Fibroma and Fertility: About Two Uncommon Case Reports and Review of the Literature
- 1. Department of Gynaecology-Obstetrics and Endoscopy, Maternity Souissi, University Hospital Center IBN SINA, University Mohammed V, Morocco
- 2. Department of Gynaecology-Obstetrics and Endocrinology, Maternity Souissi, University Hospital Center IBN SINA, University Mohammed V, Morocco
ABSTRACT
Background: Uterine fibroids are a common pathology that occurs in 20% to 50% of women of childbearing age and are the most common benign tumor. The management of a woman with fibroids and a desire for pregnancy remains controversial because of the lack until recently of clear recommendations. This disease’s impact on the couple’s ability to conceive is often mentioned in the literature but the accountability isn’t clearly established. In order to preserve fertility, practitioners must choose between no therapy or conservative treatment of fibroids consisting of myomectomy whether by hysteroscopy, laparoscopy or laparotomy depending on the number, size and topography of the myomas.
Case presentation: We hereby report two cases of patients who presented with primary infertility of respectively ten and eight years without any other etiology than a polymyomatous uterus. Laparotomy polymyomectomy performed in both patients allowed us to remove all the fibroids and allowed the patients to become pregnant and have their first child, respectively after 21- and 25-months post-surgery.
Discussion: Even today, there is insufficient evidence to conclude that uterine fibroids reduce the likelihood of pregnancy. However, it has been shown that in the case of myomas deforming the uterine cavity, myomectomy would result in pregnancy rates almost equivalent to the general population. Infertility management must of course take into account the patient as a whole, but it is legitimate to reassure the patient that her chances of pregnancy after myomectomy will improve, whether by hysteroscopy, laparoscopy or even laparotomy for large multiple myomas such as the cases presented.
KEYWORDS
- Fibroma
- Fertility
- Uterine fibroids
- Pregnancy
CITATION
ESSEBAGH Y, SLAOUI A, ZERAIDI N, LAKHDAR A, KHARBACH A, BAYDADA A (2022) Fibroma and Fertility: About Two Uncommon Case Reports and Review of the Literature. Med J Obstet Gynecol 10(1): 1157.
BACKGROUND
Uterine fibroids are a common pathology that occurs in 20% to 50% of women of childbearing age and are the most common benign tumor [1]. The management of a woman with fibroids and a desire for pregnancy remains controversial because of the lack until recently of clear recommendations [1].
This disease’s impact on the couple’s ability to conceive is often mentioned in the literature but the accountability isn’t clearly established [2]. In order to preserve fertility, practitioners must choose between no therapy or conservative treatment of fibroids consisting of myomectomy whether by hysteroscopy, laparoscopy or laparotomy depending on the number, size and topography of the myomas [2].
We hereby report two cases of patients who presented with primary infertility of respectively ten and eight years without any other etiology than a polymyomatous uterus, through which we will review the literature to analyze the impact of myomas on fertility and the possibilities of therapeutic management.
CASE PRESENTATION
First case
37-years-old patient with no notable pathological history, presented with ten years primary infertility without a determined etiology after an initial follow-up of three years then lost to follow-up, with recent onset of pelvic pain associated with menorrhagia for 6 months without other associated signs. The initial examination found a patient with normal blood pressure and heart rate but with slightly discolored conjunctiva, the gynecological examination found an increased size uterus reaching the umbilicus with presence of abdominopelvic masses mobile with the uterus without separation furrows. Initial biological assessment objectified microcytic hypochromic anemia at 9.2 g/dL and an abdominopelvic ultrasound completed with an abdominopelvic MRI allowed us to have a precise mapping of all the fibroids and to ensure the absence of any associated pathologies. The uterus measured 13 cm long by 7 cm wide with the presence of eighteen fibroids of all FIGO stages, the largest of which was of 11 cm long axis posterofundial FIGO type 2-5.
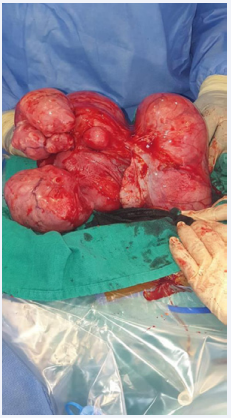
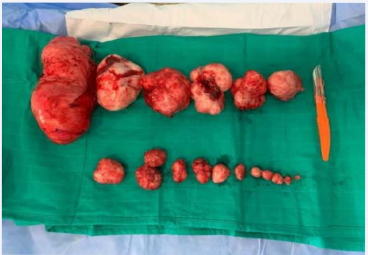
Infertility being the first consideration of the couple, it was decided by mutual agreement to perform a polymyomectomy by laparotomy. The patient was informed of the risk of hysterectomy but also of the possibility that this operation would not solve her fertility problem. The procedure allowed us to remove all eighteen fibroids using seven uterine incisions with effraction of the uterine cavity since two of the fibroids, 2 and 3 cm respectively, were of type 1 of the FIGO classification (Figure 1 and 2).
