (1978). More recently, Nile tilapia are present in more than 150 countries and used in aquaculture development. Parallel to these cultural extensions, there has been a rise in research programs and a considerable increase of these species that shows better productivity due to Nile tilapia’s relative resistance to environmental stressors such as high temperature and easy breeding compared with other species of farmed fish, and Nile tilapia is widely recognized as the most important cultured fish [20]. Information concerning different Cu sources used in fish nutrition is rather limited compared to that available for terrestrial animals; however, significant advances have been made in recent years. This study condenses the pertinent information about organic and inorganic Cu supplements to fish diets, including different effects on growth performance, tissue compositions, and antioxidant enzyme activities in Oreochromis niloticus.
MATERIALS AND METHODS
Diet preparation and composition
Nine isonitrogenous (crude protein, 32%) and isolipidic (crude lipid, 7%) trial diets were prepared with rated levels of different OC sources: control 0 Cu, 20 (2 Cu), 40 (4 Cu), 80 (8 Cu) and 120 (12 Cu) found in Cu amino acid complexes known as Availa®Cu100, and IC pellets containing CuSO4 with 7.86 mg kg−1 (2 Cu), 15.72 mg kg−1 (4 Cu), 31.43 mg kg−1 (8 Cu), and 47.15 mg kg−1 (12 Cu). The control diet (0 Cu) without supplementation of Cu contained 0.032 mg Cu kg−1 diet. The formulated experimental diets and proximate composition are shown in (Table 1). Pellets containing fish meal, soybean meal and rapeseed meal were used as the sources of protein, fish oil and soybean oil with 1:1 ratio as the lipid sources, and the main carbohydrate sources are wheat flour were formed and steamed into approximately 2.5 (1:3) mm and 4 (2:3) mm sized thick pellets in an experimental food mill, (F-26II, South China University of Technology, Guangzhou, China) and baked in an oven for 30 min at 90°C, air-dried at room temperature to a moisture content about 10%, and then stored in a freezer at -20°C before using.
Experimental design and fish culture technique: The feeding trial was made at an experimental base of Ningbo University (Zhejiang, China). NT were obtained from Freshwater Fisheries Research Centre of Chinese Academy of Fishery Sciences (FFRC), Wuxi, Jiangsu-China, a fish hatchery NT weighing between 3.2 to 3.9 g acclimated to outdoor stock tanks for two weeks and fed a commercial low protein diet containing 20% crude protein for satiation. After this adaptation NT were relocated to outdoor experimental test tanks.
At the start of the feeding trial a total of 540 NT, approximately the same size with initial body weights (IBW) between 4.30 ± 0.02 g (mean ± SD), were carefully selected and 20 fish per tank were randomly distributed into 27 circular fiberglass tanks that held 300-L of fresh water, had a diameter of 105 cm, a height of 51 cm, and was equipped with a flow-through water system. Each tank was then randomly consigned to one of the three replicates to accommodate the 9 prepared test diets. All the circular fiberglass tanks were provided with dechlorinated tap water with an exchange rate of one-third of the tank volume per day. Air stones were used for water aeration. HACH HQ30d oxygen meter from Hach Company, Loveland, USA was used to measure daily dissolved oxygen levels and water temperatures. During the trial water temperatures were maintained between 26° – 29°C, dissolved oxygen levels were measured and maintained at 8.33 ± 0.1 mg L-1, water pH levels were kept between 6.3 - 6.7, ammonia-N concentration < 0.06 mg L-1, and the photoperiod time was sustained at 12 hours of light and 12 hours of darkness.
For 8 weeks NT were observed in this study.
NT were fed manually two times a day 1) in the morning at 8:00, and 2) in the afternoon at 17:00. Food was supplied accordingly by using their weight to assure apparent satiation without overfeeding. The amount of eaten diet was recorded every day, while uneaten diets were taken by a mesh collector located under the separate drain pipe on every tank, the food was dried and weighed. Prior weighing, the fish were not fed to avoid the inclusion of food ingestion and the survival was cheked regularly.
