Oxidative Stress and Mineralocorticoid Receptor Signaling in the Brain: Possible Therapeutic Targets for Dementia
- 1. Division of Clinical Epigenetics ,University of Tokyo, Japan
- 2. Department of Clinical Laboratory, University of Tokyo Faculty of Medicine, Japan
Abstract
Glucocorticoid and mineralocorticoid receptor signals are important for memory formation, salt cravings, sympathetic tone and hypothalamic–pituitary–adrenal (HPA) axis control in the brain. Exacerbations of glucocorticoid and mineralocorticoid receptor signaling cause atherosclerosis, cognitive dysfunction, and depression. Mineralocorticoid activity is modulated by oxidative stress, and chronic stress desensitize further nongenomic mineralocorticoid receptor action. In patients with chronic diseases such as diabetes and hypertension, oxidative stress in the brain is increased, which alters the balance of the HPA axis, resulting in an elevated risk of dementia. Therefore, oxidative stress and mineralocorticoid receptor blockade are possible therapeutic targets for cognitive dysfunction.
Keywords
• Mineralocorticoid receptor
• Oxidative stress
• Brain damage
• Hypertension
Citation
Kawakami-Mori F, Shimosawa T (2014) Oxidative Stress and Mineralocorticoid Receptor Signaling in the Brain: Possible Therapeutic Targets for Dementia. Ann Clin Exp Hypertension 2(2): 1015.
INTRODUCTION
There is compelling evidence that diabetes mellitus, hypertension, ischemic brain injury, and psychiatric disease cause oxidative stress in the brain leading to cognitive deficits and dementia[1-3].The development of effective treatments for improving cognitive function is of great public health concern because of the high prevalence of hypertension and diabetes in the growing elderly population. The association between hypertension and cognitive decline has been of particular interest and there have been several clinical trials of antihypertensive drugs in the elderly, such as SCOPE, PROGRESS, HYVET, Sys-Eur [4,5];among these, only the Sys-Eur trial showed any benefit of antihypertensive drugs on preservation of cognitive function. However, these studies were limited by the relatively short observation periods and initiation of treatment late in the disease process, which precluded the ability to observe any meaningful impact on cognitive decline. In support, a recently published20- year large-scale retrospective clinical trial showed that midlife hypertension was strongly associated with cognitive decline in later life, and early intervention with antihypertensive drugs could ameliorate this process [6].High blood pressure causes cognitive decline after a long time, therefore early intervention is critical to prevent disease progression. In spite of these findings, to date there are no adequate data to indicate which antihypertensive agent is most effective. In the brain, neurotransmitters are secreted from the presynapse and bind mainly to postsynaptic receptors to open ionic channels and modulate the activity of postsynaptic cells. Glutamate is an excitatory neurotransmitter that induces cation entry by activation of the α-amino-3-hydroxy-5-methyl4-isoxazolepropionic acid (AMPA) and N-methyl-D-aspartate (NMDA) receptors on glial cells. Glutamate plays an important role in learning and in establishing memory. Meanwhile, excessive secretion of glutamate induces cell vulnerability and/ or cell death by over-excitation [7].Glutamate release is regulated by many neuro modulators that are affected by chronic diseases such as cerebral ischemia, diabetes, and hypertension. Here, we review the role of the neuro modulator glucocorticoid (GC) and oxidative stress in the development and progression of dementia.