Figure 1 Per operative aspect of the first patient’s polymyomatous uterus.
Figure 2 Macroscopic aspect of the first patient’s removed myomas.
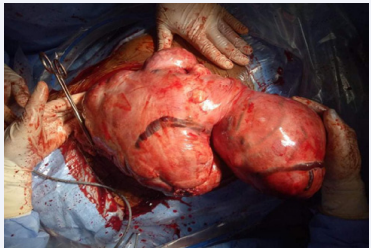
Figure 3 Per operative aspect of the second patient’s polymyomatous uterus
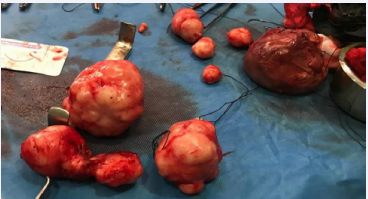
Figure 4 Macroscopic aspect of the second patient’s removed myomas.
Three layers of suture were used to close the incision: a continuous suture to close the endouterine cavity with Vicryl 1, followed by Vicryl 1 X-stitches to close the space left empty by the fibroids while ensuring hemostasis down to the surface of the uterine wall, which was sutured with a continuous suture with 3-0 PDS II. Hyalobarrier gel was inserted inside the cavity through the endouterine breach wound to prevent synechiae but also above the hysterorrhaphy sutures to prevent adhesions. The operative time was 1 hour and 13 minutes and the estimated blood loss was 785 mL. She received a first dose of 750 mg of injectable iron on day one post-surgery and a second one on an outpatient basis fifteen days later for a total cumulative dose of 1500 mg of iron. Her hemoglobin at 1 month postoperatively was 11.2 g / dL. The postoperative course was uneventful and the patient was discharged on day 2 postoperatively.
The patient initially underwent six months of oestroprogestogen contraception before resuming her pregnancy plans. Thirteen months after the operation, she became pregnant and was followed up in our facility. A planned cesarean section resulted in the birth of a healthy baby boy of 3217 g at 38 weeks of amenorrhea.
Second case
29-year-old patient with no previous pathological history presented with recent pelvic pain associated with chronic constipation and a primary infertility of eight years without a determined etiology after an initial follow-up of two years then lost to follow-up, without associated urinary signs. The general examination revealed a patient in good general condition and the gynecological examination found an enlarged uterus reaching midway to the umbilicus, bumpy in outline, mobile and painless. Initial biological assessment revealed a mild microcytic hypochromic anemia at 10.7 g/dL. Abdominopelvic ultrasound completed with an abdominopelvic MRI allowed us to have a precise mapping of all the fibroids and to ensure the absence of any associated pathologies. The enlarged uterus was 14 cm long and 8 cm wide and was the site of eleven fibroids ranging from FIGO stage 2 to stage 7, i.e., no submucosal fibroid. The most voluminous myoma was 9 cm long axis posterior corporal FIGO type 2-5, which could be the cause of the digestive symptoms by compressive effect.
Given the desire for pregnancy, the young age of the patient and the absence of a submucosal fibroid, it was decided to perform a polymyomectomy by laparotomy. The couple was informed of the risk of hysterectomy but also of the possibility that this operation would not solve her fertility problem. The intervention allowed the removal in the first case of nine myomas of which the largest was 10 cm long axis type 2-5 FIGO. For this patient there was no effraction of the uterine cavity and none of the myomas had a submucosal component. Two layers of suture were used to close the incision: Vicryl 1 X-stitches to close the space left empty by the fibroids while ensuring hemostasis down to the surface of the uterine wall which was sutured with a continuous suture with 3-0 PDS II. Hyalobarrier gel was applied above the hysterorrhaphy sutures to prevent adhesions. The operative time was 1 hour and 21 minutes and the estimated blood loss was 835 mL. She received a first dose of 750 mg of injectable iron on day one post-surgery and a second one on an outpatient basis fifteen days later for a total cumulative dose of 1500 mg of iron. Her hemoglobin at 1 month postoperatively was 12.1 g / dL. The postoperative course was uneventful and the patient was discharged on day 2 postoperatively.
