Correlation between Sleep Apnea and Blood Pressure Patterns in Ambulatory Blood Pressure Monitoring
- 1. Hypertension Center. Department of Cardiology, Hospital Británico de Buenos Aires, Buenos Aires, Argentina
- 3. Center of Respiratory Medicine, Sleep and Ventilation Unit, Hospital Británico de Buenos Aires, Buenos Aires, Argentina
- 6. Hypertension Center. Department of Cardiologu, Hospital Británico de Buenos Aires, Buenos Aires, Argentina
- 7. Center of Respiratory Medicine, Sleep and Ventilation Unit, Hospital Británico de Buenos Aires, Buenos Aires, Argentina
- 8. Hypertension Center. Department of Cardiology, Hospital Británico de Buenos Aires, Buenos Aires, Argentina
Abstract
Introduction: Guidelines suggest suspecting obstructive apnea-hypopnea syndrome (OSA) in patients with hypertension (HT) if the nocturnal blood pressure is elevated, a patient is a non-dipper or an inverse pressure pattern is observed. The objective of our work is to describe the behavior of blood pressure by ambulatory blood pressure monitoring (ABPM) in patients with OSA and HT who do not receive pharmacological treatment and CPAP.
Materials and Methods: It is a retrospective study in adults, including patients with HT and OSA without antihypertensive treatment or CPAP. Epworth Sleepiness Scale (ESS), Berlin and the STOP-BANG (SBQ) questionnaire were checked against home-based respiratory polygraphy (RP) and HT patterns through 24-h outpatient scores ABPM.
Results: We analyzed 83 patients of which 42 were men (51%) with a mean age of 44.5 years. 48 (57%) of these patients had nocturnal hypertension (NH); 30 (36%) showed daytime and nocturnal hypertension (DNH), and 5 (6.8%) had just daytime hypertension (DH). OSA clinically relevant were represented by 33.3% in the NH group, 36.6% of patients in the DNH group and 40% in the DH group (p = 0.92). Severe OSA stood for 20.8% in the NH group, 33.3% in the DNH group and 20% in the DH group (p = 0.37). We found no differences in sleepiness symptoms, in oxygen desaturation index (ODI) and desaturation time less than 90% (T < 90%).
Conclusion: The diagnosis of OSA in hypertensive patients should be suspected considering different parameters such as clinical presentation, the ABPM values and presence of comorbidities.
Keywords
Sleep apnea-Hypopnea syndrome, Hypertension, Respiratory polygraphy, Scales and questionnaires, Ambulatory blood pressure monitoring
ABBREVIATIONS
OSA: Apnea-hypopnea syndrome; HT: Hypertension; ABMP: Ambulatory blood pressure monitoring; ESS: Epworth Sleepiness Scale; SBQ: Stop-bang; RP: Respiratory polygraphy; S: Sensibility; Sp: Specificity; AUC ROC: Area under ROC curve; NH: Nocturnal hypertension; DNH: Daytime and nocturnal hypertension; DH: Daytime hypertension; AHI: Apnea-hypopnea index; ODI: Oxygen desaturation index; T<90%: Desaturation time less than 90%; BMI: Body mass index; RAAS: Renin-angiotensin-aldosterone system; BP: Blood pressure; AASM: American Academy of Sleep Medicine; NC: Neck circumference; Ev/h: events/hour; TRT: Total
recording time; PPV: Positive predictive value; NPV: Negative predictive value; WHBP: White coat high blood pressure; REM: Rapid eye movement; MBPS: Morning Blood Pressure Surge.
Citation
Schiavone M, Pronotti MV, Borsini E, Ernst G, Mariani J, et al. (2022) Correlation between Sleep Apnea and Blood Pressure Patterns in Ambulatory Blood Pressure Monitoring. Ann Clin Exp Hypertension 7(1): 1058.
INTRODUCTION
Hypertension (HT) and obstructive sleep apnea-hypopnea syndrome (OSA) are highly prevalent and frequently related [1]. HT affects 36.3% of the adult population and its’ lack of control leads to cardiovascular disease such as heart failure, acute myocardial infarction, arrhythmias, kidney failure, and stroke after years of exposure to this condition [2].
