Effects of Atrial Natriuretic Peptide on Hemodynamics, Neurohormonal Factors and Inflammatory Cytokines in Patients with Compensated Heart Failure Due to Idiopathic Dilated Cardiomyopathy
- 1. Department of Medicine, Nihon University School of Medicine, Japan
- 2. Department of Cardiovascular Medicine, Hanwa Memorial Hospital, Japan
- 3. Department of Cardiovascular Medicine, National Cardiovascular Center, Japan
Abstract
Background: A recombinant human atrial natriuretic peptide (ANP) has been reported to have multiple cardiovascular effects such as diuresis, vasodilation, and suppression of neurohormonal activity. However, few clinical investigations exist for the immune-modulating effects of ANP. Thus, we investigated if ANP modulates inflammatory cytokines as well as hemodynamics and neurohormonal factors in patients with compensated heart failure (CHF) due to idiopathic dilated cardiomyopathy (DCM).
Methods: Sixty DCM patients were recruited and divided randomly into two groups, the placebo group and the ANP group. In the ANP group, blood sampling for the measurements of norepinephrine (NE), brain natriuretic peptide (BNP), rennin (PRA), aldosterone (PAC), tumor necrosis factor-alpha (TNF-a), interleukin-6 (IL-6) and interleukin-10 (IL-10) and tissue Doppler echocardiography were performed five days before and just before continuous infusion of ANP for 96 hours and immediately and seven days after termination.
Results: ANP significantly improved left ventricular filling and decreased BNP, NE, PAC and TNF-a, IL-6 levels, whereas significantly increased IL-10 levels from 0.78 ± 0.05 pg/ml to 8.63 ± 0.64 pg/ml (p < 0.001). In ANP group, both TNF-a/IL-10 and IL-6/IL-10 decreased from 57.03 ± 4.76 to 3.41 ± 0.46 (p < 0.001) and from 6.72 ± 0.63 to 0.23 ± 0.03 (p < 0.001), respectively and these effects remained unchanged for seven days after termination.
Conclusion: ANP improved an imbalance in the cytokine network as well as hemodynamics and neurohormonal factors of CHF due to DCM.
Keywords
• Atrial natriuretic peptide (ANP)
• Heart failure
• Neurohormonal factors
• Oxidative stress
• Inflammatory cytokines
• Idiopathic dilated cardiomyopathy (DCM)
Citation
Kato M, Hashimura K, Kitakaze M, Hirayama A (2016) Effects of Atrial Natriuretic Peptide on Hemodynamics, Neurohormonal Factors and Inflammatory Cytokines in Patients with Compensated Heart Failure Due to Idiopathic Dilated Cardiomyopathy. Ann Clin Exp Hypertension 4(2): 1042.
ABBREVIATIONS
ACE-I: Angiotensin Converting Enzyme Inhibitor; ANOVA: Analysis of Variance Measures; ANP: Atrial Natriuretic Peptide; ARB: Angiotensin Receptor Blocker; BNP: Brain Natriuretic Peptide; CHF: Chronic /Compensated Heart Failure; DCM: Idiopathic Dilated Cardiomyopathy; Ea: The Peak Diastolic Velocities during Early Filling; EA: The Early Filling Peak Velocity to Atrial Filling Peak Velocity Ratio EF: Ejection Fraction; Hscrp: High Sensitivity C-Reactant Protein; IL: Interleukin; LV: Left Ventricular; NO: Nitric Oxide; NTG: Nitroglycerine; SE: Standard Error; SNS: Sympathetic Nervous System; TBARS: Thiobarbituric Acid Reactive Substances; TNF-A: Tumor Necrosis Factor-Alpha; Vp: flow Propagation Velocity
INTRODUCTION
The activation of neurohormonal factors in response to cardiac pump failure is an important underlying pathophysiology of compensated heart failure (CHF), which is supported by the fact that a blockade of either the renin-angiotensin-aldosterone system (RAAS) or the b-adrenergic system (sympathetic nervous system: SNS) increases the survival rate. In addition, the immune inflammatory system was reported to be activated in patients with CHF [1]. The pattern of cytokine expression is similar to that observed with classical neuro-hormones that are recently believed to play an important role in disease progression in CHF [2]. To the contrary, atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), nitric oxide (NO) or adenosine, are known to counteract RAAS or SNS and mediate potent cardiovascular protection [3-6]. In particular, both ANP and BNP are clinically used to treat decompensated heart failure, since accumulated evidence exists that both play a role in the pathogenesis of heart failure. Indeed, a recombinant human ANP has effects such as diuresis, vasodilatation, and suppression of neurohormonal activity [7-10] and has been used clinically for various types of acute decompensated heart failure [11], suggesting that an infusion of ANP is more appropriate in treating CHF than other vasodilator and/or inotropic drugs. The previous investigation reported the favorable hemodynamics, renal, and neurohormonal effects of ANP on the deleterious pathways of CHF, all of which contribute to improvements in decompensated heart failure and provide the rationale for the preventive effects of ANP against readmission resulting from worsening heart failure [12,13]. Few clinical investigations exist on the effects of ANP on the inflammatory factors of CHF patients with non-ischemic dilated cardiomyopathy despite the importance of cytokine networks in the pathophysiology of worsening heart failure. Therefore, we hypothesized that ANP modulates the cytokine network of patients with idiopathic dilated cardiomyopathy (DCM).
