Assessment of Anti-Sperm Antibodies Response in Mouse Experimentally Infected with Bacteria
- 1. Department of Microbiology, Panjab University, India
ABSTRACT
Introduction: In humans, anti-sperm antibodies are a common cause of infertility. 10% to 30% of infertile couples credit ASAs for their problem. The mechanisms through which ASA is produced in either sex remains elusive. The current manifestations of ASAs, which may be cross-reactive antibodies produced against microbial antigen, have been recently elucidated (bacteria, viruses, fungi, allergens). Common antigens between microorganisms and humans have long been suspected as contributing to autoimmune diseases.
Material and Methods: Serum was taken from infected animals on day 45 for agglutination testing and FITC binding experiments to detect ASAs after intraperitoneal inoculum of different bacteria in test and PBS in control. The agglutination of the bacterial strains and spermatozoa was evaluated by combining them with the serum from infected mice and control serum. FITC-binding investigations included labelling serum with the FITC dye and then incubating it with bacteria and spermatozoa in order to determine whether the ASAs were formed or not.
Results: In the agglutination test, it was observed that serum from mice infected with the bacterial strains elicits agglutination with both bacteria and spermatozoa, but no agglutination was detected in control serum. Whereas in FITC studies the PBS administrated mice’s FITC-tagged control serum exhibited no fluorescence with bacteria and spermatozoa. However, FITC-labeled test serum from mice infected with Proteus mirabilis, Serratia marcescens, and Escherichia coli fluoresced brilliantly green on bacterial surfaces and on complete spermatozoa.
Conclusion: Present research indicates that bacteria and spermatozoa have an antigenic similarity that triggers a cross-reaction.
KEYWORDS
- Anti-sperm antibodies
- Agglutination
- FITC
- Serum
- Infertility
CITATION
Divya, Jawanda IK, Soni T, Prabha V (2023) Assessment of Anti-Sperm Antibodies Response in Mouse Experimentally Infected with Bacteria. Med J Obstet Gynecol 11(1): 1165.
INTRODUCTION
Since the dawn of 1890s scientists have hypothesized that the human body mounts an immune response to sperm because of its antigenic determinants [1]. Antigenicity in many sperm- associated antigens has been found, analyzed, and shown to foster the generation of antibodies known as anti-sperm antibodies (ASAs). ASAs are characterized as Immunoglobulins IgG, IgA, and IgM that have negative impact on sperm antigen involved in reproduction [2].
Anti-sperm antibodies are one of the most prevalent causes of human infertility [3]. Around 10%–30% of infertile couples have attributed their infertility to ASAs [4]. As the contribution to idiopathic infertility (31% of all cases) remains obscure, thus, the rate may actually be larger than predicted. However, these antibodies are also found in roughly 1% to 2.5% of fertile men and 4% of viable women, suggesting that not all ASAs induce infertility [5]. Fertility is impaired only by antibodies directed against antigens involved in the fertilization process [6].
High ASAs rate was identified among prostitutes, which is concerning. Recurrence inoculations with numerous sperm antigen and/or microorganisms may be a factor to this. On the other hand, recent research examining the bedtime patterns and length of sleep-in healthy males revealed intriguing findings, indicating an increase among the incidence of ASAs in the short sleepers across all night-time patterns [7]. However, the connection between the aforementioned circumstances and the generation of ASAs has been called into question [2].
At this point in time, it is still unknown how or why women typically do not produce ASAs but some women do; the strongest correlations are that women whose male partners have ASAs in their semen are more likely to develop ASAs, and women with ASAs tend to respond solely to their partner’s sperm and not to sperm from other men [8]. Antibodies generated against ASAs in their partner’s semen, and a cytokine-driven immunological response to ASAs in their partner’s semen are the explanations for how women get ASAs as of 2017.