Steroid receptor distribution and effect in the brain
There are many ligands and receptors on neurons, in addition to neurotransmitter receptors, that modulate transmitter release and/or ionic channel activation. Among these, GC is a known neuromodulator that has an important role in cognitive memory, fear memory, and salt cravings [8, 9]. GCs bind two steroid receptors, glucocorticoid receptors (GRs) and mineralocorticoid receptors (MRs). In the brain, GRs are expressed ubiquitously, whereas MRs is localized to specific areas, such as the hippocampus, para ventricular nucleus, and brainstem. The binding affinity of corticosterone (CS) for MRs is 10-fold higher than GRs, and under normal conditions, CS preferentially binds to MRs. MRs bind not only aldosterone but also cortisol and CS with almost the same affinity. Under normal physiological conditions, the concentration of cortisol and CS is at least three orders of magnitude higher than aldosterone; therefore, the active ligands of MRs are mainly cortisol and CS in regions of the brain where 11β-hydroxysteroid dehydrogenase2(11βHSD2) is not expressed. 11βHSD2 metabolizes CS to its inactive form cortisone and in the brain, 11βHSD2 is expressed only in specific regions area such as the tractus solitarius, subcommisural organ, and ventromedial hypothalamus [10]. Hence, in most of the brain, the main MR agonist is CS.
For memory formation, GR and MR balance is important; for instance, GR is mainly for memory consolidation and MR is for memory acquisition [11].Both receptors are activated concurrently; an imbalance is associated with cognitive impairment and dysregulation of the hypothalamic–pituitary– adrenal (HPA) axis [12-14]. Imbalances can be induced by selective agonists such as dexamethasone, or chronic diseases that increase oxidative stress in the brain. Oxidative stress modulates mainly the MR signal. MRs act not only through classical transcriptional pathways, but also via short-term, non-genomic action through G-proteincoupled receptors (GPCRs). When circulating CS levels are increased by stress, CS binds membranous MRs, which are of lower affinity than intracellular MRs. These activate voltagedependent L-type Ca2+ channels and increase glutamate release from the presynapse, while simultaneously inactivating the postsynaptic K+ channel (IA current) within minutes in a nongenomic manner [15-18].
Relationship between oxidative stress and MR action
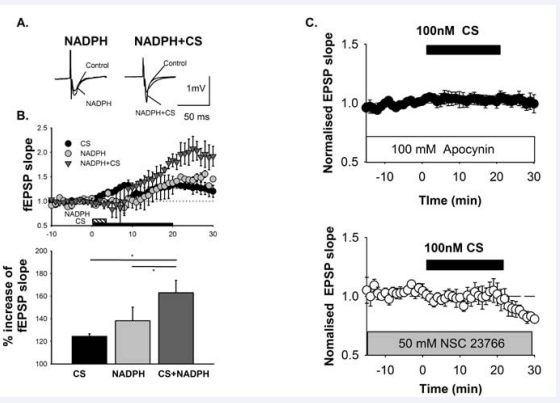
Figure 1 A) The representative data of field excitatory postsynaptic potentials fEPSPs in the CA1 region of the hippocampus induced by stimulation of the perforate pathway. B) Applying 100nM corticosterone (CS) and/or 1μM NADPH rapidly increased fEPSPs via non-genomic action. All data were obtained in the presence of 500 nM actinomycin D to confirm non-genomic action. Simultaneous application of 100nM CS and 1μM NADPH increased fEPSP remarkably. C) Non-genomic action by CS is ameliorated by an NADPH oxidase inhibitor (100μM apocynin) or Rac1 inhibitor (50μM NSC23766). These data are from (25).