METHODS
Patients
Inclusion criteria were as follows: 1) age > 18 and < 70 years, 2) stable New York Heart Association (NYHA) functional class II or III chronic heart failure resulting from idiopathic DCM as defined by normal coronary angiogram and histological finding of endomyocardial biopsy, and 3) left ventricular ejection fraction (EF) < 40%. Exclusion criteria were 1) concomitant disorder other than cardiomyopathy that could influence cytokine levels such as inflectional disease, rheumatoid arthritis, sarcoidosis, 2) severe CHF needing mechanical support, and 3) hypotension defined as systolic blood pressure < 90 mmHg.
Study design
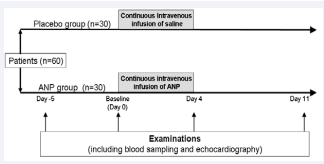
The present study is prospective, single blinded, placebo controlled, randomized study. Participants were divided randomly into two groups, the placebo group and the ANP group. To avoid influences from hospitalization, such as changes in resting and dietary conditions including salt and water intake, the examinations were performed five days before administration. Five days before administration (Day –5) and just before administration (Baseline, Day 0), examinations including blood sampling and echocardiography, were performed. Immediately after the blood sampling, a continuous intravenous infusion of either placebo (saline) or ANP was started at a dose of 0.0125 mg/ kg/min, which was increased to 0.025 mg/kg/min within three hours. The infusion continued for 96 hours. Immediatelyafter (Day 4) and seven days (Day 11) after termination of the infusion, examinations were again Performed (Figure 1).
Figure 1 Five days before (Day -5) and just before (Baseline; Day 0) the continuous intravenous infusion of ANP for 96 hours and immediately after (Day 4) and 7 days after the termination (Day 11); the examinations including blood sampling and echocardiography were performed.
To exclude the possibility of the hemodynamic effects of ANP on immune modulation, we tested whether nitroglycerine (NTG), instead of ANP, provides the identical immune modulation of ANP. To assess the effect of NTG, which is used as a ANPindependent vasodilator against decompensated heart failure, 16 patients with DCM were divided into two groups randomly, the NTG group (n = 8) and the ANP group (n = 8), and examinations were performed just before and after the 48 hours of NTG (0.25 mg/kg/min) or ANP (0.025 mg/kg/min) was administered.
Echocardiography
The echocardiographic examination was performed by skilled sonographers, who were blind to the purpose of the study, obtained echocardiographic parameters of left ventricular (LV) systolic and diastolic function, which consisted of M-mode measurements guided by transthoracic two-dimensional viewing according to the American Society of Echocardiography’s recommendations [14]. The following measurements were obtained: conventional parameters of LV dimension and LVEF by modified Simpson’s method [supplement 1]. Mitral flow velocities and interval were measured according to consensual recommendations [15]. The following measures were further obtained: early filling peak velocity (E), atrial filling peak velocity (A), and the E to A ratio (E/A). To record the longitudinal mitral annular motion, color-guided pulsed wave tissue Doppler was used, with the sample volume placed on the lateral and septal border of the mitral annulus. From spectral traces, the peak diastolic velocities during early filling (Ea) were obtained, allowing the determination of the ratio of E to Ea (E/Ea). Color M-mode Doppler echocardiography was performed in the apical 4-chamber view with the M-mode cursor aligned parallel with LV inflow and avoiding boundary regions. Adjustments were made to obtain the longest column of flow from the mitral annulus to the apex of the left ventricle. Flow propagation velocity (Vp) was measured as the slope of the first aliasing velocity from the mitral annulus in early diastole to 4 cm distally into the LV cavity [16]. All Doppler echocardiographic parameters were measured in three consecutive cardiac cycles and averaged.
Laboratory measurements
Blood was sampled between 6:00 a.m. and 7:00 a.m. from patients who were in a supine position for 30 minutes and who had fasted since 7:00 p.m. the previous night. Samples were immersed in ice water and both serum and plasma were separated from blood cells by centrifugation at 1000 ´ g for 15 minutes. Plasma was aspirated and introduced into 1.5 ml of Eppendorf tubes and stored at –20 °C until analyzed, and tubes were sent to the core laboratory at this condition.
Plasma levels of BNP were measured with a specific immunoradiometric assay using commercial kits (Shionogi, Japan). Enzyme-linked immunosorbent assay (ELISA) was performed. Tumor necrosis factor-alpha (TNF-a), interleukin-6 (IL-6), and interleukin-10 (IL-10) levels were determined on a plate reader at an optical density of 490 nm within 30 minutes using ELISA (QuantikineÒ HS, USA). High sensitivity C-reactant protein (hsCRP) levels were measured at an optical density of 450 nm using ELISA (ELISA KIT Cat. No. 1000, USA). Thiobarbituric acid reactive substances (TBARS) were measured at an optical density of 532 nm using an OXI-TEK TBARS Assay Kit. To avoid unspecific changes and to guarantee high precision in the analytical procedures, adequate conditions for sampling, storage, and measurement were ascertained in pre-study experiments [17]. Therefore, samples were immediately frozen after sampling, stored in liquid nitrogen until measurement, and analyzed in parallel for high precision.
