Two Different Neoplasms in Cougar (Puma concolor): Pheocromocytoma and Ovarian Fibroma
- 1. Parque Zoológico Municipal Quinzinho de Barros (PZMQB), Sorocaba, São Paulo, Brazil
- 2. Wild Animals Graduate Program – UNESP Botucatu, Botucatu, São Paulo, Brazil
- 3. University of Sorocaba (UNISO), Sorocaba, São Paulo, Brazil
- 4. Vetver Veterinary Services, Sorocaba, São Paulo, Brazil
- 5. Soronemer Pathological Exams, Sorocaba, São Paulo, Brazil
- 6. PHD Pathology Surgical and Molecular Pathology, São Paulo, São Paulo, Brazil
Abstract
The cougar (Puma concolor) is the second largest felid species in the Americas and the fourth largest in the world. In literature, there is abundant data on domestic animals’ neoplasms; however information on neoplasms occurring in wild animals is limited. This study was carried out on a 33 kg female, adult cougar specimen kept for approximately 16 years at Sorocaba Zoo. Hyporexia, progressive weight loss and increased abdominal volume were reported by the animal’s handler. At necropsy, ascites with serosanquinolent content, pyometra, peri-mammary lipoma and presence of a mass in the left adrenal region and in the left ovary region were observed. The adrenal formation was 7 x 7 cm, dense and vinaceous in color. Samples of all organs and masses were collected and stored in 10% formalin and, afterwards, processed by the routine technique for histopathological evaluation. The immunohistochemical examination of the adrenal mass showed an infiltrative growth of neuroendocrine origin, positive for Cromagranin and Synaptophysin and negative for AE1 and AE3 (cytokeratin), compatible with pheochromocytoma. The zoos prolong the life expectancy of their animals, when compared to that of wildlife individuals, resulting in higher incidence rates of neoplasms. Preventive veterinary examinations of wild animal collections remain a good strategy for conserving biodiversity. Further studies should be carried out to enable diagnosis in early stages, thus allowing a favorable prognosis for affected animals, with the possibility of therapeutic or surgical interventions. Data about neoplasms occurring in wild animals ex situ, might help in the conservation of species in situ.
Keywords
• Felid
• Immunohistochemistry
• Pathology
• Zoo
INTRODUCTION
The cougar (Puma concolor) has a uniformly colored pelage, varying in the dorsal region from yellow brown to reddish, with the belly and the inner part of the limbs being lighter. The average weight of an adult male can vary between 40 and 72 kg, while in females it varies from 34 to 48 kg [1]. Puppies are born with a dense pelage that varies from gray to beige, speckled with large brown spots. Its life expectancy is of 8 to 10 years, reaching up to 20 years when kept under human care [2]. The cougar is one of largest felid species in the world. It is the most widespread terrestrial mammal in the Neotropical region, originally found from southern Canada to the southern tip of the South American continent [3]. Regarding the conservation status in nature, this species is considered of least concern according to the International Union for Conservation of Nature and Natural Resources (IUCN) [4]. The effective population size has been calculated around 4,000 individuals and in three generations, or 21 years, it is estimated that a decline of more than 10% of the national subpopulation could occur due to the suppression and fragmentation of their habitat for agricultural expansion and mining; in addition to the exploitation of wood for charcoal. Besides, the elimination of individuals by hunting; retaliation for predation of domestic animals; fires (mainly in sugarcane fields); and motor-vehicle collisions also significantly contribute to population reduction in several areas [5]. In literature, there is abundant data on domestic animals’ neoplasms; however information on neoplasms occurring in wild animals is limited. Most studies in wild animals refer to a single species or a single type of tumor [6-10]. Biopsy a necropsy examination is a valuable tool for diagnosis [11]. Histological classification with the aid of immunohistochemical staining results in a much more accurate diagnosis. Neoplasm studies in wild animals with necropsy examination associated with immunohistochemistry have been rarely reported. Ovarian tumors are classified into three categories: epithelial, germ cell, and stromal. Fibroma, thecoma and gonadoblastoma are tumors of the sex cords that are part of the stroma [12]. Fibroma is a benign tumor of firm consistency, whitish, and spherical or ovoid in shape. Ovarian fibroids are rare in most animal species, although frequent in humans [13]. Pheochromocytomas originate from chromaffin cells present in the adrenal medullary region, capable of producing, storing, and secreting catecholamines (eg, epinephrine, norepinephrine). Ante-mortem diagnosis is complex and rare, requiring a high index of suspicion by the veterinarian staff, due to the paroxysmal and nonspecific nature of clinical signs and lack of specific and sensitive diagnostic tools [14], demonstrating the importance of necropsy examination as an essential tool for the diagnosis of neoplasms in wild animals under human care. Sorocaba Municipal Zoo has an estimated squad of 1,250 wild animals, divided into 340 different species in an area of 12.8 hectares. This work discusses the carcinogenesis of two neoplasms, a unilateral ovarian fibroma and a unilateral pheochromocytoma in an adult female puma (Puma concolor), confirmed by post-mortem histopathological results. This study reports two rare neoplasms in wild felids, demonstrating the importance of research in wild animals.