Every 2 days one-third of every tank’s water was substituted with freshwater. All tanks were systematically cleaned to reduce algae and fungal formation when the fish were removed for evaluation. NT from every tank were evaluated by weight for growth monitoring. Prior to weighing NT were not fed as scheduled to avoid the inclusion of the weight of food ingested this Sampled NT then were returned to their particular tank.
Every 2 weeks NT were counted for survival.
Sampling and proximate chemical analysis
At the end of 56 days feeding time, the NT were starved for twelve hours according to [21], the NT in each tank were counted and weighted to determine the growth performance, food utilization, and survival rate which included final body weight (FBW), weight gain (WG), specific growth rate (SGR), food intake (FI), food conversion ratio (FCR), food efficiency (FE) and protein efficiency ratios (PER), and then anesthetized in a benzocaine bath (50 mg L-1) as termed by [22]. Five fish from each tank were collected to calculate the proximate chemical analysis of whole body composition agreeing to the standard methods [23] for moisture, total protein, total lipids and ash content. The content of moisture was measured by drying the samples to constant weights at 105°C using a drying oven (GCA, model 18EM, Precision Scientific group, Chicago, Illinois, USA). The content of protein was estimated by multiplying nitrogen content by 6.25 and measured using Kjeldahl’s method and apparatus (FOSS Tecator TM8400, Hoganos, Sweden). The lipid content was determined by the ether extraction in multi-unit extractions Soxtec System HT (FOSS Tecator HT6, Hoganos, Sweden), and ash content was determined by burning the dry fish samples using a muffle furnace (Thermolyne Corporation, Dubuque, Iowa, USA) at 550 °C for 6 hours. Three other NT per tank were randomly selected and weighed with an electronic scale. A measurement board was used to record the standard length (mm). Then, the NT were dissected in the ice to obtain the viscera weight, the liver weight, to determine values for the hepatosomatic index (HSI), viscerosomatic index (VSI) and condition factor (CF). Liver samples were quickly frozen at −80 °C for enzymatic determinations. Remaining NT were butchered to obtain dorsal muscle and bone, which was sealed separately in plastic bags and stored at -20 °C for microelement compositions analysis.
Tissue mineral concentration analysis
The mineral contents of diet, muscle, liver and bones were analyzed for Mg, Ca, Mn, Fe, Cu and Zn using an atomic absorption spectrometry (AA6800, Shimadzu Corporation, Japan).
Antioxidant enzyme activity and lipid peroxidation assays
Liver enzymatic activity and lipid peroxidation levels were analysed with commercial assay kits (Nanjing Jian Cheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions. All enzyme activities were measured simultaneously in triplicate for each sample using a Fluorescence Spectrometer (Perkin-Elmer, LS 55). The determination of SOD Activity was performed by the method of [24]. The sampled autoxidation rates were measured at 550 nm. A unit of SOD activity was calculated using the amount of superoxide dismutase required to inhibit the 50% reduction of nitroblue tetrazolium (NBT). Catalase (CAT) activity was measured according to the method of [25], which is based on H2 O2 degradation by the action of CAT contained in the examined samples. After 10 μL of homogenate was added to the reagent, the sample was incubated for 60 s at 37°C. CAT activity was measured and read at 405 nm by observing the change on absorbance of samples. A unit of CAT activity was performed as the amount of CAT needed to transform 1 μmol of H2 O2 min-1. The activity of MDA was defined in homogenate by the colorimetric method described by [26]. The technique principle was spectrophotometric measurement of the pink color formed through the reaction of thiobarbituric-acidreacting substances (TBARS) measured at 532 nm. The activity of GPx was measured according to the method of [27]. A unit of GPx activity was measured as the amount of glutathione peroxidase needed to oxidize 1 μmoL of NADPH min−1 and was completed conferring to the method of [28], in which the decomposition of H2 O2 was followed spectrophotometrically at 240 nm.