Statistical analysis
Numerical variables are expressed as means ± standard error of the mean (SE). The data in individual stages (Days 4 and 11) were tested just before the administration of ANP (Baseline, Day 0) using a paired-sample t test. A two-sided p value < 0.05 was considered statistically significant. Obtained parameters were compared during different conditions with repeated analysis of variance measures (ANOVA). The survival curves were based on Kaplan-Meier analysis.
This investigation conformed to the ethical guidelines of the 1975 Helsinki Declaration. The institutional scientific and ethical committees approved the protocol, and each patient provided written informed consent to participate in the study.
RESULTS
Patients characteristics
Sixty patients were enrolled and randomized in this study. At the time of enrollment, all patients were observed with no clinical evidence of fluid overload judged by elevated jugular venous pressure, pulmonary rates, or peripheral edema, and had already received oral diuretics, spironolactone, angiotensin-converting enzyme inhibitors (ACE-I), angiotensin receptor blockers (ARB) or beta-adrenergic receptor blockers (beta-blocker). Medication remained unchanged throughout the study period. All patients were classified as NYHA functional class II (n = 41) or III (n = 19) CHF (Table 1). Mean EF, blood pressure, and mean plasma levels of BNP at Day –5 were 31.4 ± 1.8 and 32.2 ± 1.5%, 106 ± 4/68 ± 2 mmHg and 108 ± 3/72 ± 2 mmHg, and 612 ± 92 and 599 ± 86 pg/ ml in the placebo and ANP groups, respectively.
Clinical course and 1-year prognosis
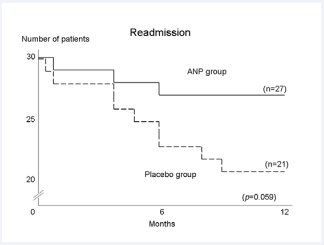
ANP was administered for 96 hours to all 30 patients in the ANP group. Two patients experienced episodes of symptomatic systemic hypotension. The ANP infusion improved NYHA class from 2.29 ± 0.46 to 2.14 ± 0.36 (p = 0.043), although none of the patients changed their baseline cardiac medications during the study period. These improvements in NYHA class continued for an additional one week after the termination of the infusion. Figure (4) shows the 1-year prognosis of the patients. In the ANP group, 3 (10%) of the patients were readmitted while 9 (30 %) in the placebo group (p = 0.059) because of worsening heart failure. Although there was no significant difference, the 1-year mortality was 2 (6.7%) in the ANP group versus 6 (20%) in the placebo group, and the cause of all death was worsening of heart failure.
Figure 4 Kaplan-Meier survival curves (end point of readmission) of patients in the ANP group and the placebo group. Readmission of patients were 3 (10 %) in the ANP group vs. 9 (30 %) in the placebo group (p = 0.059). All of them were readmitted by worsening heart failure.
Echocardiography
Ninety-six hours after administration of either a placebo or ANP, no significant changes in conventional echocardiographic parameters, including LV diameter and LVEF, were observed (Tables 2,3). Although the mean values of E or the E/A ratio did not change between Baseline and Day 4 in the ANP group, Ea and the E/Ea ratio changed significantly between Baseline and Day 4. The average of septal and lateral Ea and Vp increased from 5.0 ± 0.3 cm/s to 7.3 ± 0.5 cm/s (p < 0.001) and 39.9 ± 1.2 cm/s to 59.0 ± 1.5 cm/s (p < 0.001) in the ANP group, respectively. On the contrary, the E/Ea ratio and the E/Vp ratio decreased from 14.2 ± 0.7 to 9.3 ± 0.6 (p < 0.001) and 1.78 ± 0.08 to 1.15 ± 0.07 (p < 0.001), respectively. Furthermore, at Day 11 of the ANP group, these parameters remained almost unchanged (Table 3).
Plasma levels of BNP
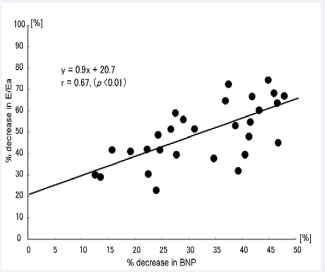
Plasma levels of BNP did not significantly change between Day –5 and Baseline for administration of both placebo and ANP. This suggests that the hospitalization had no influence. However, on Day 4 of ANP, plasma levels of BNP significantly decreased and this reduction lasted until Day 11 (Table 5) in the ANP group. In addition to the decreases in plasma levels of BNP and the E/Ea ratio, which reflect LV end-diastolic pressure [18], a significant correlation (r = 0.67, p < 0.01) existed between the percentage changes in these variables between Baseline and Day 4 (Figure 2).
Figure 2 Correlation between percentage decreases in plasma levels of BNP and the E/Ea ratio. There was a significant correlation between the percentage changes in these variables between Baseline (Day 0) and Day 4.