CASE PRESENTATION
This study was carried out on a 33 kg female, adult cougar (Puma concolor) specimen kept for approximately 16 years at Sorocaba Zoo. Hyporexia, progressive weight loss and increased abdominal volume were reported by the animal’s handler. The patient was forwarded to the Zoo Veterinary Hospital for collection of biological material and clinical, ultrasound and radiographic examination. The anesthetic protocol used was a combination of 10% ketamine hydrochloride at a dose of 8 mg/kg and 5% xylazine hydrochloride at a dose of 0.5 mg/kg, administered intramuscularly through physical restraint in a press cage. Clinical examination revealed a low body condition score of the animal and a large mass in the mid-abdominal region with approximately 40 cm in diameter.
The ultrasound examination revealed an irregular heterogeneous formation, occupying the entire mid-abdomen, overlapping intestinal loops, lymph nodes, reproductive system, adrenals, pancreas and spleen, impossibilitating its origin. The radiographic examination of the abdomen and thorax, in ventrodorsal and latero-lateral projection in right decubitus, showed an area of greater radiopacity of soft tissues distributed throughout the abdomen, making it difficult to visualize other organs, which may be compatible with abdominal mass. In the chest, there were no metastatic nodules in lung parenchyma that could be observed in radiographic evaluation, although areas of mineral radiopacity were distributed throughout the lung parenchyma compatible with osteomas, which are common in senility.
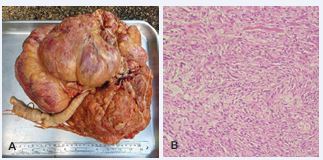
The veterinary staff opted for the surgical removal of the mass, but the animal died before the procedure. At necropsy, ascites with serosanquinolent content, pyometra, peri-mammary lipoma and presence of a mass in the left adrenal region and in the left ovary region were observed. The adrenal formation was 7 x 7 cm, dense and vinaceous in color. Samples of all organs and masses were collected and stored in 10% formalin and, afterwards, processed by the routine technique for histopathological evaluation.Histopathological examination of the ovary showed an ovarian stroma with expansive growth formed by fusocellular cells with irregular nuclei and hyperchromasia, granular eosinophilic cytoplasm and bands of connective tissue and neovascularization, compatible with ovarian fibroma (Figure 1).
Figure 1 A) Ovarian fibroma. Ovary mass with 5 kg, 40 cm in length and 40 cm in width in the largest axes, cavitations on section and extensive vascularization B) Histopathological section shows expansive growth formed by fusocellular cells with irregular nuclei hyperchromasia and granular eosinophilic cytoplasm. 20X, H&E.
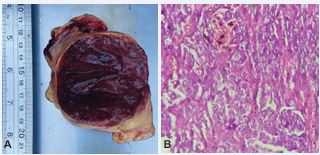
The adrenal histopathological examination showed an infiltrative growth lesion of neuroendocrine origin consisting of cellular arrangements in solid and trabecular blocks, hyperchromic, voluminous and irregular nuclei of eosinophilic cytoplasm, and vascular proliferation permeating neoplastic cells, compatible with pheochromocytoma (Figure 2).