The patient initially underwent six months of oestroprogestogen contraception before resuming her pregnancy plans. Seventeen months after the operation, she became pregnant and was followed up in our facility. A planned cesarean section resulted in the birth of a healthy baby girl of 3370 g at 38 weeks of amenorrhea.
DISCUSSION
Uterine fibroids also known as myomas or leiomyomas are benign monoclonal mesenchymal tumors arising from smooth muscle cells organized within an adjacent structurally normal myometrium [1,2]. Fibroids most commonly affect the uterine body and more rarely the cervix or isthmus. One of the greatest challenges in the diagnosis of myoma is its differentiation from uterine sarcoma, MRI can help but it is only the pathological study that confirms the diagnosis [2].
The pathophysiology of fibroid formation is poorly understood with many leads exist but not yet conclusive [3]. Premenopausal age, African ancestry, age at menarche and reproductive history are the most important risk factors and have the characteristic of being not modifiable [4]. The formation and growth of these benign tumors are likely to be the result of a genetic predisposition illustrated by the fact that more than 40% of first-degree relatives of women with fibroids will develop the same condition during their lifetime, to which endogenous factors such as sex steroid hormones and growth factors are added to promote the process of angiogenesis [5]. Cytogenetic abnormalities are not mandatory in the formation of fibroids as 50% of them do not have any cytogenetic abnormalities [6]. Two stages are essential for the formation and development of a fibroid within a normal myometrium: the first being the transformation of a normal myometrial cell into an abnormal myocyte capable of multiplication and the second involves the concept of monoclonality of fibroids which explains an anarchic multiplication from a single cell that can lead to a clinical tumor [7].
Clinically myomas may be asymptomatic or be revealed by numerous functional signs such as menorrhagia, metrorrhagia, pelvic masses, pelvic pain which may be chronic as a feeling of heaviness like our first case or acute such as torsion or expulsive colic. Fibroids’ compressive effect can also be revealing with urinary disorders like pollakiuria like our second case or towards the rectum with constipation [8]. Ultrasound is the most widely used modality because of its availability, ease of use and cost effectiveness. This modality is particularly useful for assessing myoma growth and adnexa [9]. Contrast ultrasound (saline or gel) are highly accurate diagnostic procedures that allow detection of submucosal lesions [10,11]. In women with large fibroids, diagnostic imaging occasionally reveals hydronephrosis but complete ureteral obstruction is extremely rare [12]. CT is of limited value in delineating the location of myomas in relation to the endometrium or myometrium [13]. MRI is the most accurate modality for evaluation of the adnexa [14], and uterus, providing information on the size, location, number, and perfusion of leiomyomas, as well as the presence of other uterine pathology (including adenomyosis and/or adenomyomas) allowing us to have a complete mapping of the uterus but above all to be prepared for a possible sarcoma [15,16].
It has been reported that 5% to 10% of cases of infertility prior to medical management are associated with the presence of uterine myomas [17], which are considered the sole factor in infertility in 1% to 3% of cases [18,19]. In the general population, the prevalence of infertility varies between 10% and 15% of cases [20]. Already in 1981, Buttram et al. [18], reported that myomas could be the sole factor in infertility. This observation was then objectified in many other studies [21,22]. It is accepted that intramural or cavity-distorting myomas are often associated with impaired fertility [23]. Several studies have looked at fibroid-related infertility and the issue is still being debated to this day [23,24]. Their impact on fertility can be approached either by comparing women with myomas to women without presenting with a pregnancy project or by comparing the results of myomectomy to those of therapeutic abstention in patients with myomas suffering of infertility. The first one is difficult with numerous selection biases hard to overcome without systematically including all patients from the beginning of their pregnancy project. The studies therefore focused on the second possible comparison: to treat or not to treat fibroids in the context of infertility.
