Improved Hypertension Control in Elderly Outpatients with Comorbidities
- 1. Department of Medical Sciences, Division of Nephrology and Dialysis, IRCCS “Casa Sollievo della Sofferenza”, San Giovanni Rotondo, Foggia, Italy
- 2. Unit of Biostatistics, IRCCS “Casa Sollievo della Sofferenza”, San Giovanni Rotondo, Foggia, Italy
- 0. These Authors equally contribute to this work
Abstract
With the aim to investigate whether the prevalence of blood pressure (BP) control in the elderly changes over time and according to the presence of comorbidities, as much as 123 elderly hypertensive outpatients were selected and followed for at least one year and within four years from their enrollment. BP control was considered to be achieved when BP was < 140/90 mmHg (< 130/80 mmHg for patients with type 2 diabetes mellitus and/or chronic kidney disease). Estimates of prevalence of BP control (PBPC) over follow-up time were derived using generalized random-effects patternmixture models for longitudinal studies. At four years of follow-up the estimated PBPC increased from 58.5% (baseline) to 85.5% (p for trend=0.010), and was lower in patients with type 2 diabetes mellitus and/or chronic kidney disease (from 38.2% to 68.3%, p for trend=0.062) than in patients with other comorbidities (from 76.4% to 94.5%, p for trend=0.020). Compliancy with the doctor plays a key role for BP control, even for patients with comorbidities.
Citation
Del Giudice A, Fontana A, Aucella F (2016) Improved Hypertension Control in Elderly Outpatients with Comorbidities. Ann Clin Exp Hypertension 4(2): 1040
Keywords
• Blood pressure
• Chronic kidney disease
• Elderly
• Type 2 diabetes mellitus
INTRODUCTION
Hypertension is one of the most important risk factors for coronary heart disease (CHD), congestive heart failure (CHF), stroke, renal insufficiency and cardiovascular mortality in all age groups [1]. Therefore, it is a major factor contributing to global disease burden.
Globally, the rate of blood pressure (BP) control in hypertensive subjects is low, especially in rural populations of developing countries (8.2-20%) [2-3] with lower rates in men (9.8%) than in women (16.2%) [4].
On the other hand, in developed countries, the percentage of individuals with controlled hypertension is higher than those in developing countries and varies from 27% in England [5] to 30% in Germany [6], 36% in France [7], 57% in Italy [8], 50-59% in the United States [5, 9-11], 66.8% in Canada [5].
Moreover, the percentage of US adult hypertensive patients with comorbidities (diabetes mellitus, chronic kidney disease, dyslipidemia, metabolic syndrome, stroke, congestive heart failure, coronary artery disease), in whom the hypertension is controlled is lower than that of US patients without comorbidities. Indeed, BP control rates were 52.9% in total sample, 64.6% in patients without comorbidities, 61.2% and 35.3% in patients with diabetes mellitus (BP target < 140/90 mmHg and < 130/80 mmHg, respectively), 42.2% and 23.2% in patients with chronic kidney disease (BP target < 140/90 mmHg and < 130/80 mmHg, respectively) [12].
In the elderly, the prevalence of hypertension increases progressively with age. Among the 65-year-old subjects enrolled in the Framingham Heart Study, who initially had an optimal, normal or normal-high blood pressure (BP), a progressive development of hypertension in the subsequent 4 years of observation was registered [13]. Currently estimated around 60-80%, this prevalence is destined to grow, according to the expected growth of the population older than 65 years of age. Furthermore, as the life expectancy increases, this also happens for the prevalence of age-related comorbidities, with the consequence that the managing of hypertension in the elderly patients with multiple comorbidities become even more challenging for clinicians.
In this population, as well as in patients with type 2 diabetes mellitus (T2DM) and chronic kidney disease (CKD), the guidelines of the principal scientific societies are recommending new BP targets [14-17] but the debate is still open [18-22].
This opinion seems even more reliable if referred to old or very old people [14,23].
In this article, we aim to evaluate the prevalence of blood pressure control (PBPC) of a retrospective cohort study, defined from a hypertensive elderly outpatient population with various comorbidities, carried out by the ambulatory dedicated to hypertension of our nephrology unit. In detail, we sought to investigate whether PBPC of hypertensive treated elderly Caucasians outpatients (evaluated by the same doctor and in the same environment) changes over time, especially for patients with T2DM and/or CKD (which represented the group with the most difficult treatment management) [24] compared to patients without T2DM and CKD (i.e. with other comorbidities), and comparing results using different BP targets guidelines.
