Effect of the Endocrine Disrupter BPA on the Rabbit Fetal Gonocyte Population
- 1. Department of Cell Biology and Physiology, Institute of Biomedical Research, UNAM, Mexico
Abstract
Bisphenol-A (BPA), an environmental pollutant with estrogen-like activity, is extensively used in polycarbonate plastics and epoxy resins, leading to widespread human exposure through ingestion, inhalation, and dermal contact. Despite its detection in pregnant women, amniotic fluid, placenta, breast milk, and cord blood, the impact of BPA on reproductive health remains unclear. Previous studies in murine models indicate potential effects on the male and female reproductive systems during developmental and adult exposure. However, conflicting results and the considerable morphogenetic differences between murine and human gonads raise concerns about the translatability of findings. Here, we explore the rabbit as an alternative model due to its physiological similarities to humans in embryonic development. Gonocytes, essential for germline cell production, express pluripotency genes OCT4, NANOG, and SOX2, with a unique regulation pattern during fetal development. In this work, we investigate the influence of BPA on the expression of these genes in rabbit fetal testes, aiming to establish rabbits as an alternative model for studying male reproductive diseases. The findings suggest a potential synergistic effect with Leydig cell-mediated steroidogenesis, contributing to a more comprehensive understanding of gonocyte differentiation in larger mammals and highlighting rabbits as a valuable model for male reproductive studies.
Simple Summary: The widespread use of the environmental pollutant Bisphenol-A (BPA) raises concerns about its potential impact on reproductive health, including testis cancer. Despite murine studies indicating BPA’s influence on the male and female reproductive systems, contradictory results and uncertainties persist, especially concerning the fetal testis. This study proposes the rabbit as an alternative model for assessing BPA’s effects on gonocyte differentiation during fetal testis development. Gonocytes, vital for genetic transmission, express pluripotent genes (OCT4, NANOG, SOX2) during development. Through molecular and immunocytochemical techniques, alterations in OCT4, NANOG, and SOX2 expression are observed in rabbit testes exposed to BPA, suggesting the rabbit is a potential alternative model for comprehending gonocyte differentiation in larger mammals and investigating male reproductive diseases. This experimental model contributes to understanding the heterogeneous development mechanisms of gonocytes and highlights the rabbit’s potential as an alternative model in studying the impact of endocrine disruptors on male reproductive health.
Keyword
- BPA; Pluripotency; Gonocytes, Rabbit development, Leydig cell, reproduction
Citation
Collazo-Saldaña P, Marmolejo-Valencia A, Merchant-Larios H (2024) Effect of the Endocrine Disrupter BPA on the Rabbit Fetal Gonocyte Population. J Vet Med Res 11(2): 1268.
INTRODUCTION
Bisphenol-A (BPA) is an environmental pollutant with estrogen-like activity [1,2] often used to produce polycarbonate plastics and epoxy resins for canned food products. Approximately 8 billion tons of BPA are produced worldwide yearly [3], and most of it becomes part of our daily life due to widespread exposure through ingestion, inhalation, and dermal contact. Thus, it is not surprising that high levels of BPA have been detected in the worldwide population. Moreover, BPA has also been detected in pregnant women, human amniotic fluid, placenta, human breast milk, and cord blood, suggesting a possible effect on embryonic development and reproductive health [4,5].
The effect of BPA on reproductive health is still unclear despite studies in murine models. Recent works suggest that BPA affects the male and female reproductive systems during developmental and adult exposure [6-8], suggesting a strong influence of BPA during embryonic development and the differentiation of gonads [9]. However, some of these works showed contradictory results in vivo animal studies, with high variations in the time of exposure, stage of embryonic development, BPA concentration, and time of evaluation, specifically in the fetal testis in which it is still unclear how BPA could alter the male germ cells known as gonocytes [6,7,10,11]; nevertheless, all evidence of these results has been in mice, and the morphogenetic patterns of the gonads between mice and humans differ considerably [12-15]. Therefore, there has been growing concern regarding the extensive use of rodents as models for assessing the effects of endocrine disruptors on the human fetal testis [15,16].
The rabbit is an animal that has been widely studied in embryology. Its genes and physiology are very similar to humans, and even the gonadal pattern of development and gene expression resembles that of humans [17-19]. These similarities could make the rabbit an alternative animal model to study the effects of gonocyte differentiation. There is an increase in studies using rabbits in many fields, and technologies have been successfully implemented [19-22].
Gonocytes are specialized cells that can differentiate into spermatogonia stem cells that produce the rest of germline cells that can pass on genetic material from one generation to another. During gonad differentiation, they can express genes of pluripotency, such as OCT4, NANOG, and SOX2, like pluripotent stem cells (PSCs) [23-25], which can differentiate into the three primary germ cells of the embryo. This specific expression allows gonocytes to have a high proliferation rate to colonize the mammalian gonad and prevent their differentiation towards the somatic lineage. Eventually, the transcription factors of OCT4, NANOG, and SOX2 are repressed in the gonocyte nucleus when they begin the G0/G1 phase of the cellular cycle, called mitotic arrest [26,27]. In murine testes, the downregulation of these factors occurs in two days (E.12.5- E.14.5) [27,28], while in humans, these expressions are regulated over several months during the fetal period [29-32]. We considered these three genes in this work because they control the pluripotent network [33].