Plasma levels of norepinephrine
Plasma levels of norepinephrine (NE) decreased from 1069 ± 114 pg/ml to 431 ± 47 pg/ml (p < 0.001) between Baseline and Day 4. This reduction lasted to Day 11 (529 ± 54 pg/ml, p < 0.001) in the ANP group (Table 5). However, no significant changes were observed in the placebo group (Table 4).
Plasma renin activity and plasma aldosterone concentration
Mean plasma renin activity (PRA) tended to decrease from 12.7 ± 2.4 ngAI/ml•h (Baseline, Day 0) to 9.5 ± 2.1 ngAI/ml•h (Day 4). However, a statistically reliable change was not observed between Baseline and Day 4 in the ANP group. On the other hand, plasma aldosterone concentration (PAC) decreased from 23.3 ± 2.5 pg/ml to 10.5 ± 1.1 pg/ml (p < 0.05) and this reduction lasted until Day 11 (to 9.9 ± 2.3 pg/ml, p < 0.05 vs. Baseline) (Table 5) in the ANP group.
Plasma levels of cytokines and hscrp
Plasma levels of TNF-a decreased from 39.6 ± 1.8 pg/ml to 25.1 ± 1.5 pg/ml (p < 0.001). Plasma levels of IL-6 decreased from 4.60 ± 0.24 pg/ml to 1.60 ± 0.13 pg/ml (p < 0.001) between Baseline and Day 4 of the administration of ANP. To the contrary, plasma levels of IL-10 dramatically increased from 0.78 ± 0.05 pg/ ml to 8.63 ± 0.64 pg/ml (p < 0.001) in the ANP group. All of these effect parameters lasted to Day 11 in the ANP group (Table 5). On the contrary, no significant changes were observed in the placebo group (Table 4). Plasma levels of hsCRP decreased from 106.8 ± 7.8 ng/ml to 24.7 ± 3.7 ng/ml (p < 0.001) between Baseline and Day 4 in the ANP group (Table 5). No significant change occurred at Baseline and Day 4 in the placebo group (Table 4).
Marker for oxidative stress
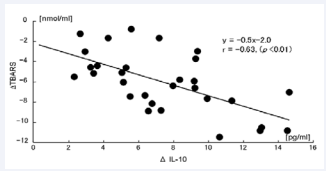
TBARS levels significantly decreased from 17.0 ± 0.6 nmol/ml to 10.8 ± 0.3 nmol/ml (p < 0.001) between Baseline and Day 4 and remained to Day 11 (to 10.7 ± 0.4 nmol/ml, p < 0.001 vs. Baseline) in the ANP group (Table 5). Furthermore, the differences in the decrease of TBARS levels correlated with those of IL-10 levels (r = 0.63, p < 0.01) between Baseline and Day 4 in the ANP group (Figure 3).
Figure 3 Differences on decrease of TBARS levels were correlated with those of IL-10 levels between Baseline (Day 0) and Day 4.
Comparison of inflammatory factors between ANP and NTG
Although both systemic blood pressure and plasma BNP levels decreased in the NTG and the ANP groups, the plasma TNF-a, IL-6, and TBARS levels did not change significantly in the NTG group (Table 6).
Table 1: Patients Characteristic at Day -5.
| placebo | ANP | p | |
| Age; years | 56 ± 4 | 59 ± 3 | NS |
| Gender; male/female | 19 / 11 | 18 / 12 | NS |
| NYHA; II/III | 20 / 10 | 21 / 9 | NS |
| Heart rate; bpm | 64 ± 4 | 66 ± 2 | NS |
| Systolic BP; mmHg | 106 ± 4 | 108 ± 3 | NS |
| Diastolic BP; mmHg | 68 ± 2 | 72 ± 2 | NS |
| EF; % | 31.4 ± 1.8 | 32.2 ± 1.5 | NS |
| BNP; pg/ml | 612 ± 92 | 599 ± 86 | NS |
| Medication use; n (%) | |||
| Loop diuretics | 26 (87) | 23 (77) | NS |
| ACE-I or ARB | 18 (60) | 20 (67) | NS |
| Beta-blocker | 24 (80) | 27 (90) | NS |
| Spironolactone | 18 (60) | 15 (50) | NS |
| Data are presented as mean±SE. Abbreviations: NYHA: New York Heart Association Functional Class; BP: Blood Pressure; EF: Ejection Fraction; BNP: Brain Natriuretic Peptide; ACE-I: Angiotensin-Converting Enzyme Inhibitor; ARB:Angiotensin Receptor Blocker. |
|||
Table 2: Changes in Clinical and Echocardiographic Parameters in the placebo group.