With this study we would provide our contribution to the scientific community in order to better understand how well the blood pressure of a hypertensive elderly compliant outpatient could be controlled when attending a nephrology unit and to better assess the appropriateness of the use of recommending new BP targets.
MATERIALS AND METHODS
Patients’ recruitment
This was a retrospective cohort study fulfilling the Declaration of Helsinki (available at: www.wma.net/en), the guidelines for Good Clinical Practice (available at: www.ema.europa.eu) and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. The approval of the study for experiments using human subjects was obtained from the local ethics committees on human experimentation. All subjects included in this study were Caucasians with hypertension, with most individuals living in Central and Southern Italy and were enrolled at their first visit, among outpatients consecutively attending the Nephrology Unit of the Scientific Institute “Casa Sollievo della Sofferenza” in San Giovanni Rotondo (Apulia, Central Southern Italy) from 1 October 2009 to 30 September 2014.
Inclusion/exclusion criteria
Inclusion criteria were: 1) age ≥ 65 years 2) the presence of a diagnosed hypertension at the first visit. Exclusion criteria were: 1) no follow-up available (i.e. occasional outpatients who attended only the first visit); 2) patients who were not followed for at least one year. Therefore, a total of 482 hypertensive elderly outpatients attended the Nephrology Unit. Having excluded those who were visited only one time or were not followed for at least one year (n=359), 123 ordinary and compliant elderly hypertensive outpatients (25.5% of the initial cohort) constituted the eligible sample for the present analysis.
Follow-up ascertain
Dates of visits (recorded into an electronic database) were used to determine the follow-up time. Baseline was defined as the patients’ enrollment (i.e. date of the first visit). The followup was defined as the time between baseline and the date of the last visit. As already stated into “Inclusion/exclusion criteria”, patients were followed for at least one year, in order to discard both occasional and noncompliant outpatients and provide more robustness to our findings. Although outpatients were recommended to periodically attend the nephrology unit (i.e. for a mean of 3-6 months between each visit, depending on their conditions), it was not possible to guarantee a constant and rigorous surveillance for each patient at the follow-up, which come voluntarily.
Clinical assessment
The following clinical-pathological characteristics were recorded for each patient: age, gender, body-mass index (BMI), waist circumference, BP levels, serum glucose, glycosylated haemoglobin, estimated glomerular filtration rate (eGFR), calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [25], lipid profile, number and type of comorbidities, number and class of antihypertensive drugs prescribed.
T2DM diagnosis was made according to American Diabetes Association (ADA) 2013 criteria [15]. Chronic kidney disease (CKD) was defined as an eGFR <60 mL/min/1.73 m2 . No patients with CKD stage 5 (eGFR<15 mL/min/1.73 m2 ) were found into our sample. Blood pressure was always measured by the same physician (A.D.), with an oscillometric semi-automatic sphygmomanometer (MicrolifeAfib; Micro life AG, 9443 Widnau, Switzerland), after the subjects had been sitting for at least 5 minutes, taking at least 3 BP measurements spaced 2 minutes apart and considering the average BP value; the BP measurements were repeated after 2 minutes in standing position. Hypertension was defined as sitting BP levels ≥140/90 mmHg and/or use of antihypertensive drugs. Patients whose sitting BP levels were <140/90 mmHg and patients having T2DM and/or CKD as comorbidities, whose sitting BP levels were <130/80 mmHg, were considered as having achieved the BP control (i.e. standard criteria) [26-27].
Furthermore, in compliance with the Eighth Joint National Committee (JNC 8) guidelines [16], in patients with T2DM and/or CKD hypertension was considered to be controlled when sitting BP levels were <140/90 mmHg.
Study size (power calculation)
Assuming a referral population prevalence of BP control at baseline of 60%, a sample size of 27 subjects achieved 90% of statistical power to detect a difference in prevalence of controlled BP between baseline and end of follow-up of (at least) 25.0%, using a one-sided binomial test and assuming a type I error rate of 0.05.