OSA is an emerging health problem that affects more than 15% of men and 10% of women in the adult population [3], and it is associated with higher morbidity and mortality attributable to traffic, work and domestic accidents. It is also related to the development of cardiovascular and cerebrovascular complications [4,5]. Previous studies carried out in our country described that approximately 50% of individuals with OSA are hypertensive [6,7]. Near 44% of the hypertensive patients who consulted to the cardiologist had moderate to severe OSA and were candidates for treatment with CPAP [8]. HT is related to the severity of OSA. It has been shown the possibility of developing HT increases by 1% with the increase per unit of AHI [9-11]. Individuals with AHI > 15 events/hour (ev/h) are more likely to be hypertensive than individuals of similar age and body weight without OSA. It has been observed that the greater the severity of OSA, the greater amount and dosages of antihypertensive drugs required to achieve control of blood pressure [12]. Upper airway obstruction generates intermittent periods of hypoxia and awakenings, dynamic changes in intra-thoracic pressure, and blood pressure oscillation during sleep, product of sympathetic activation and the renin-angiotensin-aldosterone system (RAAS) [13].
Until now, published data has shown that OSA can aggravate nocturnal blood pressure variability, so it is unquestionable to look for this disorder in those patients with nocturnal hypertension and an altered circadian pattern (non-dipper or inverse pattern). The same happens in cases of hidden hypertension, usually underestimated in OSA and which frequently manifests as well (42% to 80%) [14-16].
Most of the guidelines suggest suspecting OSA in patients with HT if nocturnal blood pressure is elevated or if a non-dipper or inverse pattern is observed [16-18], not considering the use of validated questionnaires used in sleep medicine such as: Berlin, Epworth (ESS) and the STOP BANG (SBQ). These have been shown to be tools that present a high discriminative power when used together with ABMP for the diagnosis of patients with OSA. The Berlin questionnaire has greater sensitivity to identify those with an AHI> 5 ev/h, and SBQ with the ESS have greater sensitivity to distinguish individuals with moderate to severe OSA [7,19].
The objective of our work is to describe the behavior of blood pressure in patients with OSA and HT who do not receive pharmacological treatment and CPAP.
MATERIALS AND METHODS
Ethics declarations
This study was approved by the Institutional Review Board pursuant to the Declaration of Helsinki (Approval No. CRIHB #849 – March 2018).
Design
This is a descriptive study conducted in adults referred to an HT center between August 2019 and December 2019.
Population
The study included patients between 18 and 80 years of age who attended an HT center to confirm their hypertension diagnosis. They underwent a clinical evaluation and were asked to systematically complete sleep questionnaires. A onenight self-administered respiratory polygraphy (RP) was performed by level III devices of the American Academy of Sleep Medicine (AASM) on each patient. Finally, ABPM was requested. Hypertensive patients treated with medication and those with a previous diagnosis of OSA were excluded. The questionnaires and sleep study data were correlated with the data obtained from the
patient’s anamnesis (history and risk factors), anthropometric data, office BP taking, and biochemical blood test analysis and 24-h ABPM values.
Basic clinical examination
Data gathered included history and risk factors (through systematic anamnesis), anthropometric data, body mass index (BMI), neck circumference (NC), office blood pressure (BP) and blood tests. HT diagnosis was confirmed through 24-h outpatient ambulatory blood pressure monitoring (ABPM).
Blood pressure measurement
BP was measured using an automatic blood pressure monitor (OMRON 7220) after a 2-min rest. Physicians recorded the average of 3 different readings measured at 1-min intervals.
Ambulatory blood pressure monitoring (ABPM)
24-h ABPM was performed with Spacelabs Ultralite monitor (model 90217, SpaceLabs, Redmond, WA). Measurements were programmed every 15 minutes during the day (8:00 AM to 11:00 PM) and every 30 minutes during the night (11:00 PM to 8:00 AM). Normal daytime and nighttime BP values were ≤ 135/85 mmHg and ≤ 120/70 mmHg, respectively. HT diagnosis was confirmed after ABPM. RP and ABPM were conducted on consecutive nights.
Questionnaires
All participants systematically completed validated questionnaires Berlin, ESS, and SBQ [11,12] in their Spanish version. These were administered by cardiology technicians.