Calculations and statistical analysis
The growth performance and food utilization of NT were determined as follows:
WG, g = FBW (g) – IBW (g) WG, % = 100 x (FBW (g) – IBW (g)) / IBW (g); SGR, % day-1 = 100 [ln (FBW (g)) – ln (IBW (g))] / (day);o you want to FER = wet WG (g) / dry FI (g); FCR = FI (g) / WG (g); PER = wet WG (g) / PI (g); HIS, % = 100 x [liver weight (g) / body weight (g)]; VSI, % = 100 x [viscera weight (g) / body weight (g)]; CF = 100 × [FBW (g) / body length (cm)]: Survival (%) = 100 × [final fish number / initial fish number].
The obtained data were using one-way ANOVA and mean separations were established by using the multiple range test of Duncan’s. Results were presented by means ± SD and the significance level was set at P < 0.05. All statistical calculations were completed with SPSS 18.0.0 for windows (SPSS Inc., Chicago, IL, USA) as defined by [29].
RESULTS
Growth performance
Growth performance of Nile tilapia were significantly affected by organic and inorganic copper (Table 2). Fish in all dietary treatments were progressively developed with time, and the highest final weight was recorded in the 8th week. The maximum FBW, WG, SGR and PER of Nile tilapia were obtained in fish fed diets containing IC8 where the FBW was (27.92g±0.87), WG (23.61g±0.86), WG (548.14%±18.6), SGR (3.34%±0.05) and PER (3.08±0.11); and OC8 where the FBW was (27.17g±0.62), WG (22.87g±0.62), WG (531.41%±13.73), SGR (3.29%±0.04) and PER (2.94±0.06), whereas the poorest growth performance was obtained with fish fed in group OC12 and IC12 diet (P < 0.05). Results of the 8-week feeding trials no mortality was noted in any of the dietary organic and inorganic copper
Feed utilization
NT test groups feed utilization under different treatments are shown in (Table 3). Statistically significant differences were found in FI, FCR and FER between all the treatments (P < 0.05). The lowest FCR was found with the fish fed group IC4 (1.01±0.04) and OC8 (1.06±0.01) diet (P < 0.05), although the highest was found in fish fed control (1.59±0.05) following OC12 (1.44±0.06) and IC12 (1.4±0.04). FER was significantly increased with elevating dietary OC levels up to 80 mg kg-1 and 15.72 mg kg-1 for IC and then remained (P < 0.05). No significant difference was recorded on CF (P ? 0.05). The feeding rate did not influence the HIS, or VSI (P > 0.05).
Diet, whole body mineral and chemical composition
The whole body of fish chemical composition is showing in (Table 4). The moisture content, crude protein and crude lipids were not affected the whole body of fish among the dietary treatments (P > 0.05).
The contents of mineral salts, such as Mg, Ca, Mn, Fe, and Zn were almost similar in the formulated diets (Table 5). However, Cu content was significantly increased (P < 0.05) in OC2 up to OC12 and IC2 up to IC12 supplemented diets fed Nile tilapia.
Tissue biochemical compositions
For tissue mineral compositions (liver, muscle and bone) in (Table 6). Significantly different was found in Mg in muscle, Ca in bone and Cu in liver (P < 0.05). Mg content in muscle was significantly higher in fish diet fed OC12 (27.48±0.59) and IC8 (27.89±0.23) than fish fed with control diet; Ca content in bone was significantly higher in fish diet fed OC8 (233.2±13.69) and IC12 (128.13±24.28) than fish fed with control diet; and the highest Cu content was high in OC12 (1.55±0.01) and IC12 (1.47±0.02). Dietary both inorganic and organic copper levels showed no significant effect on Mn, Fe and Zn contents in liver, muscle and bone tissues (P>0.05).