Recent findings explicating the existing states of ASAs that may be cross-reactive antibodies generated against microbial antigen (bacteria, viruses, fungi, allergens). Antigens shared by bacteria and humans have long been known to be associated with autoimmune illness. In the course of evolution, the heat-shock proteins (HSPs) of bacteria and humans alike have remained strikingly similar. Some clinical problems, such as rheumatoid arthritis, infertility, and atherosclerosis, may be caused in part by cross-reactivity based on HSPs [9].
The studies on the role of bacteria and viruses in the generation of ASA’s are being extensively explored [10]. This field has advanced much in recent years, yet there are still many areas in which progress has still to be made. Our knowledge on anti- sperm antibodies will likely advance more quickly in the near future as a result of the enormous increase in facts of immune system.
MATERIAL AND METHODS
Animals and Ethics
In the present study, sexually mature, 5-6 weeks old male (25 ± 2g) and 4-5 weeks old female (22 ± 2g) BALB/c mice were used. The animals were housed in polypropylene cages (6 animals per cage) bedded with clean husk made with rice. All the cages were kept in the well aerated, temperature (29-30°C ± humidity (40- 55%) controlled animal room of Department of Microbiology, Panjab University, Chandigarh in which a 12h light/12h dark cycle was maintained. The nutritional levels of animals were adjusted to meet maintenance requirements. They were fed with pellets (a concoction of 20-21% crude protein, 4% fat, 5.0-7.5% crude fibre, 8-9% ash, 1.0-1.5% calcium, 0.6-0.8% phosphorus and 50% nitrogen free extract) (M/s Ashirwad Industries Pvt. Ltd.) which is certified mice feed and drinking water was available ad libitum.
Before commencement of the experiments, all the mice had 7-10 days of adaptation period. All the procedures using animals were reviewed and approved by the Institutional Animal Ethics Committee of Panjab University, Chandigarh, India (PU/45/99/ CPCSEA/IAEC/2021/657). The study and all procedures were carried out in accordance with the guidelines for the care and use of laboratory animals of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India.
Microorganisms
The standard microorganisms viz., Proteus mirabilis (MTCC 425), Serratia marcescens (MTCC 7641), Escherichia coli (MTCC 1687) were used in the present study. The standard strains procured from Microbial Type Culture Collection (MTCC), Institute of Microbial Technology, Sector 39, Chandigarh, India were already available in the laboratory.
Preparation of inoculum
Proteus mirabilis was grown in nutrient broth (10mL) and Serratia marcescens (MTCC 7641) and Escherichia coli (MTCC 1687) in BHI broth (10ml) at 37°C under shaking. The cell cultures were centrifuged at 10,000rpm for 20min for extracting cell pellet. The pellets so obtained were washed twice with PBS and resuspended in same buffer.
The number of organisms expressed as colony forming units (cfu) per ml of PBS was measured by making successive dilutions of the washed cell pellets in PBS and plated on NA/BHI agar. Next day, the number of colonies for each bacterium was counted, multiplied by the dilution factor to yield cfu/mL. The final cfu for inoculum was set at 108cfu/100μl of PBS.
Generation of bacteria induced anti-sperm antibodies to elucidate cross-reactivity towards bacteria and sperm
100μL of prepared bacterial inoculum of the microorganisms was injected intraperitoneally per mouse per dose. Next five booster injections were given on 3rd, 7th, 14th, 21st and 42nd day for generation of bacteria induced ASAs. Serum was collected on day 45 i.e., 3 days after the final booster. Blood was drawn from the mice via retro-orbital route and allowed to clot for 1h at 37ºC, followed by centrifugation at 5000g for 10min for the serum collection. For the control serum, the same procedure was carried out using PBS instead of bacterial strains administered into the control group. Serum aliquots were kept at 4ºC for immediate use and at -20ºC for long term storage
Sperm preparation: Trans-abdominal vasal excision was done on male mice shortly after cervical dislocation. Carefully removing the vas deferens, it was placed in 500 μL of phosphate- buffered saline in a glass dish (PBS; pH 7.2, 50 mM). The spermatozoa were released into PBS with a little squeeze of the ducts. The mouse sperm preparations utilised in this research were chosen based on the recommendations made by the World Health Organization (2010) for normal human seminal parameters. Therefore, only high-sperm-count preparations were employed. With a count of 40×106 spermatozoa in 1 mL, a percentage of 50% motility, 60% viability, and 60% normal morphology is considered good.