Self-administered home-based respiratory polygraphy
RPs were conducted using Apnea Link Plus™ devices (ResMed™ Australia). These polygraphy devices have 5 channels and 3 basic signals, pulse oximeter, thoracic effort, and nasal pressure cannula; level III device; American Academy of Sleep Medicine [14]. Only recordings with a total recording time > 240 minutes after manual analysis were considered valid. Apnea was defined as a decrease in airflow by >80% of baseline for ≥ 10 s, and hypopnea as a 50% decrease for ≥ 10 s associated with ≥ 3% oxygen desaturation [16]. AHI was calculated as the count of apneas + hypopneas per hour, events/hour (ev/h), of valid total
recording time (TRT). Patients were classified as non-OSA (AHI < 5), mild-OSA (AHI between ≥ 5 and < 15), moderate-OSA (AHI between ≥ 15 and < 30) and severe-OSA (AHI ≥ 30) patients. The same threshold was used for the rate of desaturations in ev/h. The saturation time below 90% was expressed as a percentage of TTR.
Statistical analysis
For categorical variables, results were presented as percentages. For numerical variables, results were presented as standard deviation or mean value (±). Differences were compared using Fisher’s exact test, Mann-Whitney test, or χ2. Calculations made included sensitivity (S) and specificity (Sp), positive predictive value (PPV) and negative predictive value (NPV).
Statistical analysis was performed using Graph Pad Prism-7™ software.
RESULTS
Of the 500 initially evaluated patients, 83 were included for presenting HT and OSA, without antihypertensive treatment or CPAP, after excluding those with white coat high blood pressure (WHBP) and met an acceptable home sleep testing. The patients were grouped according to the hypertension profile by ABPM in daytime (DH), nocturnal (NH) and daytime nocturnal (DNH). There were 5, 48 and 30 patients in each group, respectively (Figure 1).
Figure 1: Patients flowchart. WHBP: White coat high blood pressure; HT: Hypertension; OSA: Apnea-hypopnea syndrome.
The mean age was 44.5 years, 51% (n= 44) were men. Their mean BMI was 34.6 kg/m2. 68% (n=33) of the patients with NH, 60% (n=3) with DH and 56% (n=17) with DNH demonstrated to have a BMI greater than 30 (p = 0.55). The mean total cholesterol was 193.1 mg/dl, but there were no statistically significant differences across groups. OSA symptoms (by ESS and SBQ questionnaires) were quite similar between groups. (Table 1) shows demographic characteristics of the study population.
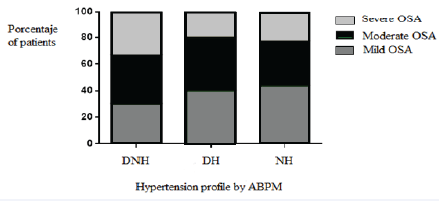
The prevalence of OSA in the 3 groups worked similarly: AHI > 15 ev/h showed in 36.6% (n=10.9) of the DNH group, 40% (n=2) of the DH group and 33.3% (n=15.8) of the NH group (p = 0.92). Severe OSA (AHI > 30 ev/h): 33.3% (n=9,9) of the DNH group, 20% (n=1) of the DH group and 20.8% (n=9,9) of the NH group (p = 0.37).
Figure 2: OSA prevalence between HT groups. OSA: apneahypopnea syndrome; Daytime-nocturnal hypertension; DH: Daytime hypertension; NH: Nocturnal hypertension; ABPM: ambulatory blood pressure monitoring.
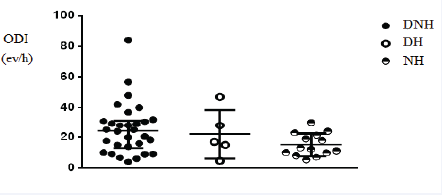
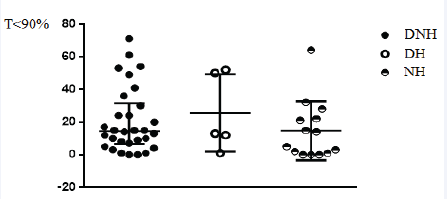
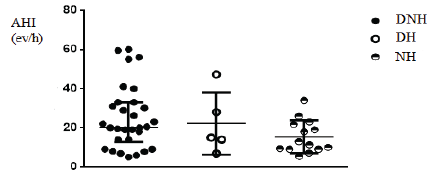
Figure 2 shows OSA prevalence. (Figure 3) shows oxygen saturation index (ODI). (Figure 4) shows desaturation time less than 90% (T< 90%) and (Figure 5) shows apnea-hypopnea index. There were no significant differences in these severity indicators between HT patterns groups (p = 0.19, p = 0.11, p=0.49, respectively).