Statistical methods
Baseline patients’ characteristics were reported as mean ± standard deviation (SD)or frequencies and percentages, for continuous and categorical variables, respectively. Comparisons of baseline characteristics between two groups of patients were performed using Mann-Whitney U test or Chi-square test (or Fisher exact test as appropriate) for continuous and categorical variables, respectively.
Changes in PBPC over time were assessed using generalized random-effects pattern-mixture models for longitudinal studies with non-ignorable missing data (i.e. a class of models which account for a comprehensive and statistically rigorous treatment of dropout mechanisms) [28,29].
Following Little RJA (1995) approach [28], subjects were divided on the basis of the following monotone missing-data pattern of dropout: “OMMM”, “OOMM”, “OOOM”, “OOOO”, where O denotes being observed and M being missing at 1, 2, 3, 4 years, respectively (e.g. “OMMM” denotes a pattern for subjects who were observed for the first year but not for the next three years, whereas “OOMM” denotes a pattern for subjects who were observed for the first and second year but not for the next two years, and so on). Pattern “OOOO” denotes outpatients who completed data across time (completers), whereas other patterns denotes dropout patients.
A pattern-mixture model was performed in order to model the presence of the BP target, defined according to standard criteria, and included: the follow-up time and the indicator group variable (which specify whether the patient had T2DM and/or CKD rather than other comorbidities) as continuous and categorical covariates into the model respectively, groupby-time variable (two-way interaction term), the missing data pattern as categorical covariate, missing pattern-by-group and missing pattern-by-time (two-way interaction terms) and missing pattern-by-group-by-time (three-way interaction term). Within this framework, the binomial distribution was assumed to model the presence of the BP target, along with the logistic transformation used as the canonical link function. As patients’ evaluations were performed at unequally-spaced visits during the follow-up, spatial-power covariance structure was used to account for the correlation between time occasions [30]. Another pattern-mixture model was performed for patients with T2DM and/or CKD subgroup, in order to model the presence of the BP target, defined according to JNC 8 guidelines [16] and included: the follow-up time, the missing data pattern and the missing pattern-by-time.
To yield overall population estimates, averaging over the missing-data patterns, for regression parameters (i.e. follow-up time, indicator group and group-by-time), the approach described by Hedecker et al., [29] was followed, using the sample proportion of each monotone missing pattern as population weights. Such weights were hence used to form linear combinations of the least squares means (at specific follow-up time). PBPC means were further estimated, for the whole sample and for each subgroup, along with their 95% confidence interval (95% CI).
Moreover, observed and estimated PBPC means were graphically represented over follow-up times. Estimated PBPC means were plotted both for completers and dropouts, respectively. Observed PBPC means were calculated categorizing the whole follow-up times into 8 equal-length intervals and determining the proportion of patients with target condition within each bin. The presence of a linear trend for the estimated means over time was assessed by looking at the significance of the estimated regression coefficients (p for trend). Moreover, the presence of a non-linear trend over follow-up time was also evaluated with the inclusion of the squared follow-up time as continuous variable into the model.
Two-sided p-values <0.05 were considered for statistical significance. All analyses were performed using SAS Software, Release 9.3 (SAS Institute, Cary, NC, USA).
RESULTS
Baseline demographic, clinical-pathological and pharmacological treatment characteristics of the enrolled outpatients are summarized in Table (1). Specifically, 58 of the 123 patients (47.2%) had T2DM and/or CKD and, among them, 14 (24.1%) had T2DM only, 31 (53.5%) had CKD only, 13 (22.4%) had both T2DM and CKD.
If compared to patients with other comorbidities, patients with T2DM and/or CKD were mostly obese (p=0.046) with a higher waist circumference (p=0.007), had a lower total mean cholesterol (p=0.030), higher serum glucose (p=0.008) and creatinine (p<0.001), a lower mean eGFR (p<0.001) and a higher mean number of concomitant comorbidities (p<0.001). Moreover, they were more treated with ACE inhibitors only (p=0.021), statins (p=0.043), calcium supplements (p=0.024), D vitamin supplements (p=0.003), and received more drugs during the follow-up (p<0.001) than those with other comorbidities. Patients were followed for a mean of 2.7 years and perceived a mean of 5.5 visits.