A few studies have demonstrated how OCT4, NANOG, and SOX2 can upregulate mRNA expression levels in embryonic stem cells (ESCs) treated with BPA in vitro [34]. Furthermore, previous studies have shown that rabbits can respond to treatments with estrogens or androgens during the fetal period, making them a potential alternative model to study male reproductive diseases [35]. In this study, we aimed to determine the capacity of BPA to alter the expression profile of OCT4, NANOG, and SOX2 in the testes of rabbits exposed to this endocrine disruptor during the fetal period. The rabbits were exposed to BPA orally and reached the embryos through the placental barrier [4]. Then, the testes of the treated embryos were collected at different developmental stages. Quantitative PCR (qPCR) was used to measure OCT4, NANOG, and SOX2 mRNA expression levels. Additionally, immunofluorescence was used to show the presence or absence of the proteins of these three factors during gonocyte development in control and treated animals. This experimental model could help to understand the heterogeneous development mechanisms of gonocyte differentiation in larger mammals than murine and establish rabbits as a potential alternative model to study male reproductive diseases.
MATERIALS AND METHODS
Animals
The female and male New Zealand rabbits used for this project were obtained from the Facultad de Estudios Superiores de Cuautitlán Itzcalli, UNAM. The rabbits were maintained under similar conditions of light-dark photoperiod (12:12 hours) and were provided with ad libitum food and water. The bottles used in this work were BPA-free. As rabbits have mount-induced reflex ovulation, copulation day was considered day “0” or 0 dpc.
Sample collection
Testes were collected at 21 dpc, 26 dpc, 31 dpc, 15 days post- partum (dpp), 30 dpp, and adulthood of 6 months based on a previous study [17], which established the sexual development of rabbit gonads, and observations of the proliferation marker Ki67 during critical stages of gonadal development in rabbits (results not published). The gonads were quickly dissected and put into PBS (10 mM Na2HPO4 and 137 mM NaCl, pH 7.4) containing 0.1% diethylpyrocarbonate (DEPC). The samples were fixed with 4% PFA or frozen with dry ice for immunofluorescence or RNA extraction, respectively.
Treatment
From 15 dpc until 30 dpc, pregnant female rabbits were treated with BPA (Sigma Aldrich, USA) dissolved in 2 mL of a vehicle composed of 12.5% ethanol, 14% glucose, and the remaining water. The control group received only the vehicle. The Lowestt Observed Adverse Effect Level (LOAEL) dose was established by the Environmental Protection Agency (EPA) in 1988, and it was 50 mg/kg body weight/day for experimental animals through oral administration. Additionally, a study by Fang et al. [36], using the same species and exposure route, reported similar concentrations of unconjugated BPA (40 ng/ mL) in rabbit serum as have been reported in human serum [37- 39].
Quantitative RT-PCR
RNA extraction was performed following the supplier’s instructions. During the extraction of nucleic acids, the samples were treated with DNase I (RNase-Free, DNase Set, Qiagen) to remove only the genomic DNA and isolate all RNA. For cDNA synthesis, Transcriptor Reverse Transcriptase (Roche) was used on 1 or 2 µg of total RNA, using the specification and protocol recommended by the supplier, including random primers (Invitrogen) and oligo (dt)18 (Thermo Scientific). The reaction for qRT-PCR contained a total volume of 10 µL, using 5 µL of SYBR Select Master Mix (2X) (Applied Biosystems), 1 µL of each oligo (Forward and Reverse) [10 mM], 1 µL of cDNA (20 ng/µL) as template, and finally, 2 µL of ultrapure water (Pisa). ΔΔCt was used to quantify the relative expression. As previously reported, primers and melting temperatures were used [22]. Primers used for qPCR are shown in Supplementary Figure 1 (A) Immunocytochemistry for Sertoli cells marker GATA4 (green) and OCT4 (red) within the seminiferous cords (white delimited area) in the testis of 21 dpc. Scale bar =50 µm. (B) Immunocytochemistry for pluripotency markers OCT4 (red) and SOX2 (green) in gonocytes (white square area). (C) Immunocytochemistry for OCT4A and NANOG in the nucleus of gonocytes (white square area). DAPI was used for nuclear detection.
The products were cloned and sequenced to verify fragment authenticity at the Instituto de Investigaciones Biomédicas, UNAM.
Immunofluorescence
Samples at 21, 26, and 31 dpc together with 15, 30, and 180 dpp were treated with 4% of paraformaldehyde (PFA) overnight at 4 °C. PFA-fixed samples were dehydrated with different gradients of saccharose and included in OCT Compound (Tissue-Tek) at -70°C. Slices (30 mm thick) were obtained using a cryomicrotome (Kedee) and were exposed in heat buffers to expose the antigens, and the specifications of antibodies used are in Supplementary Figure 2.Immunohistochemistry for OCT4 (red) and SOX2 (green) in the rabbit testis at 21 dpc. Each column represents a different z-stack. Gonocytes with double staining for OCT4/SOX2 are highlighted in the yellow square. Gonocytes with only staining for OCT4 are described in the white circle. TOTO was used for nuclei detection. Scale bar = µm.