Assessing the impact of bacteria elicited anti-sperm antibodies on bacteria and spermatozoa
Proteus mirabilis, Serratia marcescens, E. coli were grown overnight in their respective NB/BHI broth (10mL). To harvest cell pellet, the cell cultures were centrifuged at 10,000rpm for 20 minutes. These pellets were then washed three times with PBS and resuspended in the same buffer (1mL). Concentration of 108cfu/mL of each organism was prepared. 20μl of the serum sample was taken in a micro-centrifuge tube and mixed with 20μl of the bacterial sample prepared. Furthermore, a single drop of Safranin dye was added in the mixture of serum sample and bacterial culture. Following that, the smear was prepared by spreading the suspension on a slide and allowed to air dry. The air-dried smear was then observed under the microscope at 400x and 1000x magnification with immersion oil for the agglutination of bacteria.
Extraction of spermatozoa was done as per the procedure described in preceding section 1.6. 20μl of the serum sample and 20μl of the sperm sample was taken in a micro-centrifuge tube and mixed with the auto-pipette. After that, the smear was made by spreading a drop of the suspension on a slide and allowed to dry. Under the microscope, different fields were examined after adding one drop of eosin stain at 400X and with immersion oil at 1000X magnification to check the agglutinated spermatozoa.
Binding studies carried out with fluorescein isothiocyanate (FITC)-labelled serum
The FITC-Protein Labelling Kit (Bangalore Genei Pvt. Ltd.), as instructed, was used to conjugate test serum and control serum (1 mg/mL) with FITC at a F: P ratio of 1:1. The following suspensions were made for the binding experiments using mouse spermatozoa or bacteria.
The bacterial cultures were grown for 6-8 hours in nutrient broth, centrifuged at 7267g for 10 minutes at 4?, rinsed thoroughly in PBS (50 mM, pH 7.2), and finally suspended in 1 mL PBS, while the sperm preparation was washed twice with PBS and the pellet was finally suspended in 500 μl PBS (50mM, pH 7.2).
To each 100 μl bacterial or spermatozoa suspension, the same volume of labelled serum (100 μl) was added, and the mixtures were incubated at 37? for 1 hour. After that 100 μl of 3% formaldehyde was poured to it. The reaction mixture was rinsed three times with PBS after the incubation period ended. One last step included suspending the pellet in 50 μl PBS (50 mM, pH 7.2). Under a fluorescence microscope, a wet mount was examined. So as to exclude out autofluorescence, a control was also performed using unlabeled serum with bacteria and spermatozoa.
RESULTS
Determination of cross-reactivity between anti- bacterial hyperimmune serum towards spermatozoa and bacteria via agglutination
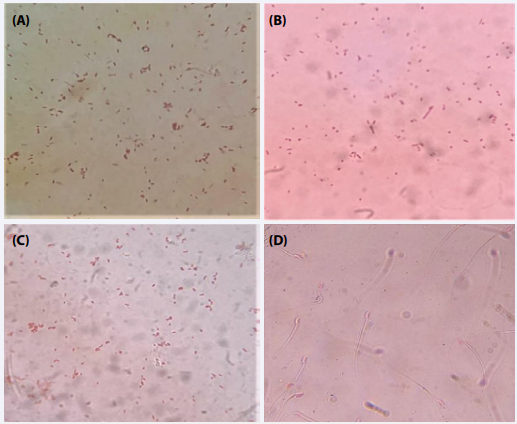
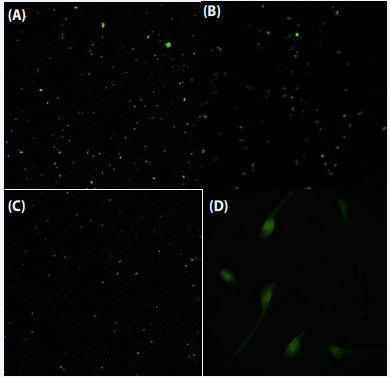
Control serum: Agglutination test was carried out using various bacterial cultures viz. Proteus mirabilis, Serratia marcescens, and Escherichia with control serum of PBS inoculated mice to evaluate the presence of antigens. The results revealed no agglutination between the bacteria and the control serum (Figure 1 A-C).