The estimated prevalence rates of BP control (along with 95% CI) over the 4 years after the baseline in the whole sample and in patients subgroups are reported in Table (2).
As shown, of the 123 recruited outpatients, 70 (56.9%) were followed within 2 years, 49 (39.8%) were followed within 3 years and only 31 (25.2%) were followed for the whole follow-up period of 4 years. Hence, 53 (43.1%) patients belonged to “OMMM” missing pattern, 21 (17.1%) patients belonged to “OOMM” missing pattern, 18 (14.6%) patients belonged to “OOOM” missing pattern and 31 (25.2%) patients eventually belonged to “OOOO” missing pattern (completers). As 31 outpatients were completers, the study achieved a post-hoc statistical power of 91.8%.
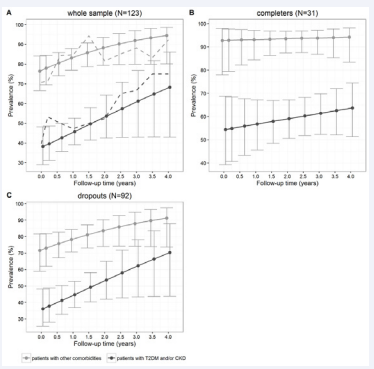
The observed mean prevalence of BP control ascertained in the whole sample, according to standard criteria, increased from 56.1% to 66.7%, 68.8%, 78.1% and 84.0% at baseline and after 1, 2, 3 and 4 years, respectively. When averaging over the missingdata patterns, the overall PBPC means estimates (derived from the generalized random-effects model) increased from 58.5% (95%CI: 50.3-66.1%) to 66.8% (95%CI: 61.3-71.9%), 74.2% (95%CI: 66.1-81.0%), 80.5% (95%CI: 68.7-88.6%) and 85.5% (95%CI: 70.8-93.5%) at baseline and after 1, 2, 3 and 4 years, respectively (p for trend=0.010). As expected, the estimated prevalence rates were higher in the subgroup of patients with other comorbidities (from 76.4% (95%CI: 66.6-84.1%) to 94.5% (95%CI: 80.1-98.6%), at baseline and at the end of the follow-up respectively, p for trend=0.020) than in patients with T2DM and/or CKD (from 38.2% (95%CI: 29.0-48.2%) to 68.3% (95%CI: 43.0-86.1%), at baseline and at the end of the followup respectively, p for trend=0.062). All such estimates accounted for missing data patterns. Although the baseline prevalence rates were significantly different between the two groups (p<0.001), the trend over time was not differential (p=0.954) (i.e. the prevalence of BP control significantly increased within the two subgroups with the same slope). Plots of observed and estimated PBPC means (standard criteria) over follow-up were also shown in Figure (1) for the whole sample, for completers and dropouts, separately.
Figure 1 Plots of observed (dashed lines) and estimated (solid lines) controlled blood pressure prevalence rates (standard criteria) during followup, in patients with diabetes and/or CKD and in patients with other co morbidities, in the whole sample (panel A), completers (panel B) and dropouts (panel C), respectively. Error bars represented 95% confidence interval around point estimates.
Furthermore, using 140/90 mmHg as the recommended cut-off (JNC 8 criteria) [18], for patients with T2DM and/or CKD only, the estimated PBPC means over time increased from 63.5% (95%CI: 53.2-72.7%) to 77.5% (95%CI: 71.0-82.9%), 87.2% (95%CI: 78.8-92.6%), 93.1% (95%CI: 83.7-97.3%) and 96.4% (95%CI: 87.4-99.0%) at baseline and after 1, 2, 3 and 4 years, respectively (p for trend=0.002). The further inclusion of the squared follow-up time into GLMMs resulted statistically non-significant (data not shown) suggesting that the linear trend component was sufficient to adequately investigate the evolution of PBPC over the follow-up time.