| Day -5 | Baseline | Day 4 | Day 11 | ANOVA | |
| Heart rate; bpm | 64 ± 4 | 64 ± 2 | 63 ± 4 | 63 ± 2 | NS |
| sBP; mmHg | 106 ± 4 | 106 ± 2 | 105 ± 6 | 106 ± 8 | NS |
| dBP; mmHg | 68 ± 2 | 66 ± 6 | 66 ± 4 | 67 ± 2 | NS |
| LVDd; mm | 62.1 ± 1.8 | 62.4 ± 0.8 | 62.6 ± 1.4 | 62.2 ± 0.5 | NS |
| LVDs; mm | 52.2 ± 0.4 | 52.6 ± 1.2 | 52.1 ± 1.8 | 52.8 ± 1.6 | NS |
| EF; % | 31.4 ± 1.8 | 31.6 ± 2.1 | 31.2 ± 1.2 | 31.4 ± 1.6 | NS |
| peak E; cm/s | 68 ± 8 | 68 ± 6 | 67 ± 15 | 67 ± 3 | NS |
| peak A; cm/s | 52 ± 2 | 54 ± 3 | 58 ± 3 | 58 ± 3 | NS |
| E/A | 1.31 ± 0.18 | 1.26 ± 0.22 | 1.15 ± 0.16 | 1.16 ± 0.18 | NS |
| Ea; cm/s | 4.4 ± 1.6 | 4.6 ± 1.5 | 4.3 ± 1.5 | 4.5 ± 0.5 | NS |
| E/Ea | 15.5 ± 1.8 | 14.8 ± 2.2 | 15.6 ± 1.8 | 14.9 ± 1.4 | NS |
| Vp; cm/s | 40.8 ± 2.2 | 40.9 ± 1.8 | 39.0 ± 1.5 | 40.5 ± 1.7. | NS |
| E/Vp | 1.67 ± 0.24 | 1.66 ± 0.12 | 1.72 ± 0.14 | 1.65 ± 0.24 | NS |
| Data are presented as mean value±SE. Abbreviations: ANOVA: P Value of Repeated Measure Analysis Of Variance; NS: Not Significant; SBP: Systolic Blood Pressure; Dbp Diastolic BP; LVD: End-Diastolic Left Ventricular Diameter; Ds: End-Systolic Diameter; EF: Ejection Fraction; E: Early Filling Trans Mitral Flow Velocity; A: Atrial Filling Velocity; Ea: Early Filling Mitral Annular Velocity; Vp: Flow Propagation Velocity |
|||||
Table 3: Changes in Clinical and Echocardiographic Parameters in the ANP group.
| Day -5 | Baseline | Day 4 | Day 11 | ANOVA | |
| Heart rate; bpm | 66 ± 2 | 66 ± 2 | 63 ± 2¶ | 66 ± 2 | <0.001 |
| sBP; mmHg | 108 ± 3 | 108 ± 2 | 104 ± 2† | 107 ± 2 | <0.05 |
| dBP; mmHg | 72 ± 2 | 73 ± 2 | 70 ± 2 | 73 ± 2 | NS |
| LVDd; mm | 61.1 ± 1.5 | 60.8 ± 1.4 | 60.6 ± 1.4 | 61.2 ± 1.5 | NS |
| LVDs; mm | 50.8 ± 1.6 | 50.6 ± 1.6 | 50.1 ± 1.5 | 50.8 ± 1.6 | NS |
| EF; % | 32.2 ± 1.5 | 32.5 ± 1.5 | 33.2 ± 1.5 | 32.8 ± 1.5 | NS |
| peak E; cm/s | 72 ± 3 | 71 ± 3 | 68 ± 15 | 68 ± 3 | NS |
| peak A; cm/s | 59 ± 4 | 60 ± 3 | 68 ± 3 | 68 ± 3 | NS |
| E/A | 1.22 ± 0.13 | 1.18 ± 0.12 | 1.03 ± 0.06 | 1.04 ± 0.06 | NS |
| Ea; cm/s | 4.6 ± 0.3 | 5.0 ± 0.3 | 7.3 ± 0.5¶ | 6.5 ± 0.5¶ | <0.001 |
| E/Ea | 15.6 ± 0.8 | 14.2 ± 0.7 | 9.3 ± 0.6¶ | 10.5 ± 0.4¶ | <0.001 |
| Vp; cm/s | 39.2 ± 1.2 | 39.9 ± 1.2 | 59.0 ± 1.5¶ | 51.5 ± 1.4¶ | <0.001 |
| E/Vp | 1.83 ± 0.08 | 1.78 ± 0.08 | 1.15 ± 0.07¶ | 1.32 ± 0.07 | <0.001 |
| Data are presented as mean value±SE. ANOVA p value of repeated measure analysis of variance; ¶ p<0.001; † p<0.05 Abbreviations: Vs: Baseline; NS: Not Significant; SBP: Systolic Blood Pressure; DBP: Diastolic BP; LVDD: End-Diastolic Left Ventricular Diameter; DS: End-Systolic Diameter; EF: Ejection Fraction; E: Early Filling Trans Mitral Flow Velocity; A: Atrial Filling Velocity; EA: Early Filling Mitral Annular Velocity; Vp: Flow Propagation Velocity |
|||||
Table 4: Changes in Biochemical Parameters in the placebo group.