Figure 2 A) Pheochromocytoma. Adrenal mass with 7 cm long and 7 cm wide, dense, and vinaceous in color. B) Histopathological section shows an infiltrative growth lesion of neuroendocrine origin consisting of cellular arrangements in solid and trabecular blocks, hyperchromic, voluminous and irregular nuclei of eosinophilic cytoplasm. 40X, H&E.
DISCUSSION
In literature, most studies in wild animals portray a single species or a single kind of tumor [9]. There are reports of neoplasms in the nervous [15,13], liver [17,18], respiratory [17,19], gastrointestinal [20,21], reproductive [22], endocrine [17,23,9], and skin [24,25], systems of cougars. Nevertheless, reports of endocrine system neoplasms are rare in wild felids.
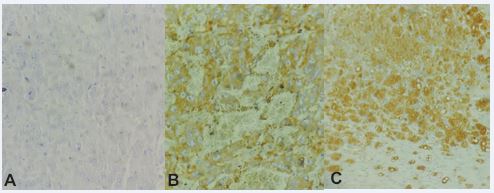
Figure 3 A) Infiltrative growth of neuroendocrine origin. Negative for AE1 and AE3 (cytokeratin) 40X, H&E. B) Infiltrative growth of neuroendocrine origin. Positive for Synaptophysin 40X, H&E. C) Infiltrative growth of neuroendocrine origin. Positive for Chromagranin 20X, H&E.
Pheochromocytomas are commonly reported in ruminants, canids, and new world primates, but there are rare reports of adrenal pheochromocytoma in wild felids, although some studies have already been reported in clouded leopard (Neofelis nebulosa) [26], tiger (Panthera tigris) [27], and jaguar (Pantehra onca) [28]. Pheochromocytomas are often large (10 cm or more in diameter), firm and encapsulated, and typically replace most of the affected adrenal gland [26], similar to what is described in this report. According to Corner (2017), 25% of pheochromocytomas are bilateral and 20% showed metastasis, unlike our report in which the neoplasm was unilateral and did not present metastasis.
The diagnosis of pheochromocytoma confirmed by immunohistochemistry demonstrated positive neuroendocrine origin for Chromagranin and Synaptophysin, corroborating other studies [29]. Confirmation of the diagnosis by immunohistochemistry shows the importance of performing additional tests for accurate diagnosis. Study demonstrated that ultrasound exam does not have 100% diagnostic accuracy in the case of pheochromocytoma [30], as only masses larger than 2 cm in diameter can be detected [31]. However, confirmation of the topography, as well as details of the textural aspect and involvement of adjacent tissues, helps in the differential diagnosis. There is a relationship between the increase in life expectancy of wild cats in zoos or under humans care and the greater incidence of neoplasms diagnosed. The animal subject of this report was of high age, which is a factor that might have influenced the onset and development of the tumors [32]. Comparative studies are a useful tool to learn more about the risk of specific species to different neoplasms and to better tailor the preventive care, monitoring, and treatment of the animals [10]. Studies have found a good statistical correlation between air pollution and cancer incidence [33,34]. Environmental pollution by genotoxic agents implies an increase in the rate of mutations. There are two possibilities as to which cell type undergoes the mutation. In germinated cells, mutations can increase the incidence of genetic diseases in future generations. In somatic cells the adverse effect will be an increase in the number of cancer cases [35].
The vast majority of cancers arise independently in each individual animal in response to multiple ranges of causal factors, including chemical or irradiating carcinogens, oxygen free radicals associated with aging, and interactions of these with oncogenes and tumor-suppressing genes and viruses [36]. As with many human cancers, some wildlife cancers are attributable to environmental contaminants. For example, the cancers in belga whales (Delphinapterus leucas) that are related to polycyclic aromatic hydrocarbons [37] and mesotheliomas those are strongly associated with prolonged exposure to pollutants, the asbestos fibers [27].
CONCLUSION
Clinical aspects and occurrence of neoplasms are still unknown in many species of wild animals. Further studies should be carried out to enable diagnosis in early stages, thus allowing a favorable prognosis for affected animals, with the possibility of therapeutic or surgical interventions. Data about neoplasms occurring in wild animals ex situ, might help in the conservation of species. The post-mortem examination and the results of the histopathological and immunohistochemical tests were decisive for the diagnosis of the patient’s cause of death. According to Brazilian legislation, post-mortem examinations must be carried out in wild animals from a zoo. The zoos prolong the life expectancy of their animals, when compared to that of wildlife individuals, resulting in higher incidence rates of neoplasms. Preventive veterinary examinations of wild animal collections remain a good strategy for conserving biodiversity.