Table 1: Baseline patients’ clinical-pathological characteristics and comparisons between patients according to the presence of type 2 diabetes (T2DM) and /or chronic kidney disease (CKD).
| Variable | All (N=123) | T2DM and/or CKD (N=58) | Other comorbidities (N=65) | p-value* |
| Age (years) | 72.8 ± 5.6 | 73.5 ± 5.9 | 72.1 ± 5.2 | 0.159 |
| Males - N (%) | 46 (37.4) | 22 (37.9) | 24 (36.9) | 0.908 |
| BMI (Kg/m2) | 29.6 ± 5.2 | 29.9 ± 5.2 | 29.3 ± 5.3 | 0.336 |
| Obesity - N (%) | 50 (40.7) | 29 (50.0) | 21 (32.3) | 0.046 |
| Systolic Blood Pressure (mmHg) | 134.3 ± 19.8 | 134.2 ± 21.3 | 134.4 ± 18.6 | 0.938 |
| Diastolic Blood Pressure (mmHg) | 74.9 ± 10.3 | 73.8 ± 10.3 | 76.0 ± 10.4 | 0.353 |
| Total cholesterol (mg/dL) | 192.7 ± 46.4 | 182.5 ± 51.8 | 201.0 ± 40.2 | 0.030 |
| HDL cholesterol (mg/dL) | 56.4 ± 15.3 | 54.0 ± 14.6 | 58.4 ± 15.8 | 0.315 |
| LDL cholesterol (mg/dL) | 116.4 ± 42.8 | 116.0 ± 48.7 | 116.7 ± 37.9 | 0.676 |
| Glycated heamoglobin (%) | 6.8 ± 1.2 | 7.1 ± 1.1 | 5.5 ± 0.0 | 0.288 |
| Triglycerides (mg/dL) | 133.1 ± 63.2 | 127.3 ± 49.1 | 136.9 ± 71.3 | 0.994 |
| Creatinine (mg/dL) | 1.1 ± 0.6 | 1.4 ± 0.6 | 0.7 ± 0.2 | < 0.001 |
| Glucose (mg/dL) | 108.4 ± 24.1 | 120.6 ± 31.2 | 100.0 ± 12.3 | 0.008 |
| eGFR (mL/min/1.73m2) | 66.0 ± 24.7 | 51.6 ± 22.8 | 83.9 ± 12.2 | <0.001 |
| Comorbidities | ||||
| COPD - N (%) | 9 (7.3) | 5 (8.6) | 4 (6.2) | 0.734# |
| Dyslipidemia - N (%) | 74 (60.2) | 32 (55.2) | 42 (64.6) | 0.286 |
| CHD - N (%) | 10 (8.1) | 7 (12.1) | 3 (4.6) | 0.188# |
| Dysthyroidism - N (%) | 15 (12.2) | 7 (12.1) | 8 (12.3) | 0.968 |
| Atrial fibrillation – N (%) | 8 (6.5) | 5 (8.6) | 3 (4.6) | 0.474# |
| Diabetic nephropathy - N (%) | 3 (2.4) | 3 (5.2) | 0 (0.0) | 0.102# |
| Metabolic syndrome – N (%) | 28 (22.8) | 11 (19.0) | 17 (26.2) | 0.343 |
| Left ventricular hypertrophy - N (%) | 96 (78.0) | 42 (72.4) | 54 (83.1) | 0.154 |
| Number of comorbidities | 9.2 ± 3.4 | 10.8 ± 3.2 | 7.8 ± 3.0 | <0.001 |
| Pharmacological treatments | ||||
| ACEIs only - N (%) | 23 (18.7) | 16 (27.6) | 7 (10.8) | 0.021# |
| ACEIs and CCBs - N (%) | 2 (1.6) | 1 (1.7) | 1 (1.5) | 1.000# |
| ACEIs and HCTZ - N (%) | 20 (16.3) | 9 (15.5) | 11 (16.9) | 1.000# |
| ARBs only - N (%) | 36 (29.3) | 19 (32.8) | 17 (26.2) | 0.435# |
| ARBs and CCBs - N (%) | 5 (4.1) | 3 (5.2) | 2 (3.1) | 0.666# |
| ARBs and HCTZ - N (%) | 51 (41.5) | 23 (39.7) | 28 (43.1) | 0.718# |
| Use of CCBs only - N (%) | 46 (37.4) | 22 (37.9) | 24 (36.9) | 0.908 |
| Use of beta-blockers - N (%) | 51 (41.5) | 22 (37.9) | 29 (44.6) | 0.453 |
| Use of diuretics - N (%) | 33 (26.8) | 20 (34.5) | 13 (20.0) | 0.070 |
| Use of antiplatelets- N (%) | 84 (68.3) | 41 (70.7) | 43 (66.2) | 0.589 |
| Use of statins - N (%) | 56 (45.5) | 32 (55.2) | 24 (36.9) | 0.043 |
| Use of calcium supplement - N (%) | 11 (8.9) | 9 (15.5) | 2 (3.1) | 0.024# |
| Use of D vitamin supplement - N (%) | 28 (22.8) | 20 (34.5) | 8 (12.3) | 0.003 |
| Number of prescribed drugs | 4.3 ± 2.4 | 5.4 ± 2.5 | 3.3 ± 2.0 | <0.001 |
| Follow-up time | ||||
| Number of visits (per patient) | 5.5 ± 3.0 | 5.7 ± 3.1 | 5.2 ± 2.8 | 0.457 |
| Follow-up (years) | 2.7 ± 1.3 | 2.8 ± 1.3 | 2.5 ± 1.3 | 0.235 |