| Day -5 | Baseline | Day 4 | Day 11 | p value | |
| BNP; pg/ml | 612 ± 92 | 588 ± 58 | 544 ± 88 | 575 ± 86 | NS |
| NE; pg/ml | 1165 ± 301 | 948 ± 224 | 934 ± 67 | 989 ± 115 | NS |
| PRA; ngAI/ml·h | 14.2 ± 4.6 | 14.7 ± 1.4 | 14.5 ± 1.1 | 14.9 ± 2.6 | NS |
| PAC; pg/ml | 26.2 ± 1.8 | 23.8 ± 1.4 | 22.5 ± 1.4 | 22.5 ± 1.3 | NS |
| TNF-α; pg/ml | 39.8 ± 1.1 | 37.4 ± 0.6 | 36.1 ± 1.1 | 37.2 ± 0.8 | NS |
| IL-6; pg/ml | 5.02 ± 0.89 | 4.88 ± 0.87 | 4.72 ± 0.92 | 4.49 ± 0.85 | NS |
| IL-10; pg/ml | 2.01 ± 1.15 | 1.98 ± 0.85 | 2.03 ± 1.06 | 2.78 ± 1.28 | NS |
| hsCRP; ng/ml | 121.8 ± 8.1 | 108.1 ± 8.2 | 88.5 ± 6.6 | 69.5 ± 8.9 | <0.05 |
| TBARS; nmol/ml | 18.2 ± 2.1 | 17.6 ± 1.4 | 17.2 ± 0.8 | 17.7 ± 0.8 | NS |
| Data are presented as mean±SE. ¶ P<0.001; † p<0.05 Abbreviations: Vs: Baseline; BNP: Brain Natriuretic Peptide; NE: Norepinephrine; PRA: Plasma Renin Activity; PAC: Plasma Aldosterone Concentration; TNF-Α: Tumor Necrosis Factor-Alpha; IL: Interleukin; Hscrp: High Sensitivity C - reactive protein; TBARS: Thiobarbituric Acid Reactive Substances |
|||||
Table 5: Changes in Biochemical Parameters in the ANP group.
| Day -5 | Baseline | Day 4 | Day 11 | p value | |
| BNP; pg/ml | 599 ± 86 | 506 ± 73 | 255 ± 37¶ | 275 ± 46¶ | NS |
| NE; pg/ml | 1072 ± 201 | 1069 ± 114 | 431 ± 47¶ | 529 ± 54¶ | NS |
| PRA; ngAI/ml·h | 11.2 ± 3.6 | 12.7 ± 2.4 | 9.5 ± 2.1 | 9.9 ± 2.4 | NS |
| PAC; pg/ml | 24.2 ± 2.8 | 23.3 ± 2.5 | 10.5 ± 1.1† | 12.5 ± 1.3† | NS |
| TNF-α; pg/ml | 38.2 ± 2.1 | 39.6 ± 1.8 | 25.1 ± 1.5¶ | 25.3 ± 1.5¶ | NS |
| IL-6; pg/ml | 4.82 ± 0.68 | 4.60 ± 0.24 | 1.60 ± 0.13¶ | 1.39 ± 0.11¶ | NS |
| IL-10; pg/ml | 1.21 ± 0.12 | 0.78 ± 0.05 | 8.63 ± 0.64¶ | 7.12 ± 0.71¶ | NS |
| hsCRP; ng/ml | 112.3 ± 6.8 | 106.8 ± 7.8 | 24.7 ± 3.7¶ | 19.4 ± 3.3¶ | <0.05 |
| TBARS; nmol/ml | 16.8 ± 1.2 | 17.0 ± 0.6 | 10.8 ± 0.3¶ | 10.7 ± 0.4¶ | NS |
| Data are presented as mean±SE. ¶ P<0.001; † p<0.05 Abbreviations: Vs: Baseline; BNP: Brain Natriuretic Peptide; NE: Norepinephrine; PRA: Plasma Renin Activity; PAC: Plasma Aldosterone Concentration; TNF-Α: Tumor Necrosis Factor-Alpha; IL: Interleukin; Hscrp: High Sensitivity C - reactive protein; TBARS: Thiobarbituric Acid Reactive Substances |
|||||
Table 6: Changes in Biochemical Parameters Compared with ANP and NTG.
| ANP group (n=8) | NTG group (n=8) | |||
| before | after | before | after | |
| sBP; mmHg | 106 ± 4 | 104 ± 10 | 106 ± 6 | 102 ± 8 |
| dBP; mmHg | 68 ± 2 | 66 ± 4 | 68 ± 4 | 64 ± 8 |
| BNP; pg/ml | 508 ± 66 | 248 ± 26¶ | 502 ± 78 | 268 ± 36¶ |
| NE; pg/ml | 1055 ± 201 | 483 ± 224¶ | 1033± 217 | 729 ± 54¶ |
| PRA; ngAI/ml·h | 12.2 ± 1.6 | 9.7 ± 2.9† | 11.5 ± 3.2 | 10.5 ± 1.4 |
| PAC; pg/ml | 22.8 ± 3.8 | 12.1 ± 2.2† | 21.5 ± 2.1 | 22.5 ± 1.3 |
| TNF-α; pg/ml | 39.9 ± 3.1 | 24.4 ± 2.8¶ | 38.1 ± 2.1 | 35.3 ± 3.5 |
| IL-6; pg/ml | 4.51 ± 1.02 | 1.60 ± 0.34¶ | 4.65 ± 0.13 | 3.39 ± 0.11† |
| IL-10; pg/ml | 1.21 ± 0.12 | 5.18 ± 0.82¶ | 1.84 ± 1.08 | 2.41 ±1.45¶ |
| hsCRP; ng/ml | 102.8 ± 5.2 | 26.8 ± 8.8¶ | 104.4 ± 8.7 | 79.4 ±9.3† |
| TBARS; nmol/ml | 15.8 ± 8.2 | 10.9 ± 1.4¶ | 16.8 ± 6.3 | 17.7 ± 5.4 |
| Data are presented as mean±SE. ¶ P<0.001; † p<0.05 vs. before; Abbreviations: BNP: Brain Natriuretic Peptide; NE: Norepinephrine; PRA: Plasma Renin Activity; PAC: Plasma Aldosterone Concentration; TNF-Α: Tumor Necrosis Factor-Alpha; IL: Interleukin; Hscrp: High Sensitivity C - Reactive Protein; TBARS: Thiobarbituric Acid Reactive Substances. |
||||
Table 7: The Ratio of Pro- to Anti-inflammatory Cytokine in the ANP group.