Antioxidant enzyme activities
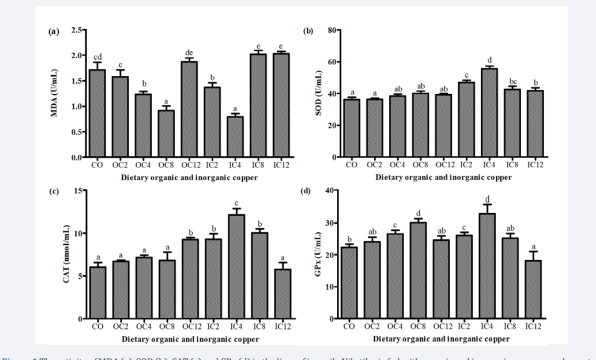
The enzyme activity levels in liver of Nile tilapia fed with dietary organic and inorganic copper are presented in [Figure 1 (a), (b), (c) and (d)]. The activity of MDA was decreased by increasing the dietary organic copper up to OC8, but significantly higher in fish fed OC12 diet compare to the same group. At the same time decreased by increasing the dietary inorganic copper group level up to IC4 and then significantly increased in fish fed diets containing IC8 and IC12 but showing non-significant different between the two groups. The highest MDA were found in fish fed diets OC12, IC8 and IC12 groups. SOD activity in the liver was significantly enhanced in the OC4, OC8, OC12, IC2, IC8 and IC12 groups than in the control, with the maximum SOD activity recorded in the IC4 group. According to the CAT concentration in the liver, CAT activity significantly improved in the OC12 group compare to the other group in organic copper diets. Regarding to the inorganic copper groups, CAT activity increased up to IC4 and then decreased significantly from IC8 up to IC12 groups. The highest CAT activity was found in fish fed diet having IC4. The highest GPx activity was found in fish fed OC8 and IC4 diets, coming with OC4 and IC2 diets, the lowest is IC12 diet (P < 0.05).
Figure 1 The activity of MDA (a), SOD (b). CAT (c) and GPx (d) in the liver of juvenile Nile tilapia fed with organic and inorganic copskr supplemented diets for 8 weeks. Values mean ± SD with different superscripts are significantly different (P 0.05).
DISCUSSION
Growth performance, feed utilization and body composition
The role of copper is as essential in the metabolism and growth of fish as in any living organism. Although, according to [30], a slightly higher than normal copper concentration for growth and reproduction may have irreversible effects. In this study, no mortality was found during the experiment. A similar finding [31] has shown that dietary copper has no effect on Nile tilapia survival. The study of [32, 33] have shown that the toxic effects of copper on fish and their metabolism depend on many factors, namely: copper concentration; water quality such as: temperature, salinity, pH, hardness, suspended solids and organic matter; and interactions with other elements; organisms, size, age species and fish affected, as well as basic exposure of copper.
The present study is the first report on dietary organic Cu levels in Nile tilapia, although several studies have reported results from other copper sources in Nile tilapia. Various results indicated that organic and inorganic graded levels of copper have significant effect on the growth performance (Table 2). The fish food having 80 mg Availa-Cu100 kg-1 and 15.72 mg CuSO4 kg-1 had the maximum ratio of FBW, WG, SGR and PER of Nile tilapia. [34] reported the similar result as in juvenile beluga had the optimum dietary Cu levels between the range of 10.3 and 13.1 mg Cu kg-1 diet, when copper sulphate is used as inorganic copper. In the present work the growth performance of Nile tilapia fed 120 mg Availa-Cu 100 kg-1 and 47.15 mg CuSO4 kg-1 significantly decreased in terms of growth rate and food conversion ratio compared to those fed 80 mg Availa-Cu100 kg-1 and 15.72 mg CuSO4 kg-1 (Tables 2 and 3). The results can be explained that the high dietary copper levels reduce the growth. A previous study showed that the growth performance of Nile tilapia fed 1000 and 1500 mg Cu kg−1 effected by the toxicity of dietary copper addition [8]. Copper is also an essential micro elements in aquatic organism, its requirements have been adapted for certain species of shrimp. [35] reported that in pacific white shrimp (Litopenaeus vannamei), dietary Cu hydroxychloride is effective copper source; however, CuSO4 plays different metabolic appearance and revealed no measurable impact on growth performance of shrimp. According to [36], juvenile Malabar grouper (Epinephelus malabaricus), was significantly higher WG with 2.55 mg kg-1 and lower with 0.19, 8.99 and 12.81 mg Cu kg-1 [37] when the diet was supplemented with organic copper (Cu peptide); though, by the same fish species, diet supplemented with 4.37 and 6.56 mg kg-1 CuSO4 improves growth and food utilization [36]. In large yellow croaker (Larimichthys croceus), the higher concentration of Cu reduced growth performance [38]. [39] studied on Gibel carp (Carassius gibelio) stated that fish fed diets containing of 3–6 mg kg-1 copper level had obviously higher WG and SGR, besides relatively lower FCR. According to the results of the present study, fish fed diet containing 120 Availa-Cu100 mg kg-1 and 47.15 CuSO4 mg kg-1 significantly reduced WG, SGR and higher FCR, with agreement of (Damasceno et al., 2016) research that higher concentrations of copper than required effects the growth and food utilization of NT.