| Continuous variables are reported as mean ± standard deviation, categorical variables as frequencies (percentage); COPD: Chronic Obstructive Pulmonary Disease; CHD: Coronary Heart Disease; ACEIs: Angiotensin-Converting Enzyme Inhibitors; ARBs: Angiotensin Receptor Blockers; CCBs: Calcium Channel Blockers; * p from Mann-Whitney U test or Chi-square test for continuous and categorical variables respectively; #p from Fisher exact test | ||||
DISCUSSION
We show that blood pressure control was achieved in 58.5% of the elderly hypertensive outpatients at baseline and in 85.5% at the end of the fourth year of follow-up (estimated means). These control rates are higher than those published in the scientific literature as they were mainly population-based estimates [11].
A French population-based study carried out between 2005 and 2007 [7] showed that, among treated hypertensive participants aged 65-74 years, only 19.0% of men and 27.4% of women had their BP controlled (at BP levels of <140/90 mmHg, or <130/80 mmHg in diabetics); these values slightly increased later, reaching 20.2% in men and 29.0% in women when the BP target among all patients, including diabetics, was set at <140/90 mmHg.
Instead, BP control rates in subjects aged 60 years or older from the United States were not constant, since they varied from 51.4% to 56% [5,31] in the period 2007-2010 and were 50.8% in the years 2011-2014 [11].
In patients with T2DM, the estimated prevalence of hypertension ranges from 40% to 80%. The BP target of <130/80 mmHg, which was previously recommended, is currently considered not evidence-based [32].
For instance, in the ACCORD-BP trial [33], which compared outcomes associated with ‘lower’ (<120 mmHg) or ‘standard’ (<140 mmHg) systolic blood pressure (SBP) targets in 4,734 participants, the only significant benefit in the group assigned to ‘lower’ SBP was a reduction in the incidence of stroke; and trying to achieve the ‘lower’ SBP target was associated with a significant increase in the number of other serious adverse events.
Therefore, evidence from randomized trials did not support the use of BP targets lower than the standard targets in people with elevated blood pressure and diabetes [34], and other BP targets have recently been proposed: <140/80 mmHg [35], <140/85 mmHg [14], <140/90 mmHg [15,17].
Similarly, at least 85% of patients with CKD stage 3 or higher presents hypertension, and also in these patients the ideal BP targets to achieve in order to consider the BP controlled is still debated [20-21,27,35-38].
As the results of the recent meta-analyses and current evidence showed that the achievement of a BP control at <130/80 mmHg did not fully protect against the risk of development of the principal cardiovascular outcomes, the most recent guidelines have backtracked on stricter BP control in patients with CKD and recommend the same BP targets as in other patient groups [16,39], occasionally advocating stricter control only in welldefined subgroups of patient, such as those with proteinuria. In other words, stricter systolic BP control is associated with higher all-cause mortality in patients with CKD, and not only uncontrolled hypertension is deleterious but also overzealous lowering of BP could be harmful. Such evidence should encourage clinicians to prefer a personalized approach for the treatment in this hypertensive population [19-21].
Currently a BP target of <140/90 mmHg for all CKD patients, independently of the levels of proteinuria [16,17,39] is recommended.