30 µm thick serial sections of longitudinally oriented testis were alternately placed on of five slides to include adjacent sections of each testis. It is essential to mention that the OCT4 protein used for the current study was searched to detect the pluripotency isoform OCT4A (reviewed in Wang and Dai 2010). The primary antibody was incubated overnight and then washed three times with PBS. Then, the second antibody, Alexa Fluor (Invitrogen), was diluted 1:200 in PBS and incubated for 20 minutes at room temperature. The nucleus was stained with DAPI (4′,6-diamidino-2-phenylindole) 3mM diluted 1:500 in PBS for 10 minutes. Finally, the slides were covered with Dako (DAKO) and observed using confocal microscopy (Nikon Storm).
Cell counting
To determine the number of gonocytes positive for OCT4A and SOX2 proteins in the nucleus, we took z-stacks using confocal microscopy. Labeled cells were identified in four confocal stacks f 2.5 µm optical sections. Each slide contained four slices of 30 µm thick, and each slice was divided into three different fields. Once we obtained the microphotographs with a 60x objective, we delimited an area of approximately 10,000 μm2 of seminiferous cords. We multiplied that number for the total optical sections (10 µm) to obtain a total volume of 10,000 μm³ for each z-stack. We analyzed nine z-stacks for each rabbit; four were used in each treatment. We considered a positive mark in the nucleus of gonocytes when at least two consecutive focal planes of the z-stack colocalized with DAPI staining to avoid artifacts.
Statistics
Data was shown as mean and standard error (SE). All data was analyzed in the Gradprism 8 program. Firstly, we ran a normality probe to verify the expected behavior of our data. Then, we compared different ages and groups using the Two- way ANOVA test. We used multiple Bonferroni comparisons to discriminate between significant differences among ages and groups. All rabbits were sacrificed according to the Norma Oficial Mexicana NOM-062-ZOO-19 “Specific techniques for the care, use, and handling of laboratory animals.”
RESULTS
Transcription factor OCT4 is expressed in gonocytes
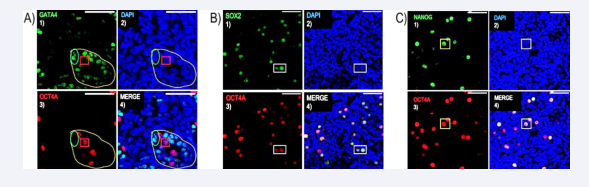
To determine whether OCT4A is a specific marker of gonocytes in rabbits, we conducted double immunofluorescence on the entire testis to identify the localization of this transcription factor within the seminiferous cords Consistent with human observations, our results revealed that OCT4 protein expression in the seminiferous cord is confined to gonocytes, as evidenced by the absence of colocalization with the GATA4 marker, which is specific for Sertoli cells [Figure 1A]. Subsequently, we performed double immunofluorescence using SOX2/OCT4A [Figure 1B] and NANOG/OCT4A [Figure 1C] antibodies to assess whether these transcription factors are also expressed in gonocytes. Confocal microscopy images demonstrated the specific expression of all three pluripotency markers in the nuclei of rabbit gonocytes [Figure 1B-C].
Figure 1: OCT4 protein expression detected on rabbits’ gonocytes.
(A) Immunocytochemistry for Sertoli cells marker GATA4 (green) and OCT4 (red) within the seminiferous cords (white delimited area) in the testis of 21 dpc. Scale bar =50 µm. (B) Immunocytochemistry for pluripotency markers OCT4 (red) and SOX2 (green) in gonocytes (white square area). (C) Immunocytochemistry for OCT4A and NANOG in the nucleus of gonocytes (white square area). DAPI was used for nuclear detection.
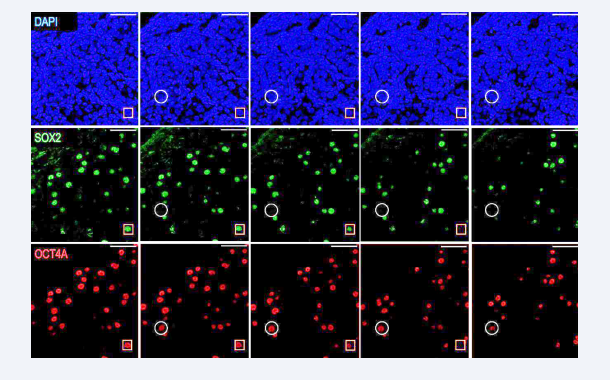
Interestingly, based on confocal microscopy images and the analyzed z-stacks, the double immunofluorescence of OCT4A/ SOX2 exhibited a non-perfect match [Figure 2],
Figure 2: Heterochronic expression of pluripotency factors in gonocytes.
Immunohistochemistry for OCT4 (red) and SOX2 (green) in the rabbit testis at 21 dpc. Each column represents a different z-stack. Gonocytes with double staining for OCT4/SOX2 are highlighted in the yellow square. Gonocytes with only staining for OCT4 are described in the white circle. TOTO was used for nuclei detection. Scale bar = µm.
revealing the co- expression of both OCT4 and SOX2 in some nuclei. In contrast, only OCT4 was observed in others. This discrepancy could suggest that the expression of these factors is heterochronic in gonocytes, mirroring what is observed in human fetal testes.