Similarly, the agglutination test between the mouse spermatozoa and the control serum was observed under the microscope. Immediately after mixing, the observations showed the presence of motile spermatozoa in the wet mount showing no agglutination of spermatozoa with control serum (Figure 1 D).
Figure 1 Photomicrographs showing no agglutination of bacteria with control serum; Proteus mirabilis (A); Serratia marcescens (B); Escherichia coli (C); mouse spermatozoa (D).
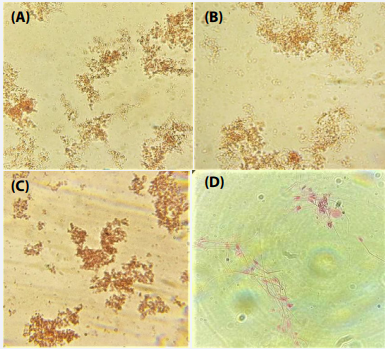
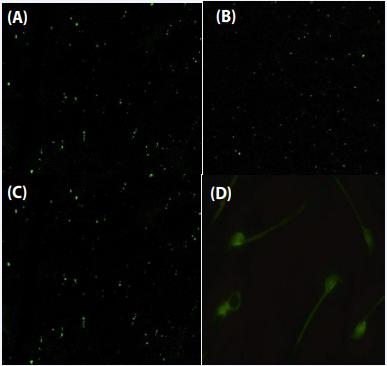
Proteus mirabilis: Agglutination test were performed against Proteus mirabilis, Serratia marcescens, E. coli and mouse spermatozoa with the test serum from mice infected with Proteus mirabilis. Microscopic inspection of bacterial samples with test serum showed instant clumping of bacteria resulting in a high degree of agglutination (Figure 2 A-C). Similarly, to analyze the agglutination of spermatozoa, distinctive fields were seen under the microscope. The observations revealed that spermatozoa also agglutinated with the test serum (Figure 2 D).
Figure 2 Photomicrographs showing agglutination of bacteria and spermatozoa with serum of Proteus mirabilis inoculated mice; Proteus mirabilis (A); Serratia marcescens (B); Escherichia coli (C); mouse spermatozoa (D) .
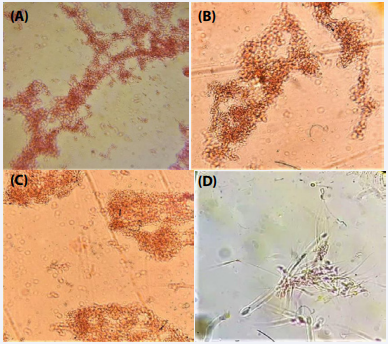
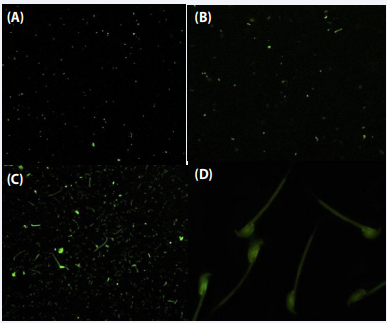
Serratiamarcescens: Under 40x magnification, examinations of Proteus mirabilis, Serratia marcescens, and Escherichia coli showed that the bacteria instantly formed clusters after being exposed to the test serum from mice administered with Serratia marcescens. Test serum-treated spermatozoa were also found to be clumped together. (Figure 3A-D).