Figure 3: Oxygen desaturation index between HT groups. ODI: Oxygen desaturation index; DNH: Daytime-nocturnal hypertension; DH: Daytime hypertension; NH: Nocturnal hypertension.
DISCUSSION
Figure 4: Desaturation time less than 90% in relation to HT patterns. T<90%: Desaturation time less than 90%; DNH: Daytimenocturnal hypertension; DH: Daytime hypertension; NH: Nocturnal hypertension.
In our study the prevalence of OSA was similar in the 3 groups. Although there were not statistically differences between them in terms of severity and symptoms evaluated by the questionnaires.
Figure 5: Apnea-hypopnea index in relation to HT patterns. AHI: Apnea-hypopnea index; DNH: Daytime-nocturnal hypertension; DH: Daytime hypertension; NH: Nocturnal hypertension.
In 24-hour recordings, BP in OSA frequently features a “nondipping” profile, ie, <10% fall from day to night, which may increase cardiovascular risk and occurrence of major cardiovascular events in the nocturnal hours (20). Research has shown a strong association between sleep apnea and nocturnal hypertension [21,22], and guidelines indicate that polysomnography should be considered in patients with nocturnal hypertensive and nondipping profile.
Table 1: Demographic characteristics of the study population.
| Daytime-nocturnal HT | Daytime HT | Nocturnal HT | p | |
|---|---|---|---|---|
| N | 30 | 5 | 48 | |
| Age | 49.5 (43.5-60.5) | 33 (32-65.5) | 51 (43-62.7) | 0.43 |
| Men | 70% (n=21) | 40% (n=2) | 44% (n=21) | 0.65 |
| BMI | 33 (28-38.5) | 34.7 (27.3-41) | 36.2 (30.4-42.3) | 0.13 |
| BMI > 30 | 56% (n=17) | 60% (n=3) | 68% (n=33) | 0.55 |
| Cholesterol | 209 (182.3-224.5) | 172 (150-219.5) | 198.5 (166.5-215.9) | 0.28 |
| ESS | 6.5 (3.7-9) | 12.5 (6.2-19.5) | 5 (2-9) | 0.08 |
| ESS > 10 | 10% (n=3) | 40% (n=2) | 16% (n=8) | 0.4 |
| SBQ > 5 | 33% (n=10) | 40% (n=2) | 18% (n=9) | 0.2 |
| HT: hypertension; DNH: Daytime-nocturnal HT; DH: Daytime HT; NHT: Nocturnal HT; BMI: Body mass index; ESS: Epworth Sleepiness Scale; SBQ: Stop-Bang Scale | ||||
On the other hand, some information about the diurnal BP changes in OSA has been obtained with measurements taken in the evening and the next morning, after awakening. There is some evidence about the association of daytime HT and OSA and their mechanism, but it seems that increase in morning BP in those patients could potentially relate to the circadian variations in arterial tone mediated by α1-adrenoreceptor activity with peak vascular resistance during the morning hours. Increased morning levels of catecholamines (because of repetitive sympathetic activation during the night in these patients) and relative increase in REM sleep is associated with peripheral vasoconstrictive activity in the last third of the sleep period [23]. According to this methodology, the morning BP levels in OSA tend to exceed the evening ones. Most studies have reported that the evening to-morning BP difference was correlated with OSA severity. (Table 2).
Table 2: Studies based on morning and evening BP measurements.