Bulletti et al. [24], found a significantly higher delivery rate after myomectomy compared with a control group of non- operated patients with myomas with respectively 42% versus 11%. Casini et al. [25], did not find any significant improvement in the pregnancy rate after myomectomy for intramural or subserous myomas but in case of submucosal or intramural myomas with a submucosal component, myomectomy showed a benefit compared to therapeutic abstention with a pregnancy rate of respectively 43% versus 27% and 36% versus 15% [25]. In the prospective study by Shokeir et al. [26], the authors found a significant difference in terms of pregnancy rate % in patients under 35 yearsofagewith submucosal myoma astheonlyinfertility factor in favor of myomectomy compared with abstention with respectively 64% versus 28 [26]. According to the same authors, the size, number and location of myomas did not influence the pregnancy rate [26]. Indeed, another prospective study by Bulletti et al. [27], demonstrated the interest of myomectomy in the case of large intramural myomas over 5 cm long axis. Thus, patients who have undergone myomectomy have significantly increased rates of conception (33 % versus 15 %), and full-term pregnancy (25 % versus 12 %) [27]. Several retrospective studies showed an average conception rate of over 50 % after myomectomy patients suffering of infertily, which is superposable on the pregnancy rate of 48 % found in the meta-analysis by Donnez et al. [28]. This conception rate is identical whether the operation is carried out by laparotomy or laparoscopy [29-32]. In our case, both patients underwent myomectomy by laparotomy, both succeeded in having a pregnancy spontaneously. Finally, a systematic review [33], published in 2017 objectified that in women with cavity- distorting myomas, myomectomy may be considered to optimize pregnancy outcomes but did not reduce miscarriage rates.
CONCLUSION
Even today, there is insufficient evidence to conclude that uterine fibroids reduce the likelihood of pregnancy. However, it has been shown that in the case of myomas deforming the uterine cavity, myomectomy would result in pregnancy rates almost equivalent to the general population. Infertility management must of course take into account the patient as a whole, but it is legitimate to reassure the patient that her chances of pregnancy after myomectomy will improve, whether by hysteroscopy, laparoscopy or even laparotomy for large multiple myomas such as the cases presented.
DECLARATIONS
Guarantor of Submission
The corresponding author is the guarantor of submission.
Availability of data and materials
Supporting material is available if further analysis is needed.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor- in-Chief of this journal.
Ethics approval and consent to participate
Ethics approval has been obtained to proceed with the current study. Written informed consent was obtained from the patient for participation in this publication.
REFERENCES
- Verkauf BS. Myomectomy for fertility enhancement and preservation. Fertil Steril 1992; 58: 1-15.
- Suzuki A, Aoki M, Miyagawa C, Murakami K, Takaya H, Kotani Y, et al. Differential Diagnosis of Uterine Leiomyoma and Uterine Sarcoma using Magnetic Resonance Images: A Literature Review. Healthcare (Basel). 2019; 7: 158.
- Borahay MA, Al-Hendy A, Kilic GS, Boehning D. Signaling Pathways in Leiomyoma: Understanding Pathobiology and Implications for Therapy. Mol Med. 2015; 21: 242-256.
- Wise LA, Laughlin-Tommaso SK. Epidemiology of Uterine Fibroids: From Menarche to Menopause. Clin Obstet Gynecol. 2016; 59: 2-24.
- Haney AF. Clinical decision making regarding leiomyomata: what we need in the next millenium. Environ Health Perspect. 2000; 108: 835- 839.
- Hennig Y, Wanschura S, Deichert U, Bartnitzke S, Bullerdiek J. Rearrangements of the high mobility group protein family genes and the molecular genetic origin of uterine leiomyomas and endometrial polyps. Mol Hum Reprod. 1996; 2: 277-283.
- Aaronson SA. Growth factors and cancer. Science. 1991; 254: 1146- 1153.
- Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update. 2016; 22: 665-686.
- Cantuaria GH, Anglioli R, Frost L, Duncan R, Penalver MA. Comparison of bimanual examination with ultrasound before hysterectomy for uterine leiomyoma. Obstet Gynecol. 1998; 92: 109e12
- Farquhar C, Ekeroma A, Furness S, Arroll B. A systematic review of transvaginal ultrasonography, sonohysterography and hysteroscopy for the investigation of abnormal uterine bleeding in premenopausal women. Acta Obstet Gynecol Scand. 2003; 82: 493e504.
- Makris N, Kalmantis K, Startados N, Papadimitriou A, Mantzaris G, Antsaklis A. Three dimensional hysterosonography versus hysteroscopy for the detection of intracavitary uterine abnormalities. Int J Gynecol Obstet. 2007; 95: 6e9.
- Vercellini P, Crosignani PG, Mangioni C, Imparato E, Ferrari A, De Giorgi O. Treatment with a gonadotrophin releasing hormone agonist before hysterectomy for leiomyomas: results of a multicentre, randomized controlled trial. Br J Obstet Gynaecol.1998; 105: 1148e54.