Figure 3 Photomicrographs showing agglutination of bacteria and spermatozoa with serum of Serratia marcescens infected mice; Proteus mirabilis (A); Serratia marcescens (B); Escherichia coli (C); mouse spermatozoa (D).
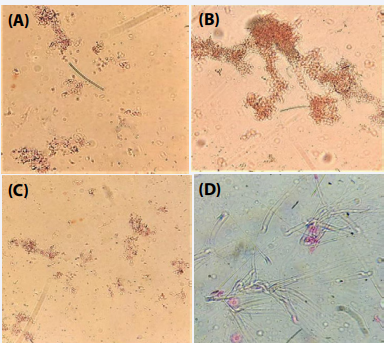
Escherichia coli: Examination on the basis of agglutination test was carried out using the test serum from mice infected with Escherichia coli with the different bacteria and mouse spermatozoa. Proteus mirabilis, Escherichia coli and Serratia marcescens with the test serum sample exhibited agglutination (Figure 4 A-C). Similarly, mouse spermatozoa showed considerable agglutination as depicted in Figure 4 D.
Figure 4 Photomicrographs showing agglutination of bacteria and spermatozoa with serum of mice infected with Escherichia coli; Proteus mirabilis (A); Serratia marcescens (B); Escherichia coli (C); mouse spermatozoa (D).
Examination of antigenic sharing between anti- bacterial hyperimmune serum towards spermatozoa and bacteria via FITC-binding studies
Control serum: Fluorescence microscopy was carried out to detect the existence of cross-reacting antigens on mouse spermatozoa and various bacteria using FITC-tagged control serum. FITC-tagged control serum displayed no fluorescence, demonstrating the absence of antigens (Figure 5).
Figure 5 Flourescent microscpy of FITC labelled control serum with Proteus mirabilis (A); Serratia marcescens (B); Escherichia coli (C); mouse spermatozoa (D).
Proteus mirabilis: FITC-labelled serum from Proteus mirabilis infected mice were used in fluorescence microscopy investigations to assess the binding with spermatozoa and various bacteria. The presence of intense green fluorescence on bacterial surfaces of Proteus mirabilis, Serratia marcescens and Escherichia coli and on the entire spermatozoa was observed. Thus, indicating the existence of a shared antibody-binding antigen on the surfaces of bacteria as well as on spermatozoa (Figure 6).
Figure 6 Vizualization of antigens on bacteria and mouse spermatozoa using FITC-tagged serum from mouse infected with Proteus mirabilis. Flourescent microscpy of FITC-labelled test serum with Proteus mirabilis (A); Serratia marcescens (B); Escherichia coli (C); mouse spermatozoa (D) .
Serratia marcescens: Further using FITC-tagged serum of Serratia marcescens infected mice resulted in fluorescence on both sperm (head and tail) and bacteria, demonstrating the presence of cross-reacting antigens (Figure 7).
Figure 7 Vizualization of antigens on bacteria and mouse spermatozoa using FITC-tagged serum from mouse immunized by Serratia marcescens. Flourescent microscpy of FITC lablled test serum with Proteus mirabilis (A); Serratia marcescens (B); Escherichia coli (C); mouse spermatozoa (D).
Escherichia coli: Similarly, FITC tagged test serum from mouse immunized with Escherichia coli when incubated with Proteus mirabilis, Serratia marcescens, Escherichia coli and mouse spermatozoa showed brilliant green fluorescence, which represented the existence of shared antibody binding on both the spermatozoa and bacterial surface (Figure 8).
Figure 8 Vizualization of shared antigenic determinants on bacteria and mouse spermatozoa using FITC tagged serum from mice infected by Escherichia coli. Flourescent microscpy of FITC lablled test serum with Proteus mirabilis (A); Serratia marcescens (B); Escherichia coli (C); mouse spermatozoa.