REFERENCES
- Sunquist ME & Sunquist FC. Family Felidae (cats). In: Wilson, D.E. & Mittermeier, R.A. (eds.). Handbook of the mammals of the world. Carnivores. Lynx Editions. 2009; 1: 727.
- Adania C H, Silva JC, Fellipe PAN. CARNIVORA – FELIDAE (Onça, Suçuarana, Jaguatirica e Gato-do-Mato). In: Cubas ZS, Silva JCR, Catão-Dias JL. Tratado de animais selvagens – medicina veterinária, 2ª edn. Roca. 2014; 779-818.
- Currier MJ. Felis concolor. Mammalian Species. 1983; 200: 1-7.
- Nielsen C, Thompson D, Kelly M. & Lopez-Gonzalez CA. 2015. Puma concolor (errata version published in 2016). The IUCN Red List of Threatened Species 2015.
- De Azevedo FC, Lemos FG, De Almeida LB, De Campos CB, De Mello BB, De Paula RC, et al. Avaliação do risco de extinção da Onça-parda Puma concolor (Linnaeus, 1771) no Brasil. Biodiversidade Brasileira – BioBrasil. 2013; 107-121.
- Newman SJ, Smith SA. Marine mammal neoplasia: a review. Vet Pathol. 2006; 43: 865-80.
- Sykes JMT, Trupkiewicz JG. Reptile neoplasia at the PhiladelphiaZoological Garden, 1901-2002. J Zoo Wildl Med. 2006; 37: 11-9.
- Suedmeyer WK, Johnson G. Survey of neoplasia in red kangaroos (Macropus rufus), 1992- 2002, in a zoological collection. J Zoo Wildl Med. 2007; 38: 231-9.
- Owston MA, Ramsay EC, Rotstein DS. Neoplasia in felids at the Knoxville Zoological Gardens, 1979–2003. J Zoo Wildl Med. 2008; 39: 608-613.
- Moresco A, Muñoz KE, Gutiérrez F, Arias-Bernal L, Yarto-Jaramillo E, Teixeira RHF, et al. V. Taxonomic distribution of neoplasia among non- domestic felid species under managed care. Animals. 2020; 10: 1-10.
- Effron M, Griner L, Benirschke K. Nature and rate of neoplasia found in captive wild mammals, birds, and reptiles at necropsy. J Natl Cancer Inst. 1977; 59: 185-198.
- Giordano LA, Giordano MV, Oliveira R. Adnexal tumors in adolescence/ Adnexal tumors in adolescents. Adolescent Health. 2009; 6: 48-52.
- Seaman WJ. Canine ovarian fibroma associated with prolonged exposure to mibolerone. Toxicol Pathol. 1985; 13: 177-180.
- De Queiroz DLM, Arruda GNG, Lourenço PVA, Diógenes YP, DosSantos MH, Cabral LAR. (2017). FEOCROMOCITOMA EM PEQUENOSANIMAIS. Revista De Ciência Veterinária E Saúde Pública. 2017; 4: 166-175.
- Kondo H, Leone AM, Erlacher-Reid C, Gary J, Kiupel M, Farina LL, et al. Medium-grade astrocytoma in a cougar (Puma concolor). J Zoo Wildl Med. 956-960; 43: 956-960.
- Duhamelle A, Langlois I, Pey P, Tremblay J, Ruel H, Parent J, et al. Malignant paraganglioma in a cougar (Puma concolor). J Zoo Wildl Med. 2014; 45: 994-998.
- Kennedy GA, Strafuss AC. Multiple neoplasia in an aged cougar. TheJournal of Zoo Animal Medicine. 1976; 7: 24-26.
- Werner PR, Chiquito M, Pachaly JR. Estudo retrospectivo das neoplasias diagnosticadas em animais selvagens ou exóticos pelo Serviço de Patologia do Hospital Veterinário da Universidade Federal do Paraná entre 1974 e 1996. Archives of Veterinary Science. 1998; 3.