BPA increases the expression of pluripotent markers in gonocytes
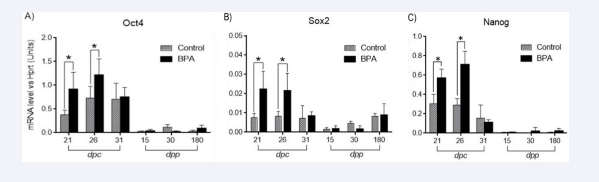
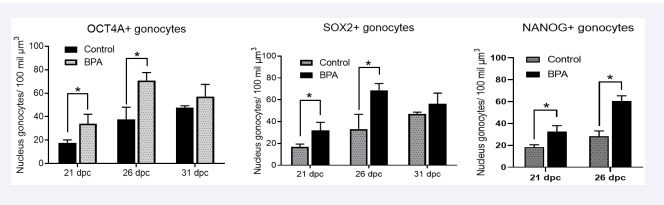
To assess whether BPA affects the expression of pluripotency markers in gonocytes, the mRNA expression levels of the three primary pluripotent markers—OCT4, SOX2, and NANOG—were analyzed. The expression profile of OCT4 showed the highest levels at 26 dpc for both the control and BPA groups. We observed that at days post-partum, the mRNA levels of this transcription factor decreased and remained low until adulthood. Testes treated with BPA showed significantly higher expression levels at 21 and 26 dpc when compared with control animals. At dpp, the expression levels in both groups were similar; therefore, no significant differences were observed between them [Figure 3A].
Figure 3: Gene expression analysis with RT-qPCR of the three pluripotency genes expressed in developing rabbit testes. (A) OCT4 (A), SOX2 (B), and (C) NANOG (C) at different days of development (black bars). Significant expression levels increase in BPA-treated animals compared with control testes at 21 and 26 dpc (grey bars). Data was normalized by HPRT expression. *p < 0.05. n=5 independent experiments. Bars represent SD.
In contrast to OCT4, the expression levels of SOX2 remained similar in all ages in the control groups. Higher expression levels of SOX2 were observed in animals treated with BPA only during the fetal period (21 and 26 dpc). No significant differences were observed between the control and BPA groups at 31 dpc, 15 dpp, and 30 dpp [Figure 3B].
Similar to OCT4, the expression profile of NANOG revealed higher expression at 21 and 26 dpc in both control and BPA groups. Meanwhile, lower expression was detected at 15 dpp for both groups [Figure 3C]. Consistent with the expression levels of OCT4 and Sox2, a significant difference in the expression profile at 21 and 26 dpc was demonstrated [Figure 3C]. From 31 dpc, the expression levels in both groups did not show significant differences. Together, these results showed that mRNA expression levels of these three pluripotency factors were increased only during the fetal period in BPA-exposed animals, and no differences were observed after birth [Figure 3].
BPA affects the proliferative state of gonocytes in rabbit testis
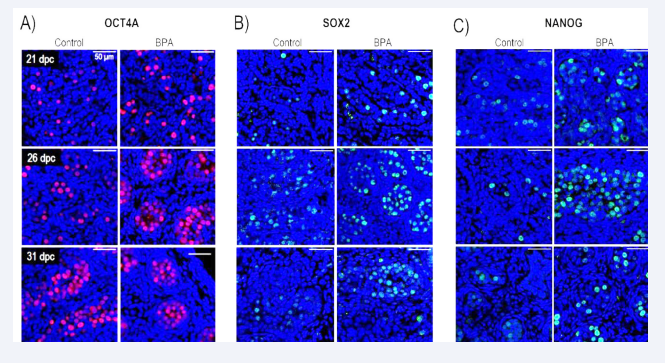
After determining the OCT4A, SOX2, and NANOG expression levels in rabbit gonocytes, we analyzed the expression patterns of these pluripotency markers at 21, 26, and 31 dpc. Similar to what was observed in the gene expression analysis, both the control and BPA groups exhibited positive staining for these factors [Figure 4].
Figure 4: Immunocytochemical detection of three pluripotency transcription factors in nuclei of gonocytes of rabbit testes treated with BPA at different days (A) OCT4A (B) SOX2 and (C) NANOG were treated at 21, 26, and 31 dpc, respectively (right columns). Controls are shown on the left columns. n = 4 independent experiments. Scale bars = 50 µm DAPI was used for nuclear detection.
Gonocytes were not positive for both groups for OCT4A and NANOG in the post-natal ages. However, the SOX2 antibody was detectable in adulthood at 180 dpp in some cells within the seminiferous tubules for both groups.
Through a standardized quantification method previously validated by Ortega-García in 2021. [22], we obtained the count of gonocytes positive for the proteins OCT4A, SOX2, and NANOG within the seminiferous cords in both control and BPA-treated animals [Figure 5].
Figure 5: Quantifying the three markers associated with pluripotency: OCT4A, SOX2, and NANOG in nuclei of gonocytes at different dpc from BPA-treated pregnant females. A) Gonocytes positive for OCT4A, b) SOX2. *p < 0.05; n = 4 independent experiments.
It was determined that there was a significant increase in the number of gonocytes expressing all three proteins at 21 and 26 dpc in animals exposed to BPA. However, at 31 dpc, the observed gonocytes showed no significant differences.