DISCUSSION
Human body elicits an immunological response towards sperm antigenic determinants. In several sperm-associated antigens, antigenicity has been discovered, studied, and demonstrated to stimulate the formation of antibodies known as anti-sperm antibodies (ASAs). Anti-sperm antibodies are immunoglobulins that impede the function of spermatozoa by interaction with sperm antigens [2].
Anti-sperm antibodies are one of the causes of immunological sterility. Quite a few studies reported that sperms get agglutinated or immobilized indicating the localization of ASAs not only on the tail of spermatozoa but also, on the head of spermatozoa, thus, preventing the sperm from effectively traversing a woman’s cervical mucous to the ovum [11].
Few investigations have contributed to our understanding of the production of ASAs as a result of a prior microbial infection in infertile individuals [12]. Consequently, a supposition proposing the role of molecular mimicry between bacteria and spermatozoa, i.e., an immune response is elicited against the intruding microorganism and antibodies may be generated against an epitope that is similar to one expressed on spermatozoa, resulting to antibody cross-reactivity, was proposed. According to certain studies human spermatozoa and other prokaryotic bacteria have been shown to share antigens, leading to the development of ASAs. As a result, it is essential to identify bacteria that share antigenic determinants with sperm.
With this perspective, the current work was designed to provide light on the molecular mimicry between bacteria and spermatozoa by producing antibodies from bacteria against a similar epitope found in both bacteria and spermatozoa. Thus, Proteus mirabilis, Serratia marcesencs, and Escherichia coli were chosen to raise polyclonal anti-sperm antibodies in the BALB/c mouse model based on a previous study conducted in our lab in which FITC labelled SIF was found to bind not only to mouse spermatozoa but also to motile and non-motile bacteria, indicating that spermatozoa and bacteria may share an antigen for SIF. When the bacterial receptor/s had a protective effect against SIF-mediated sperm impairment and the sperm receptor offered protection against SIF-mediated bacterial impairment, the homology between the SIF-binding sperm receptor and the bacterial receptor/s was shown.
In our preliminary studies, initially serum from mice infected with Proteus mirabilis, Serrata marcescens and Escherichia coli agglutinated with Proteus mirabilis, Serrata marcescens, Escherichia coli, and spermatozoa as comparative to the control serum which showed no agglutination. This demonstrated that anti-sperm antibodies produced by above mentioned bacteria were present in the serum and cross-react with Proteus mirabilis, Serrata marcescens, Escherichia coli, and spermatozoa. This suggests that bacteria and spermatozoa may have homologous antigens, which is the primary cause of cross-reactivity. Similar study was carried out by Figura et al. [13], in which they explored the ability of H. pylori-specific hyperimmune serum to cross- react with tubulin rich regions of human spermatozoa (pericentriolar and tails).
On similar note, research revealed that the proteins with high structural and sequence homology can induce antibody cross- reaction between human spermatozoa and bacteria. In addition, the antigenicity and epitope analysis indicated that these homologous proteins can be recognized by same antibody. The immunogenic proteins that stimulated cross-reaction between bacteria and spermatozoa include sperm acrosome membrane- associated protein 4, protein deglycase DJ1, UDP-N-acetyl hexosamine pyrophosphorylase [14].
The preliminary studies which were conducted by the serum of Proteus mirabilis, Serrata marcescens, and Escherichia coli showed the production of bacterial induced ASAs against some common antigen present on bacteria and spermatozoa. To further elucidate the confirmation from the previous experiments that there is some antigen-sharing between bacteria and spermatozoa, FITC binding studies were put forth. The FITC tagged control serum and test serum of each bacterium were observed with Proteus mirabilis, Serrata marcescens, and Escherichia coli and spermatozoa. The results from these binding studies showed intense fluorescence of spermatozoa as well as bacteria upon incubation with FITC-tagged test serum while the same was absent in the case of control serum. Thus, this specific binding of bacterial induced anti-sperm antibodies with spermatozoa and bacteria which further authenticated the existence of cross- reactivity of ASAs between spermatozoa and bacteria.