Food conversion ratio in this experiment was significantly different between all treatments (P <0.05). The lowest FCR was registered in the OC8 diet followed by the IC4 diet indicates the greatest food Cu qualities. Similar discovery with American catfish (Ictalurus punctatus), high copper levels slowing growth and altering FCR [40]. Poor uptake or absorption of main nutrients is unlikely as VSI, HSI and CF remained the same for all treatments throughout the experiment (Table 3). Moisture, crude protein and lipid amounts of the whole fish body were not significantly influenced by copper management (P> 0.05), (Table 4). These results suggest that dietary copper couldn’t affect digestibility and nutrient deposition. On the other side, the protein and lipid quantities of whole-body fish could be related to changes in their synthesis, muscle rate deposition and/or a diverse growth rate [41-43].
Tissue biochemical compositions
Copper is one of the crucial trace elements for many functionalities. As such, Cu is involved in the metabolism of several nutrients: carbohydrates, lipids and iron; and contributes to the formation of red blood cells, immune defenses, regulation of neurotransmitters, melanin production and especially bone mineralization. Fish is a highly protein food consumed by the population. A large proportion of consumers do eat fish due to its availability, flavor and taste, although a lower proportion do so because of their nutritional significance. Therefore, studies on the tissue biochemical composition of freshwater fish have not really attracted to the attention of fisheries research. Concentrations of Mg, Ca, Mn, Fe, Cu and Zn in tissue (liver, muscle and bone) of Nile tilapia are presented in (Table 6). As the human population consumes more fish, it is therefore important to study the heavy metal concentrations in fish. Several studies have shown that a high concentration in fish tissues can vary considerably from one species to another. This was probably due to differences in metabolism and feeding of fish patterns. This present study’s results show that fish feed diets containing OC8 and IC12 significantly increased Mg in muscle and Ca content in bone.
This present study resulted that fish feed diet containing OC8 and IC12 significantly increased Mg in muscle and Ca content in bone. It was found that amount of Cu in liver is highly significant in fish fed diet containing OC12 and IC12, while it is lower significant in fish fed diet containing OC8, IC2, IC4 and IC8. According to [8], Fe concentration in liver of Nile tilapia decreases due to the absence of dietary copper; however, the current study showed that dietary organic and inorganic copper did not affect the Fe in fish tissues. The difference of these studies could conclude that the amount of copper in diet and species are probably different. This study showed that Nile tilapia is a good source of minerals. Thus, it is fair to say that the mineral content of each species depends on the availability of these elements in their local environment, ability to absorb food and their preferential accumulation. According to the current research, the order of accumulation of Cu in the tissues is as follows: liver> muscle> bone. While in rockfish tissues, the pattern of Cu accumulation is different, it is dependent upon exposure times; however, liver tissue shows a larger storage than other tissues. The Cu accumulation order in the tissues was liver> intestine> kidneys> gill> muscle [44]. However, very low value of micronutrients was recorded as the body needs it in a small quantity and the water concentration in the body are minimum. It has consequently become important to reflect the state of fish minerals and the persistence of fish feed security prior to consumption, in addition to the dominant choice of taste, size, type and external morphology. In addition, this work has led to a better understanding the importance of fish as a respectable source of protein and minerals. Further studies are still needed for a nutritional balance that could help eliminate the toxic elements of body tissue by providing the preferred minerals, thus allowing toxic elements to be released and eliminated.