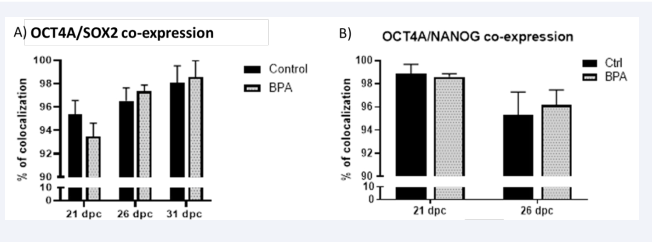
The quantitative results obtained through our cell counting method revealed an average colocalization of up to 90%, depending on age and group. However, we did not observe 100% colocalization of the gonocyte nucleus for OCT4A/SOX2 and OCT4A/NANOG [Figure 6], contrary to what was observed in mice and rats. The results obtained for OCT4A/SOX2 at 21 dpc showed an average of 95% in the control group and 93% in the BPA group, respectively. At 26 dpc, the average was 96% for the control group and 97% for the BPA group. Finally, at 31 dpc, the average was around 98% for both groups [Figure 6A].
Figure 6: Semiquantitative Co-expression of Pluripotency Markers In A), protein colocalization of OCT4A and SOX2 can be observed at different ages, and in B), the colocalization of OCT4A and NANOG, where the black bars represent control animals, and the gray bars represent animals treated with BPA (50mg/kg). The standard deviation is depicted above each bar with n=4. The percentage of colocalization ranges from 0% to 100%.
Additionally, the double immunostaining of OCT4/NANOG at 21 dpc exhibited up to 98% for both groups. In comparison, at 26 dpc, the colocalization for both groups decreased to 95% [Figure 6B].
DISCUSSION
In 1947, Alfred Jost used the rabbit to determine sexual differentiation in mammals. Despite mice and rats being the most common mammals for experimental studies, the morphogenetic pathways differ considerably in larger species, such as humans [28-30,32]. Recording the cortex and medulla establishment in the rabbit fetal gonad and the pattern of SRY and SOX9 genes (involved in gonadal sexual determination) is similar to human reports [17,40].
Fetal period (21 and 26 dpc)
During the fetal period in humans and rodents, a significant abundance of Oct4 transcripts and protein presence has been reported [32,41]. In rabbits, Oct4 mRNA expression is remarkable during the fetal period until newborns (21, 26, and 31 dpc). The number of Oct4-positive gonocytes at 26 dpc was higher than at 21 dpc in the control group [Figure 4A], consistent with mRNA levels [Figure 1A]. In humans, Oct4 transcripts and protein have been highly detected during the first and second trimester of pregnancy in the fetal testis until neonatal age and later become undetectable [29,31]. Conversely, mice have shown considerable transcript level expression from birth to adulthood and protein expression after birth [28,42,43]. These results demonstrate the variability of gonocyte development between species [28,41], highlighting the necessity of different animal models to extrapolate results more confidently to understand human gonocyte development.
Furthermore, the transcript levels of Nanog and Sox2 were downregulated in gonocytes [Figure 1B and 1C]. The expression of the three genes was also observed during the onset of mitotic arrest at 31 dpc [44]. The protein expression of SOX2 and NANOG in rabbit fetal testis showed heterogeneity. We found a small population of gonocytes that were negative or weakly positive for SOX2 protein expression since 21 dpc, suggesting that these gonocytes may belong to another population of differentiation. This result has not been described in other species besides humans [30,45,47].
In contrast to rodents and rabbits, SOX2 protein was not detected in humans during the fetal period [48]. However, SOX2 has been studied in human testis with neoplasia, and its cellular presence was observed in Spermatogonia, Sertoli cells, and spermatocytes, with expression varying between cell types according to the anti-SOX2 antibody used [49]. The results about SOX2 protein expression in gonocytes differ between species, techniques, age, conditional pathologies, and antibodies for staining gonocytes. Moreover, in fetal human cases, the samples were obtained from aborted fetuses.
Newborn and Neonatal (31 dpc and 15 dpp)
In rabbits, as shown in Figures 2-4, the transcript levels of Oct4, Sox2, and Nanog were found to be downregulated at 15 days post-partum (dpp). The regulators for these pluripotency genes may increase during mitotic arrest. Some factors, such as Nanos2 (which promotes male germ cell differentiation), have been reported to be expressed in rabbits (Saga, 2010; Daniel-Carlier et al., 2013). Studies in rodent testes have clearly described the time-lapse expression of Oct4, Nanog, and Sox2, which is observed for three days (10.5-13.5 days post-coitum, dpc), after which it is downregulated. In rabbit testes, the protein expression of OCT4 corresponds to at least 17 dpc to 10 dpp. However, the heterochrony of human testis development, similar to that of rodents or rabbits, is unclear. Delimiting these stages is complex because, in humans, this process begins at four weeks of gestation and continues until birth [30].
Childhood and Adulthood (30 dpp and 180 dpp)
In contrast with the mouse, the presence of OCT4 and NANOG proteins was not observed in the adult stages in this study [28], The expression levels of their transcripts remained relatively low [Figure 1]. However, SOX2’s mRNA expression remained similar to the fetal stage. This expression suggests that SOX2 is involved in a gene network related to the process of spermatogenesis, probably in spermatogonia. Finally, our mRNA expression profile is comparable to that obtained by Daniel-Carlier in 2013, using similar techniques but different oligonucleotides. However, we cannot yet highlight any isoform of this gene in rabbits as described in humans (reviewed in Wang and Dai [50]). The expression profiles of Nanog and Sox2 are defined for the first time in this study model, demonstrating their specific expression in gonocytes in fetal and newborn testis [Figure 1]. Unfortunately, we could not compare the results of this research project with those of the human species, as there is a lack of published data and reports on the expression of these genes and their proteins in healthy adult testes.