| Authors | Subjects under study | Findings |
|---|---|---|
| Hoffstein and Mateika (24) | 611 suspected OSA | If AHI <10: BP morning < BP evening If AHI 10- <50: BP morning = BP evening If AHI >50: BP morning > BP evening |
| Sforza and Lugaresi (25) | 253 suspected OSA | In both simple snorers and OSA: BP morning > BP evening SBP evening-to-morning rise correlated to AHI |
| Stradling et al (26) | 448 subjets from general population | ODI and mean nocturnal respiratory effort independently correlated with evening-to-morning BP rise. |
| Lavie-Nevo and Pillar (27) | 2009 suspected OSA | SBP and DBP evening-to-morning rise correlated with AHI in men, but not in women. |
| Ting et al (28) | 263 simple snorers, UARS or OSA | Subjetcs with morning:evening BP ratio >11% (“morning surge” pattern) or with mean both morning and evening BP >130mmHg (“constant high”) had higher AHI, lower lowest SaO2, longer T<90% and higer arousal index than subjects with morning:evening BP ratio <11% (“morning drop”) or both mean BP <130mmHg (“constant low”). |
| Huang et al (29) | 105 positional and 266 nonpositional OSA | Morning BP higher in nonpositiional OSA; evening BP and eveningto- morning BP difference similar in positional and nonpositional OSA. |
| Wang et al (30) | 3246 OSA +- 354 controls | Progressive increase in morning/evening mean BP with increasing OSA severity. |
| Mokros et al (31) | 454 normotensive suspected OSA | AHI linearly correlated with evening-to-morning DBP rise, but not SPB rise |
| AHI: apnea/hypopnea index; BP: blood pressure; DBP: diastolic BP; ODI: oxygen desaturation index; OSA: obstructive sleep apnea; SaO2: oxyhemoglobin saturation; SPB: systolic BP; T<90%: time spent with oxyhemoglobin saturation <90%; UARS: upper airway resistance syndrome. | ||
In the general population, when excluding patients treated for HT, the prevalence of clinical undiagnosed HT was found to be about 23% among the 27783 subjects of the IPHAF study (mean age 38.2 years), and 24% of 1637 patients in the PAMELA study (mean age 48.2 years) [32]. In 2005, Baguet et al [33] published a study which evaluated 59 patients with OSA who had their blood pressure measured in an office and outpatient using the ABPM. It was shown that hypertension in these patients was underdiagnosed since the use of ABPM which allowed finding 38% more hypertensive OSA patients, compared to taking BP in
the office. Nocturnal hypertension was more frequent in these patients, but 48% of them also had hypertension during the night and during the day, although those exclusively diurnal were not discriminated. Isolated diastolic HT was diagnosed in a high percentage of the population, especially using ABPM (44% of the hypertensives during the daytime and 73% during the nighttime).
Current knowledge suggests that BP behavior in OSA plays a role in the increased cardiovascular risk that is typical of this disorder, not only through its high diurnal and even more its nocturnal BP values but also through the characteristics of its variability. In fact, very short-term variability, nondipping BP profile and high Morning Blood Pressure Surge (MBPS), which are all common in OSA, are known risk factors. Among OSA patients studied with repeated morning and evening BP measurements, those with an MBPS showed higher high-sensitivity C-reactive protein levels. Similarly, among OSA patients studied by ABPM, non-dippers showed higher levels of IL2 and high-sensitivity C-reactive protein than dippers. Patients with both moderate– severe OSA and reverse-dipping BP profile showed worse brain white matter lesions than subjects with one condition alone. In a retrospective study, it was observed that OSA patients who had shown a nondipping-BP profile had a higher incidence of stroke, coronary artery disease, and heart failure in a 43-month period [34,35].
The objective of our work was to describe the behavior of blood pressure by ABMP in patients with OSA and HT. While most prevalent is nocturnal hypertension, there is also an association between OSA, daytime and nocturnal hypertension, as evidence shows. The complete pathophysiological mechanisms that would help us explain this finding are unknown, but we should consider that patients with moderate to severe OSA and arterial hypertension (present in any of their circadian periods) will have a poor prognosis if they are not treated early and we would need a systematic strategy to identify OSA in hypertensive patients,
not only using ABPM, but also using all available tools to study the patient globally.
LIMITATIONS
Our work was executed under a small sample size since the patients lacked treatment, both antihypertensive and any use of CPAP, which today is difficult to find in our clinical practice. In addition, using ambulatory respiratory polygraphy instead of polysomnography may underestimate 10-15% of patients with IAH. Not being able to compare to normotensive populations with OSA was another of our limitations, as was the absence of discrimination due to the type of blood pressure pattern.
CONCLUSION
The diagnosis of OSA in hypertensive patients should be suspected considering the patient in a global way, associating it with their clinical conditions evaluated by questionnaires.
DECLARATION OF INTEREST
The authors report no conflicts of interest and certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent licensing arrangements) or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript. The authors alone are responsible for the content and writing of the paper.
REFERENCES
13. Cecilia A, Wiechers U. Consecuencias metabólicas de la apnea del sueño. Centro médico ABC observatorio A. 2018; 46: 65-71.
18. Majul C, Marín M. Consenso de Hipertensión Arterial Consejo Argentino de Hipertensión Arterial “Dr. Eduardo Braun Menéndez”. Rev Argent Cardiol 2013;81:1-72.