- Mathieu A, Garner MM. A retrospective study of neoplasia in nondomestic felids in human care, with a comparative literature review. Journal of Zoo and Wildlife Medicine. 2021; 52: 413-426.
- Yanai T, Masegi T, Hosoi M, Yamazoe K, Iwasaki T, Yagi T, et al. Gastric adenocarcinoma in a cougar (Felis concolor). J Wildl Dis. 1994; 30: 603-608.
- Yamazaki Y, Aono I, Ohya T, Shibahara T, Kadota K. Gastroduodenaladenocarcinomas and rectal adenoma in a cougar (Felis concolor)infected with Helicobacter-like organisms and spirochetes. J Vet MedSci. 2002; 64: 149-53.
- Cavalli GD, Malta MCC, Costa MELT. Neoplasia mamária em onça parda (Puma concolor) e leoa (Pantera leo). Clínica Veterinária. 2008; 77: 86-90.
- Li X, Steinberg H, Wallace C, Kallfelz FA, Johnson R, Anderson WI, et al. Functional thyroid follicular adenocarcinoma in a captive mountain lion (Felis concolor). Vet Pathol. 1992; 29: 549-551.
- Schulman FY, Krafft AE, Janczewski T, Mikaelian I, Irwin J, HassingerK. Cutaneous fibropapilloma in a mountain lion (Felis concolor). J Zoo Wildl Med. 2003; 34: 179-183.
- Sandoval BJ, Che’ amat A, Sabri J, Ramli MN. Intralesional vincristine use for treatment of squamous cell carcinoma in a puma (Puma concolor). J Zoo Wildl Med. 2013; 44: 1059-1062.
- Corner S, Walsh T, Padilla L, Macneill A, Wallig M, Kiupel M, et al. Histologic and immunohistochemical characterization of pheochromocytomas in 20 clouded leopards (Neofelis nebulosa). Vet Pathol. 2017; 54: 269-276.
- Junginger J, Hansmann F, Herder V, Lehmbecker A, Peters M, Beyerbach M, et al. Pathology in Captive Wild Felids at German Zoological Gardens. PLoS One. 2015; 10: e0130573.
- CD Port, ER Maschgan, J Pond, DG Scarpelli. Multiple neoplasia in ajaguar (Panthera onca). J Comp Pathol. 1981; 91: 115–122, 1981.
- De Ramos JA. Feocromocitoma: Relato de caso. Revista Brasileira de Análises Clínicas. 2020; 52: 395-9.
- Barthez PY, Marks SL, Woo J, Feldman EC. Matteucci, M. Pheochromocytoma in dogs: 61 cases (1984-1995). J Vet Intern Med. 272-278; 5: 272-278.
- Maher ER. Pheochromocytoma in the dog and cat: diagnosis and management. Seminars in Veterinary Medical Surgery (Small Animal). 1994; 3: 158-166.
- Miranda FR, Kimura KC, Côrrea SHR, Teixeira RHF, Fedullo JLD, Barbosa HS, et al. 2003. Incidência de neoplasia em felídeos na Fundação Parque Zoológico de São Paulo – Estudo retrospectivo 1971 a 2001. In: XXVII Congresso de Zoológico do Brasil. 2003.
- Reymão MSFR, Cury PM, Lichtenfels AJFC, Lemos M, Bathlenner CN, Conceição GMS, et al. Urbana air pollution enhances the formation of urethane induced lung tumors in mice. Environ Res. 1997; 74: 150-8.
- Cangerana-Pereira FA. Estudo exploratório da influência da poluição do ar na incidência de câncer por distrito do Município de São Paulo. São Paulo. 2000. 110 f. Dissertação (Mestrado em Ciências) - Faculdade de Saúde Pública, Universidade de São Paulo, São Paulo.
- Hemminki K, Veidebaum T. Environmental Pollution and Human Exposure to Polycyclic Aromatic Hydrocarbons in the East Baltic Region. Scand J Work Environ Health. 1999; 25: 33-39.
- Ruddon RW. Cancer Biology. 4th edn. Oxford UK: Oxford UniversityPress Inc. 2007; 568.
- Mccallum H, Jones M. Infectious cancers in wildlife. ConservationMedicine: Applied Cases of Ecological Health. 2012; 270-283.