Biological implications on fetal germ cells by BPA
According to the literature, in the murine model, the onset of mitotic arrest of gonocytes is crucial for their proper development and differentiation into spermatogonia [51]. The entry into mitotic arrest of gonocytes is closely correlated with the expression of NANOS2, which begins to be detected in rabbits at 28 dpc [44]. This study observed that the number of gonocytes significantly increased in the BPA-treated group during 21 and 26 dpc before mitotic arrest), as the number of BPA-treated gonocytes normalized by 31 dpc (once mitotic arrest had begun) [Figure 5]. Thus, a regulatory mechanism in the cell cycle of gonocytes during mitotic arrest determines the adequate or necessary number of gonocytes sufficient for adult life [52-54].
It is important to note that gonocytes are susceptible to the environment, particularly their cellular niche. Therefore, if somatic cells are also vulnerable to BPA, the pathway of gonocyte alteration may be indirect through the somatic cells found in their cellular niche [12,7]. Some studies have shown the critical relationship that the process of steroidogenesis and its regulation have on the proliferation of Sertoli cells and gonocytes during fetal development through the feminization of the testis (by blocking the androgen receptor), resulting in an increase in gonocytes during their proliferative stage before mitotic arrest [55]. Recently published results using the same type of treatment as in this study determined the effect of BPA on steroidogenic cells (Leydig cells), where it was observed that 1) Leydig cell proliferation decreased in animals treated with BPA, 2) androgen receptor mRNA levels decreased with treatment, and 3) the transcripts of the enzymes CYP11A1 and 3 Βhsd (critical enzymes for the steroidogenesis process) were also down-regulated by treatment [22]. Combining all these factors contributes to this project’s observed increase in gonocytes. The results obtained in our laboratory, using the same treatment on New Zealand rabbits but with a focus on steroidogenic enzymes, have shown a lower number of Leydig cells in the testis (analyzed by Ki67, a marker of proliferation), a decrease in mRNA transcripts of CYP11A1 and 3βHSD (critical enzymes for the steroidogenic process), and an effect on mRNA androgen receptor during the fetal period. These lower transcripts may reflect the crucial impact of BPA on Leydig cells [22], which may dysregulate the control of gonocyte proliferation during the fetal period, known as the period of fetal proliferative gonocytes (PFPG).
Likely, the excess of gonocytes that increased during PFPG was generally regulated by apoptosis or another form of cellular death, as observed in neonatal rabbit gonocytes [56]. Still, this process occurred prematurely in the BPA group due to increased gonocytes [Figure 5B and 5C]. Moreover, this dysregulation could reduce gonocytes, leading to a lower number of spermatogonia and altering the morphology or number of sperm in adulthood, as demonstrated in rodent studies exposed in utero to BPA [52-54].
The effects of BPA were observed primarily during the fetal period, and no significant effects were observed during post- natal stages. In Figure 6, we illustrate the normal and theoretical conditions of gonocytes in the presence of BPA. Despite the heterogeneous population of rabbit gonocytes in fetal testis (21 and 31 dpc), we propose that some cells with unknown characteristics may be more susceptible to environmental pollutants such as BPA than others. While the typical response is apoptosis and genomic instability repair, a small population of spermatogonia cells may remain altered.
CONCLUSIONS
Cellular homeostasis is regulated by intrinsic and extrinsic signaling. Our current results indicate that BPA steroidogenic dysregulation alters the timing of both the expression of three pluripotency genes and the cell cycle of the fetal gonocyte. We suggest that BPA-altered gonocyte survivors may cause testicular tumors, including cancer in adults.
ACKNOWLEDGMENTS
This work was supported by Grant CONAHCYT 166012 to HML. PCS is a doctoral student from the Programa en Ciencias Biomédicas, Universidad Nacional Autónoma de México and received a CONAHCYT fellowship 581307.
REFERENCES
- Rubin BS. Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects. J Steroid Biochem Mol Biol. 2011; 127: 27-34.
- Le Fol V, Aït-Aïssa S, Sonavane M, Porcher JM, Balaguer P, Cravedi JP, et al. In vitro and in vivo estrogenic activity of BPA, BPF and BPS in zebrafish-specific assays. Ecotoxicol Environ Saf. 2017; 142: 150-156.
- Prins GS, Patisaul HB, Belcher SM, Vandenberg LN. CLARITY-BPA academic laboratory studies identify consistent low-dose Bisphenol A effects on multiple organ systems. Basic Clin Pharmacol Toxicol. 2019; 125 Suppl 3: 14-31.
- Balakrishnan B, Henare K, Thorstensen EB, Ponnampalam AP, Mitchell MD. Transfer of bisphenol A across the human placenta. Am J Obstet Gynecol. 2010; 202: 393.e1-397.e1.
- Kasper N, Peterson KE, Zhang Z, Ferguson KK, Sánchez BN, Cantoral A, et al. Association of Bisphenol A Exposure with Breastfeeding and Perceived Insufficient Milk Supply in Mexican Women. Matern Child Health J. 2016; 20: 1713-1719.
- Peretz J, Vrooman L, Ricke WA, Hunt PA, Ehrlich S, Hauser R, et al. Bisphenol A and reproductive health: update of experimental and human evidence, 2007-2013. Environ Health Perspect. 2014; 122:775-786.