| Baseline | Day 4 | Day 11 | ANOVA | |
| IL-6 / IL-10 | 6.72 ± 0.63 | 0.23 ± 0.03¶ | 0.31 ± 0.05¶ | <0.001 |
| TNF-α / IL-10 | 57.03 ± 4.76 | 3.41 ± 0.46¶ | 5.63 ± 0.92¶ | <0.001 |
| Data are presented as mean±SE. ¶ p<0.001 vs. Baseline; Abbreviations: IL: interleukin; TNF-α: Tumor Necrosis Factor-Alpha; ANOVA: p value of repeated measure analysis of variance. |
||||
DISCUSSION
In summary, we elucidated that short-term infusion of exogenous ANP was shown to improve LV filling in CHF patients with DCM, and to alter plasma cytokine levels. These effects of ANP on both hemodynamic improvements and modulation of the cytokine network continued for at least seven days after the termination of the ANP infusion, suggesting that part of the beneficial effects of ANP on heart failure are attributable to hemodynamic-independent after-effects.
Since the hemodynamic effects of exogenous ANP infusion disappears within a few minutes after the termination and the biologic half-life of ANP is reported at 2.8 minutes [19], the after effect of ANP was not a result of transient hemodynamic effect of ANP, but was attributable to the modulatory effect on the neurohormonal and/or the immune system that improved RAAS, SAS, and an imbalance in the cytokine network (Table 7) in CHF patients.
After-effects of ANP on Hemodynamics
Administration of ANP for a short period was not shown to improve either the LVEF or LV dimension. However, intriguingly, Ea, Vp, and the E/Ea ratio were improved and continued to improve, even several days after the temporal administration of ANP, without a significant change in E which suggests no changes in preload. Furthermore, plasma levels of BNP were found to decrease following the administration of ANP. Since the plasma levels of BNP are elevated in patients with LV dysfunction proportional to NYHA functional class and filling pressures [20], and the plasma levels of BNP predict isolated diastolic dysfunction [21], these lines of evidence indicate that ANP mainly and predominantly improves LV diastolic function in patients with CHF.
However, this observation does not necessarily indicate that ANP does not improve LV systolic function because only the systolic function using the EF or LV dimension was evaluated and these parameters are not sensitive enough to detect systolic dysfunction compared with tissue Doppler imaging, a sensitive and less load-dependent method for the assessment of the LV diastolic function. Indeed, the E/Ea ratio is strongly correlated with LV filling pressures [18,22]. If a more sensitive method of assessing LV systolic function was developed, it may be able to detect improvements in systolic dysfunction in patients with heart failure in the present study.
On the other hand, the sensitive detection of improvements in diastolic dysfunction does not imply that the present finding is less meaningful when trifle changes in diastolic function are detected, because the E/Ea ratio is known to be a good predictor for the prognosis of CHF [23]. This is because both plasma levels of BNP and the E/Ea ratio predict prognosis in patients with hypertrophic cardiomyopathy with normal EF [24].
One year prognosis of patients
Although no significant statistical change in the incidence of readmission (p = 0.059) or cardiac death (p = NS) was not observed between both groups, the number of patients with either of incidence was smaller in the ANP group than in the placebo group. This is intriguing that transient treatment with ANP may provide the long-term clinical outcome and these results in this study were supported by former investigation [13].
After-effects of ANP on Neurohormonal factors
Plasma levels of both norepinephrine and aldosterone have been found to decrease in response to the temporary administration of ANP. These changes are not attributable to the hemodynamic effects of ANP because these modulations are seen even seven days after the termination of the ANP infusion. Since both SNS and RAAS are important for the formation of CHF and they predict both mortality and morbidity in patients with CHF, ANP-induced attenuation of SNS or RAAS should be highlighted in the treatment strategy of CHF. Beta-blockers are effective for heart failure but cannot fully block RAAS. Additionally, either ACE-I or ARB is effective for heart failure but cannot block the SNS in the long term however, ANP can block both systems, which merits the treatment of CHF.