Interestingly, by applying this less restrictive BP target versus those of JNC 7 [26] to population of the National Health and Nutrition Examination Survey (NHANES) 2011/2012 [available at http://www.cdc.gov/nchs/nhanes/index.htm. Accessed November 07, 2016], Sakhuja et al., [40] reclassified the prevalence of uncontrolled hypertension and showed its drastic reduction in specific subgroups of patients: those older than 60 years with no diabetes or CKD, those with CKD with or without diabetes, and those with diabetes but without CKD.
As well as reported elsewhere [10,14-15,18-22,32-35], also in our study the presence of T2DM and CKD resulted significantly associated with a lower prevalence of BP control, achieving, however, better results than those reported into the NHANES 2011-2012. Taking into account subjects who fall in the same age-group and using the standard BP target of <130/80 mmHg, we found that the prevalence of BP control was higher in our patients with T2DM and/or CKD (68.3% vs. 35% of NHANES 2011-2012). Using the JNC 8 guidelines BP target of <140/90 mmHg [16], this prevalence was even higher (96.4%) than the one found by Sakhuja et al., (35.% of all adult patients with both CKD and DM) [40].
Table 2: Systolic and diastolic blood pressure sample means ± standard deviation and estimated prevalence rates of controlled blood pressure, along with 95% confidence interval (95% CI), within four years from the first visit (baseline), in the whole sample, in patients with type 2 diabetes (T2DM) and/or chronic kidney disease (CKD) and in patients with other comorbidities (without T2DM and CKD), respectively.
| Samples | Follow-up (years) | N° of patients | Systolic blood pressure (SBP) | Diastolic blood pressure (DBP) | Estimate (95%CI) - Standard criteria* | Estimate (95%CI) - JNC 8 criteria^ |
| All patients (N=123) | Baseline | 123 | 134.3 ± 19.8 | 74.9 ± 10.3 | 58.5 (50.3-66.1) | --- |
| 1 | 123 | 129.0 ± 15.3 | 72.3 ± 9.7 | 66.8 (61.3-71.9) | --- | |
| 2 | 70 | 126.3 ± 15.6 | 72.9 ± 11.8 | 74.2 (66.1-81.0) | --- | |
| 3 | 49 | 125.2 ± 14.2 | 71.8 ± 7.3 | 80.5 (68.7-88.6) | --- | |
| 4 | 31 | 121.7 ± 11.6 | 64.8 ± 9.3 | 85.5 (70.8-93.5) | --- | |
| p trend=0.010 | ||||||
| Patients with T2DM and/or CKD (N=58) | Baseline | 58 | 134.2 ± 21.3 | 73.8 ± 10.3 | 38.2 (29.0-48.2) | 63.5 (53.2-72.7) |
| 1 | 58 | 128.5 ± 15.1 | 73.0 ± 11.5 | 45.8 (39.1-52.6) | 77.5 (71.0-82.9) | |
| 2 | 36 | 124.4 ± 13.7 | 68.9 ± 9.7 | 53.6 (42.5-64.3) | 87.2 (78.8-92.6) | |
| 3 | 26 | 124.0 ± 15.8 | 69.7 ± 7.1 | 61.2 (43.0-76.7) | 93.1 (83.7-97.3) | |
| 4 | 16 | 123.7 ± 10.5 | 67.1 ± 9.6 | 68.3 (43.0-86.1) | 96.4 (87.4-99.0) | |
| p trend=0.062 | p trend=0.002 | |||||
| Patients with other comorbidities (N=65) | Baseline | 65 | 134.4 ± 18.6 | 76.0 ± 10.4 | 76.4 (66.6-84.1) | --- |
| 1 | 65 | 129.5 ± 15.6 | 71.6 ± 7.7 | 83.1 (76.9-87.9) | --- | |
| 2 | 34 | 127.8 ± 17.0 | 76.1 ± 12.4 | 88.2 (79.4-93.5) | --- | |
| 3 | 23 | 126.2 ± 13.0 | 73.7 ± 7.0 | 91.9 (80.0-97.0) | --- | |
| 4 | 15 | 119.8 ± 12. | 62.8 ± 8.9 | 694.5 (80.1-98.6) | --- | |
| *Hypertension was considered to be controlled when systolic blood pressure (SBP) was <140 mmHg and diastolic blood pressure (DBP) was <90 mmHg. For T2DM and/or CKD patients only, hypertension was considered to be controlled when SBP was <130 mmHg and DBP was <80 mmHg. ^Furthermore, for patients with T2DM and/or CKD only, in compliance with the Eighth Joint National Committee (JNC 8) guidelines, hypertension was considered to be controlled when SBP was <140 mmHg and DBP was <90 mmHg. Estimates were derived from generalized random-effects pattern-mixture models for longitudinal studies with non-ignorable missing data. | ||||||
STRENGTHS AND LIMITATIONS
Our study presents points of strength and limitations. The points of strength include the fact that, during the entire followup, the patients of the ambulatory dedicated to hypertension in our nephrology unit were evaluated by the same physician, in the same environment and with the same semi-automatic sphygmomanometer. All this has undoubtedly helped establish a relationship of empathy between the doctor and the patients, favoring in the former the capacity of gaining the trust and confidence of the patients in the objectives set, and in the latter the compliance with the prescribed treatment. Our results are also strengthened by the fact that they refer to subjects with multiple comorbidities, and in particular with T2DM and CKD conditions, which are widely known to have a high prevalence in the elderly and associated difficulties of treatment.