- Siracusa JS, Yin L, Measel E, Liang S, Yu X. Effects of bisphenol A and its analogs on reproductive health: A mini-review. Reprod Toxicol. 2018; 79: 96-123
- Rajkumar A, Luu T, Beal MA, Barton-Maclaren TS, Robaire B, Hales BF. Elucidation of the Effects of Bisphenol A and Structural Analogs on Germ and Steroidogenic Cells Using Single Cell High-Content Imaging. Toxicol Sci. 2021; 180, 224-238.
- Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Plastics- derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS One. 2013; 8: e55387.
- Lv Y, Li L, Fang Y, Chen P, Wu S, Chen X, et al. In utero exposure to bisphenol A disrupts fetal testis development in rats. Environ Pollut. 2019; 246: 217-224.
- Corpuz-Hilsabeck M, Culty M. Impact of endocrine disrupting chemicals and pharmaceuticals on Sertoli cell development and functions. Front Endocrinol (Lausanne). 2023; 14: 1095894.
- Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, et al. Retinoid Signaling Determines Germ Cell Fate in Mice. Science. 2006; 312: 596-600.
- Koubova J, Menke DB, Zhou Q, Cape B, Griswold MD, Page DC. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci U S A. 2006; 103: 2474-2479.
- Childs AJ, Cowan G, Kinnell HL, Anderson RA, Saunders PT. Retinoic Acid signaling and the control of meiotic entry in the human fetal gonad. PLoS One. 2011; 6: e20249.
- Eladak S, Grisin T, Moison D, Guerquin M-J, N’Tumba-Byn T, Pozzi- Gaudin S, et al. A new chapter in the bisphenol A story: bisphenol S and bisphenol F are not safe alternatives to this compound. Fertil Steril. 2015; 103: 11-21.
- N’Tumba-Byn T, Moison D, Lacroix M, Lecureuil C, Lesage L, Prud’homme SM, et al. Differential effects of bisphenol A and diethylstilbestrol on human, rat and mouse fetal leydig cell function. PLoS One. 2012; 7: e51579.
- Díaz-Hernández V, León del Río A, Zamora M, Merchant-Larios H. Expression profiles of SRY and SOX9 in rabbit gonads: the classical model of mammalian sex differentiation. Sex Dev. 2008; 2: 152-166.
- Díaz-Hernández V, Caldelas I, Merchant-Larios H. Gene Expression in the Supporting Cells at the Onset of Meiosis in Rabbit Gonads. Sex Dev. 2019; 13: 125-136.
- Táncos Z, Nemes C, Varga E, Bock I, Rungarunlert S, Tharasanit T, et al. Establishment of a rabbit induced pluripotent stem cell (biPSC) line using lentiviral delivery of human pluripotency factors. Stem Cell Res. 2017; 21: 16-18.
- Fan J, Watanabe T. Transgenic rabbits as therapeutic protein bioreactors and human disease models. Pharmacol Ther. 2003; 99: 261-282.
- Xu X, Shi D, Liu Y, Yao Y, Dai J, Xu Z, et al. In vivo repair of full-thickness cartilage defect with human iPSC-derived mesenchymal progenitor cells in a rabbit model. Exp Ther Med. 2017; 14: 239-245.
- Ortega-García A, Díaz-Hernández V, Collazo-Saldaña P, Merchant- Larios H. Bisphenol A alters differentiation of Leydig cells in the rabbit fetal testis. Int J Dev Biol. 2021; 65: 403-412.
- Palmieri SL, Peter W, Hess H, Scholer HR. Oct-4 transcription factor is differentially expressed in the mouse embryo during establishment of the first two extraembryonic cell lineages involved in implantation. Dev Biol. 1994; 166: 259-267.
- Yeom YI, Fuhrmann G, Ovitt CE, Brehm A, Ohbo K, Gross M, et al. Germline regulatory element of Oct-4 specific for the totipotent cycle of embryonal cells. Development 1996; 122: 881-894.
- Yamaguchi S, Kimura H, Tada M, Nakatsuji N, Tada T. Nanog expression in mouse germ cell development. Gene Expr Patterns. 2005; 5: 639-646.
- Hilscher B, Hilscher W, Bülthoff-Ohnolz B, Krämer U, Birke A, Pelzer H, et al. Comparative histological and autoradiographic studies of oocytes and transitional prospermatogonia during oogenesis and prespermatogenesis. Cell Tissue Res. 1974; 154: 443-470.
- Western PS, van den Bergen JA, Miles DC, Sinclair AH. Male fetal germ cell differentiation involves complex repression of the regulatory network controlling pluripotency. FASEB J. 2010; 24: 3026-3035.
- Lin ZY, Imamura M, Sano C, Nakajima R, Suzuki T, Yamadera R, et al. Molecular signatures to define spermatogenic cells in common marmoset (Callithrix jacchus). Reproduction. 2012; 143: 597-609.
- Gaskell TL, Esnal A, Robinson LLL, Anderson RA, Saunders TK.Immunohistochemical Profiling of Germ Cells Within the Human Fetal Testis: Identification of Three Subpopulations. Biol Reprod. 2004; 71: 2012-2021.
- Honecker F, Stoop H, de Krijger RR, Chris Lau YF, Bokemeyer C, Looijenga LH. Pathobiological implications of the expression of markers of testicular carcinoma in situ by fetal germ cells. J Pathol. 2004; 203: 849-857.