Effects of ANP on cytokine network
In addition to neurohormonal factors, the present study showed that ANP reduced pro-inflammatory cytokines and increased anti-inflammatory cytokines (Table 5), which may further explain the beneficial effects of ANP. Many aspects of the CHF syndrome can be explained by the known biologic effects of pro-inflammatory cytokines [1]. Pro-inflammatory cytokines, such as TNF-a and IL-6, are elevated in CHF and modulate myocardial hypertrophy, fibrosis and apoptosis [25,26]. In this study, reduction in the IL-6 level was also found. Since IL-6 is thought to be released in response to TNF-a, that both proinflammatory cytokines decreased in the same direction was reasonable.
On the other hand, IL-10 was reported to be a key component in the suppression of the production of several inflammatory cytokines, such as TNF-a, IL-6, and IL-10 [27]. Similar to proinflammatory cytokines, mRNA of IL-10 was also detected in failing myocardium. Furthermore, IL-10 was recently found to have protective effects on the development of atherosclerosis and viral myocarditis in mice [28, 29]. In addition to TNF-a and IL-6, IL-10 levels were reported to be elevated and their ratios, such as the ratio of TNF- a to IL-10, become prominent as the NYHA functional class worsens in patients with CHF. This indicates that the ratio of pro-inflammatory cytokine to antiinflammatory cytokine is more important than the absolute values of TNF- a, IL-6, and IL-10 alone [30]. Indeed, the present study demonstrated that the increases in the plasma IL-10 levels and the improvement in an imbalance in the cytokine network (Table 7) following temporal infusion of ANP suggest that ANP is a potent immune modulator. Recently, new novel biomarkers that can detect worsening heart failure have reported [31].
Effect of ANP on oxidative stress
Oxidative stress increases during CHF because of the increase in NO secondary to the activation of iNOS and the reduction in antioxidant activity. The increase in oxidative stress causes production of reactive oxygen species (ROS), which are cytotoxic, promote apoptosis and necrosis, and result in subsequent ventricular dysfunction. ANP was shown to inhibit LPS-induced iNOS in macrophages through the inactivation of NF-kB [32,33]. Moreover, IL-10 was shown to limit the production of macrophage-derived NO and ROS via inhibition of NF-kB and inhibitory kappa B-alpha (IkB) degradation [34]. Taken together with these findings, ANP might decrease TBARS as a result of, in part, an inactivation of NF-kB and, in part, enhanced release of IL-10. In the present study demonstrated that a significant correlation exists in the differences in plasma levels of IL-10 and TBARS before and after the administration of ANP (Figure 3).
Comparison of NTG with ANP on cytokine network
The mechanism of ANP against the immune system remains unclear and one simple explanation must be that the effects of ANP are attributable to the changes in hemodynamic conditions. To clarify this issue, we studied the effect of NTG which is a wellknown vasodilator used for the treatment of decompensated heart failure on the cytokines comparing with that of ANP. The hemodynamic effects of NTG compared with ANP studied in detail [35] and has reported that no significant differences were observed in terms of hemodynamic effects between both drugs, which cause vasodilation, increased coronary flow, and improve congestive heart failure.
We demonstrated that no significant changes occurred in cytokine levels in the NTG group (Table 6) despite decreasing in serum BNP level in both vasodilators. Interestingly, the decrease in the serum levels of BNP and NE were much larger in the ANP group than in the NTG group. Therefore, the effects of ANP against the immune system are not attributable to hemodynamic effects but are intrinsic effects of ANP.
Clinical implications
Whereas a specific anti-cytokine therapeutic approach has so far failed (etanercept, infliximab), recent studies of various broad-based immunomodulating agents in patients with CHF clearly suggest a potential for such therapy in these patients in addition to optimal cardiovascular treatment regimens [36]. In the present study, ANP improved an imbalance in the cytokine network in CHF, which resulted in improving CHF in both the neurohormonal condition and the hemodynamic condition suggested by echocardiographic parameters.
In addition to the beneficial effects of ACE-I and b-blockers on neurohormones, several reports show that ACE-I and/or b-blockers have beneficial effects against pro-inflammatory cytokines [37,38]. Interestingly, although the vast majority of patients in this study were on ACE-I and b-blockers for three months or longer, ANP caused a further reduction in neurohormone and pro-inflammatory cytokine levels, indicating that ANP could be of clinical use as a potent neurohormonal and immunomodulator, and would give therapeutic modality a new perspective.
STUDY LIMITATIONS
Since the study population of the present study is relatively small, a large-scale, control-matched clinical trial is warranted to strength the present findings.
CONCLUSION
Short-term intravenous infusion of exogenous recombinant human ANP improved an imbalance in the cytokine network of patients with DCM, which was associated with decreased plasma BNP levels and improvement of echocardiographic parameter of LV diastolic function. The effects may be contributed by not only a hemodynamic effect but also a neurohormonal and immunemodulating effect.
REFERENCES
10. Hunt PJ, Espiner EA, Nicholls MG, Richards AM, Yandle TG. Differing biological effects of equimolar atrial and brain natriuretic peptide infusions in normal man. J Clin Endocrinol Metab. 1996; 81: 3871- 3876.
28. Mallat Z, Besnard S, Duriez M, Deleuze V, Emmanuel F, Bureau MF , et al. Protective role of interleukin-10 in atherosclerosis. Circ Res. 1999; 85: 17-24.