Antioxidant enzyme activity and lipid peroxidation assays
Copper plays an antioxidant role to animals, it is involved in enzymes activity such as MDA, SOD, CAT and GPx; and cofactor of superoxide dismutase, a key enzyme protector against excessive oxidation phenomena. Organic copper has metabolic roles [45]. In the eyes, the amount of copper is more abundant and bound to a melanin protein and forms a copper-protein complex. Other organs such as the liver, brain and heart as well contain relatively large amounts of copper. Ceruloplasmin is a type of copperprotein complex with oxidative activity found in blood plasma. The present research investigated the antioxidants enzyme in liver of Nile tilapia fed dietary copper organic and inorganic. MDA content delivers a suitable key of lipid peroxidation [46], commonly used as the convenient index for oxidative harm to lipid and can also redirect the fish antioxidant status. In the current study, high liver MDA levels were detected in the high dietary copper groups, it may indicate dietary copper toxicity of juvenile fish. The SOD and GPx activities were significantly increased with increasing CuSO4 in diets, while decline with increasing Availa-Cu in liver of fish. In channel catfish, [47], noted that a lower amount of copper affects the activity of cytochrome oxidase in the heart, whereas copper-zinc superoxide dismutase in liver. While the consumption of a large amount of copper prevented the activity of the antioxidant enzyme and lipid synthetase [38].
In terms of animal performance, organic minerals have positive effects compared to inorganic sources because of their higher bioavailability. Numerous studies have revealed significant differences in the bioavailability of organic and inorganic minerals in different animal species with different sources of mineral elements. These studies suggest that the binding of Cu, Zn, Fe and Mn with amino acids and peptides could improve the bioavailability of these trace elements, thereby improving growth, reproduction and overall health of animals. Presently, there is not much information on the effect of dietary organic copper in fish. Therefore, these data showed that there is an additional response to Availa-Cu on fish performance. This reaction may be due to a perceived systemic effect of copper supplementation and the high bioavailability of this source of organic copper. Researchers expect the results of this research to influence the decisions of practicing nutritionists as to the advisability of adding organic copper.
Therefore, this present script offers valuable information about effects of two dietary copper sources level and showed that copper sulphate (CuSO4 ) is more bioavailable for Nile tilapia than copper amino acid complex (Availa®Cu100) and could be used as a means of selecting the copper sources.
REFERENCES
11. Okocha R, Adedeji O. Overview of copper toxicity to aquatic life. Report and Opinion. 2012; 4:57-67.
12. Sorensen EMB. Metal Poisoning in Fish. CRC Press, Boca Raton. 1991:175-234.
20. Welker T, Lim C. Use of Probiotics in Diets of Tilapia; 2011.
23. AOAC. Official Methods of Analysis. Association of Official Analytical Chemists. 2000:56.
25. Aebi H. Catalase in vitro. Methods in enzymology. 1984; 105:121-126.
27. Flohé L, Günzler WA. [12] Assays of glutathione peroxidase. Methods in enzymology. 1984; 114-120.
30. Baldwin DH, Sandahl JF, Labenia JS, Scholz NL. Sublethal effects of copper on coho salmon: Impacts on nonoverlapping receptor pathways in the peripheral olfactory nervous system. Environmental Toxicology and Chemistry. 2003; 22(10):2266-2274.