- Rajpert-De-Meyts E, Hanstein R, Jørgensen N, Graem N, Vogt PH, Skakkebaek NE. Developmental expression of POU5F1 (OCT-3/4) in normal and dysgenetic human gonads. Hum Reprod. 2004; 19: 1338- 1344.
- Gillis AJ, Stoop H, Biermann K, van Gurp RJ, Swartzman E, Cribbes S, et al. Expression and interdependencies of pluripotency factors LIN28, OCT3/4, NANOG and SOX2 in human testicular germ cells and tumours of the testis. Int J Androl. 2011; 34: e160-e174.
- Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005; 122: 947-956.
- Chen X, Xu B, Han X, Mao Z, Talbot P, Chen M, et al. Effect of bisphenol A on pluripotency of mouse embryonic stem cells and differentiation capacity in mouse embryoid bodies. Toxicol In Vitro. 2013; 27: 2249- 2255.
- Veeramachaneni DN. Germ cell atypia in undescended testes hinges on the aetiology of cryptorchidism but not the abdominal location per se. Int J Androl. 2006; 29: 235-240.
- Fang C, Ning B, Waqar AB, Niimi M, Li S, Satoh K, et al. Bisphenol A exposure induces metabolic disorders and enhances atherosclerosis in hyperlipidemic rabbits. J Appl Toxicol. 2015; 35: 1058-1070.
- Schönfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ Health Perspect. 2002; 110: A703-A707.
- Kaddar N, Bendridi N, Harthé C, de Ravel MR, Bienvenu A-L, Cuilleron C-Y, et al. Development of a radioimmunoassay for the measurement of Bisphenol A in biological samples. Anal Chim Acta. 2009; 645: 1-4.
- Christensen KL, Lorber M, Koslitz S, Brüning T, Koch HM. The contribution of diet to total bisphenol A body burden in humans: results of a 48 hour fasting study. Environ Int. 2012; 50: 7-14.
- Hanley N, Hagan D, Clement-Jones M, Ball S, Strachan T, et al: SRY, SOX9 and DAX1 expression patterns during human sex determination and gonadal development. Mech Dev. 2000; 91: 403-407.
- Western PS, Miles DC, van den Bergen JA, Burton M, Sinclair AH. Dynamic regulation of mitotic arrest in fetal male germ cells. Stem Cells. 2008; 26: 339-347.
- Pamnani M, Sinha P, Singh A, Nara S, Sachan M. Methylation of the Sox9 and Oct4 promoters and its correlation with gene expression during testicular development in the laboratory mouse. Genet Mol Biol. 2016; 39: 452-458.
- Li R, Vannitamby A, Zhang JG, Fehmel EL, Southwell BR, Hutson JM. Oct4-GFP expression during transformation of gonocytes into spermatogonial stem cells in the perinatal mouse testis. J Pediatr Surg. 2015; 50: 2084-2089.
- Daniel-Carlier N, Harscoët E, Thépot D, Auguste A, Pailhoux E, Jolivet G. Gonad differentiation in the rabbit: evidence of species-specific features. PLoS One. 2013; 8: e60451.
- Fukuda T, Hedinger C, Groscurth P. Ultrastructure of developing germ cells in the fetal human testis. Cell Tissue Res. 1975; 161: 55-70.
- Wartenberg H. Differentiation and development of the testes. 2nd ed. New York, NY: Raven. 1981.
- Holstein AF, Schutte B, Becker H, Hartmann M. Morphology of normal and malignant germ cells. Int J Androl. 1987; 10: 1-18.
- Perrett RM, Turnpenny L, Eckert JJ, O’Shea M, Sonne SB, Cameron IT,et al. The early human germ cell lineage does not express SOX2 during in vivo development or upon in vitro culture. Biol Reprod. 2008; 78: 852-858.
- Sonne SB, Perrett RM, Nielsen JE, Baxter MA, Kristensen DM, Leffers H, et al. Analysis of SOX2 expression in developing human testis and germ cell neoplasia. Int J Dev Biol. 2010; 54: 755-760.
- Wang X, Dai J. Concise review: isoforms of OCT4 contribute to the confusing diversity in stem cell biology. Stem Cells. 2010; 28: 885-893.
- Hamer G, de Rooij DG. Mutations causing specific arrests in the development of mouse primordial germ cells and gonocytes. Biol Reprod. 2018; 99: 75-86.
- Vilela J, Hartmann A, Silva EF, Cardoso T, Corcini CD, Varela AS, et al. Sperm impairments in adult vesper mice (Calomys laucha) caused by in utero exposure to bisphenol A. Andrologia. 2014; 46: 971-978.
- Hass U, Christiansen S, Boberg J, Rasmussen MG, Mandrup K, Axelstad M. Low-dose effect of developmental bisphenol A exposure on sperm count and behaviour in rats. Andrology. 2016; 4: 594-607.
- Rahman MS, Kwon WS, Karmakar PC, Yoon SJ, Ryu BY, Pang MG. Gestational Exposure to Bisphenol-A Affects the Function and Proteome Profile of F1 Spermatozoa in Adult Mice. Environ Health Perspect. 2017; 125: 238-245.
- Merlet J, Moreau E, Habert R, Racine C. Development of fetal testicular cells in androgen receptor deficient mice. Cell Cycle. 2007; 6: 2258- 2262.
- Gondos B, Byskov AG. Germ cell kinetics in the neonatal rabbit testis. Cell Tissue Res. 1981; 215: 143-151.