The main limitation of the study is the inevitable reduction in the number of patients during the follow-up. This may be ascribed to the fact that the patients’ cohort was formed retrospectively, by accessing an electronic database in which all characteristics of the patients who had been coming on a voluntarily basis to our nephrology unit from October 2009 were registered. For this reason, despite the good compliance between the doctor and the patient, it was not possible to guarantee a constant surveillance for each patient at the follow-up. It is also important to underline that as the enrollment of patients took place dynamically during the period of observation (October 2009 – September 2014), only the subjects enrolled in the first time had the opportunity to be observed for all four years. Another important limitation is that the recruited sample (i.e. elderly hypertensive outpatients) is not representative of the entire elderly hypertensive population and thus our findings should be interpreted with caution. Moreover, it should be noted that better drugs compliance was not necessarily related to a better clinical outcome. Indeed, improved BP control can be even achieved in compromised patients (e.g. in those developing heart failure or in those showing dizziness, syncope or falls as adverse effects of antihypertensive drugs) and therefore cannot suffice to assert that such a group of patients achieved a better clinical outcome. Although, regrettably, the registration of any other comparative healthy outcome was not available in our dataset (this of course represents another major limitation), we can however confidently exclude the hypothesis of patients’ early withdrawal because, as the doctor recalled, no adverse events, heart diseases or deaths events occurred during the follow-up.
However, despite these important limitations, our study highlights that the estimated mean prevalence of BP control in the whole sample increased from 58.5% to 74.2% at the second year, and reached the 85.5% after the subsequent two years, suggesting that the adherence to the treatment and the relationship of trust between the patient and the doctor should contribute to the achievement of the patient’s objectives and the stabilization of the BP target.
The study is being updated and every effort will be made to guarantee a better surveillance of the patients, who will also be invited to attend more constantly all the visits requested during the follow-up.
CONCLUSIONS
The control of hypertension in the elderly is more and more improving worldwide, even if data from the USA seem conflicting [5,10,12,26,31].
Despite the progress obtained over the years, there is still room for improvement in reaching the goal of 61.2% within 2020, set by the Healthy People 2020 [11] US project [available at: http://www.healthypeople.gov/2020/default.aspx. Accessed November 07, 2016].
In this perspective, our results, which indicated the achievement of a BP control (≤ 140/90 mmHg in the entire cohort, ≤ 130/80 mmHg in patients with T2DM or CKD) in the 74.2% of the investigated hypertensive elderly patients just at the second year of follow-up, seemed relevant if we consider that they were obtained in subjects with multiple comorbidities, who were therefore not easy to manage.
ACKNOWLEDGEMENTS
The authors thank Chiara Di Giorgio, medical translator, for her editorial assistance and language revision of the manuscript.
A.D.G, A.F: contributed to study concept, collected data and wrote drafts of the manuscript.
A.F: performed statistical analysis; A.D.G, A.F, F.A: approved the final manuscript.
REFERENCES
28. Little RJA. Modeling the drop-out mechanism in repeated-measures studies. JASA. 1995; 90: 1112-1121.
30. Diggle PJ, Heagerty P, Liang KY, Zeger SL. Analysis of longitudinal data. Oxford: Oxford University Press; 2013.