These FITC binding studies were comparable with the experiments performed by Thaper et al. [10], which confirmed that polyclonal antibodies raised in response to purified receptor component from spermatozoa (MS-SBR) were able recognize the antigenic determinants shared by both spermatozoa and bacteria. The anti-MS-SBR antibodies exhibited molecular mimicry to Sperm immobilization factor (SIF), causing the immobilization and death of mouse spermatozoa. The intriguing results obtained with these experiments might serve to fill a knowledge gap by providing substantial evidence of antigen-sharing of ASAs produced by bacteria with spermatozoa.
CONCLUSION
Based on the outcomes of current investigation, it can be concluded that the phenomenon of cross-reactivity between spermatozoa and bacteria emerged from generating antibodies against various bacteria was evinced. The anti-sperm antibodies induced by bacteria were found to cross-react not only with the spermatozoa but also with the various bacteria, indicating that the spermatozoa and bacteria share homologous antigens. However, still there is a dire need to screen other existing infectious microorganisms which might cause the formation of anti-sperm antibodies.
REFERENCES
- AS V, Dhama K, Chakraborty S, Abdul SH, Latheef S, Sharun K. Role of antisperm antibodies in infertility, pregnancy, and potential for contraceptive and antifertility vaccine designs: Research progress and pioneering vision. Vaccines. 2019; 7: 116.
- Lu JC, Huang YF, Lu NQ. Antisperm immunity and infertility. Expert review of clinical immunology. 2008; 4: 113-126.
- Mahdi BM, Salih WH, Caitano AE, Kadhum BM, Ibrahim DS. Frequency of antisperm antibodies in infertile women. J Reprod Infertil. 2011; 12: 261.
- Cui D, Han G, Shang Y, Liu C, Xia L, Li L, Yi S. Antisperm antibodies in infertile men and their effect on semen parameters: a systematic review and meta-analysis. Clin Chim Acta. 2015; 444: 29-36.
- Shetty J, Sherman NE, Herr JC. Methods of analysis of sperm antigens related to fertility. Immune Infertility. 2017; 23-47.
- Krause WK, Naz RK. Immune infertility: Springer Verlag Berlin Heidelberg. 2009.
- Silva AF, Ramalho-Santos J, Amaral S. The impact of antisperm antibodies on human male reproductive function: An update. Reproduction. 2021; 162: R55-R71.
- Clarke GN. ASA in the Female. Immune Infertility. 2017; 161-171.
- Kalaydjiev S, Dimitrova D, Mitov I, Dikov I, Nakov L. Serum sperm antibodies after diarrhoeal diseases. Andrologia. 2007; 39: 101-108.
- Thaper D, Rahi DK, Prabha V. Amelioration of sperm immobilisation factor-induced infertility by bacterial antigenic determinants cross- reacting with spermatozoa. Reprod Fertil Dev. 2019; 31: 602-612.
- Agostini A, Lucas H. Semen analysis. 9th Postgraduate course for training in reproductive medicine and reproductive biology, Geneva, Switzerland, 2011.
- Eggert-Kruse W, Buhlinger-Göpfarth N, Rohr G, Probst S, Aufenanger J, Näher H, et al. Immunology: Antibodies to Chlamydia trachomatis in semen and relationship with parameters of male fertility. Human Reprod. 1996; 11: 1408-1417.
- Figura N, Piomboni P, Ponzetto A, Gambera L, Lenzi C, Vaira D, et al. Helicobacter pylori infection and infertility. Eur J Gastroenterol Hepatol. 2002; 14: 663-669.
- Anas M, Utomo D H, Widjajanto E, Prawiro SR, Aulani’am AA, WiyasaI. Molecular Modeling for Revealing Cross-Reaction Antibody with Staphylococcus aureus and Human Spermatozoa Protein. Int J ChemTech Res. 2016; 9: 233-239.