- Bradley LD, Falcone T, Magen AB. Radiographic imaging techniques for the diagnosis of abnormal uterine bleeding. Obstet Gynecol Clin North Am. 2000; 27: 245e76.
- Adusumilli S, Hussain HK, Caoili EM, Weadock WJ, Murray JP, Johnson TD, et al. MRI of sonographically indeterminate adnexal masses. AJR Am J Roentgenol. 2006; 187: 732e40.
- Dueholm M, Lundorf E, Hansen ES, Ledertoug S, Olesen F. Accuracy of magnetic resonance imaging and transvaginal ultrasonography in the diagnosis, mapping, and measurement of uterine myomas. Am J Obstet Gynecol. 2002; 186: 409e15.
- Stamatopoulos CP, Mikos T, Grimbizis GF, Dimitriadis AS, Efstratiou I, Stamatopoulos P, et al. Value of magnetic resonance imaging in diagnosis of adenomyosis and myomas of the uterus. J Min Invas Gynecol. 2012; 19: 620e6.
- Stovall DW, Parrish SB, Van Voorhis BJ, Hahn SJ, Sparks AE, Syrop CH. Uterine leiomyomas reduce the efficacy of assisted reproduction cycles: results of a matched follow-up study. Hum Reprod. 1998; 13: 192-197.
- Buttram Jr. VC, Reiter RC. Uterine leiomyomata: etiology, symptomatology, and management. Fertil Steril. 1981; 36: 433-445.
- Narayan R. Rajat, Goswamy K. Treatment of submucous fibroids, and outcome of assisted conception. J Am Assoc Gynecol Laparosc. 1994; 1: 307-311.
- Cramer DW, Walker AM, Schiff I. Statistical methods in evaluating the outcome of infertility therapy. Fertil Steril. 1979; 32: 80-86.
- Rubin IC. Uterine fibromyomas and sterility. Clin Obstet Gynecol 1958; 1: 501-518.
- Hasan F, Arumugam K, Sivanesaratnam V. Uterine leiomyomata in pregnancy. Int J Gynaecol Obstet. 1991; 34: 45-47.
- Tian YC, Wu JH, Wang HM, Dai YM. Improved Fertility Following Enucleation of Intramural Myomas in Infertile Women. Chin Med J (Engl). 2017; 130: 1648-1653.
- Donnez J, Jadoul P. What are the implications of myomas on fertility? A need for a debate? Hum Reprod. 2002; 17: 1424-1430.
- Bulletti C, De Ziegler D, Polli V, Flamigni C. The role of leiomyomas in infertility. J Am Assoc Gynecol Laparosc. 1999; 6: 441-445.
- Casini ML, Rossi F, Agostini R, Unfer V. Effects of the position of fibroids on fertility. Gynecol Endocrinol. 2006; 22: 106-109.
- Shokeir T, El-Shafei M, Yousef H, Allam AF, Sadek E. Submucous myomas and their implications in the pregnancy rates of patients with otherwise unexplained primary infertility undergoing hysterosco- pic myomectomy: a randomized matched control study. Fertil Steril. 2010; 94: 724-729.
- Bulletti C, De Ziegler D, Levi Setti P, Cicinelli E, Polli V, Stefanetti M. Myomas, pregnancy outcome, and in vitro fertilization. Ann N Y Acad Sci. 2004; 1034: 84-92.
- Shue S, Radeva M, Falcone T. Comparison of long-term fertility outcomes after myomectomy: relationship with number of myomas removed. J Minim Invasive Gynecol. 2018; 25: 1002-1008.
- Campo S, Campo V, Gambadauro P. Reproductive outcome before and after laparoscopic or abdominal myomectomy for subserous or intra- mural myomas. Eur J Obstet Gynecol Reprod Biol. 2003; 110: 215-219.
- Flyckt R, Soto E, Nutter B, Falcone T. Comparison of long-term fer- tility and bleeding outcomes after robotic-assisted, laparoscopic, and abdominal myomectomy. Obstet Gynecol Int. 2016; 2016: 2789201.
- Seracchioli R, Rossi S, Govoni F, Rossi E, Venturoli S, Bulletti C, et al. Fertility and obstetric outcome after laparoscopic myomectomy of large myomata: a randomized comparison with abdominal myomec- tomy. Hum Reprod. 2000; 15: 2663-2668.
- Practice Committee of the American Society for Reproductive Medicine. Removal of myomas in asymptomatic patients to improve fertility and/or reduce miscarriage rate: a guideline. Fertil Steril. 2017; 108: 416-425.