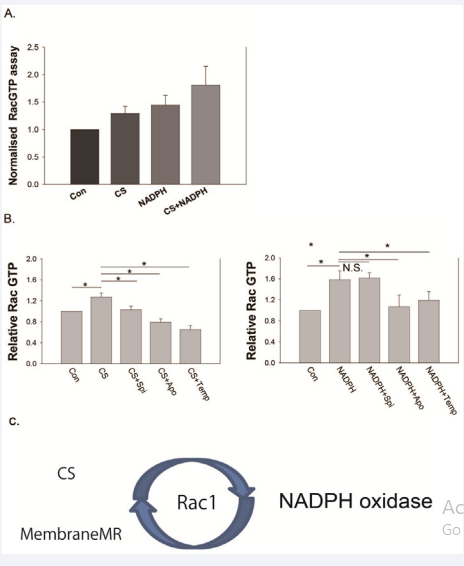
In peripheral tissue, MR controls sodium transport and induces inflammation, fibrosis, and hypertrophy. This action is independent of serum ligand concentration, and is potentiated by oxidative stress, which has been a paradoxical problem regarding MRs for a long time [19, 20]. One factor that exacerbates MR action under conditions of oxidative stress is 11βHSD1/2[19, 21, 22], and another is Rac1 (23, 24). 11βHSD1 converts cortisone to CS or deoxycorticosterone, which is NADPH-dependent and then11βHSD2 metabolize CS to cortisone, which is also NADPHdependent. Oxidative stress activates 11βHSD1 and inactivates 11βHSD2, resulting in increased concentrations of CS in the tissue. In contrast, Rac1 GTPase, a member of the Rho family GTPases, activates MR nuclear translocation, MR-dependent transcription, and MR-dependent signals such as serum and glucocorticoidregulated kinase 1(SgK1) [23,24]. Rac1 is dispensable for the non-genomic action of MR in the brain. The Rac1 inhibitor, NSC23766, and the NADPH oxidase inhibitor apocynin block the non-genomic action of CS, rapid potentiation of field excitatory postsynaptic potential (EPSP), and ERK1/2 phosphorylation in hippocampal neurons. Furthermore, simultaneous stimulation of MR and NADPH oxidase increases potentiation of field EPSPs (Figure 1) and ERK pathway activation remarkably. Meanwhile, the non-genomic action of MR activates Rac1 GTP, and induces MR translocation (Figure2). These results indicate that both CS elevation and oxidative stress induce MR activation, and oxidative stress is subsequently further increased by MR activation [24] in a feedback loop. The physiological roles of non-genomic action of MRs include memory retrieval [26-29], negative feedback with the HPA axis via hippocampal activation, suppression of adrenocorticotropic hormone(ACTH) and CS [30],and possibly ,effects on transition to genomic action [31]. It is plausible that oxidative stress modulates the physiological functions of the non-genomic action of MR leading to pathological consequences of cognitive dysfunction and multiple organ dysfunction.
Figure 2 A) Ten min after addition of corticosterone (CS) and/or NADPH, Rac1 GTPase was activated in the CA1 region of the hippocampus. CS and NADPH had an additive effect. B) Rac1 activation by CS and/or NADPH was blocked by 1μM spironolactone, apocynin, and Tempol. C) Rac1 is a key factor in the feedback loop between mineralocorticoid receptors (MR) and NADPH oxidase.
MR blockade in the brain and cognitive function
GC elevation is commonly observed in patients with poorly controlled diabetes and depression. In fetuses, a high salt diet changes glucocorticoid metabolism in the tissues and affects the HPA axis [32,33]. HPA axis dysregulation and/or set point changes are partially induced by oxidative stress; glucocorticoid excess further induces oxidative stress via the MR pathway and glutamate release in the brain, causing neuron damage. A mouse model of diabetes showed impaired memory; treatment of these mice with an MR blocker that can pass through blood brain barrier, protected cognitive functions [34]. On the other hand, treatment with a high dose of MR blocker impaired selective attention and working memory performance but enhanced long term memory in healthy humans [35,36].It is possible that MR blockade increased serum cortisol and changed the MR/GR activation ratio[37].
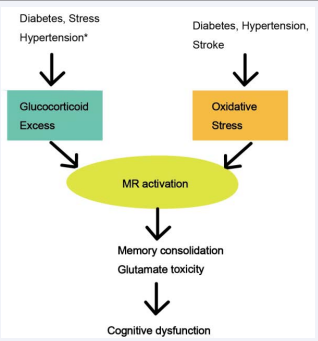
Figure 3 Chronic oxidative stress and excess corticosterone (CS) induce mineralocorticoid receptor (MR) activation that leads to memory impairment.
Rac1 inhibition and cognitive function
In our study, a Rac1 inhibitor attenuated the non-genomic action of MR and NADPH in the CA1 region of the hippocampus [25].While Rac1 GTPase is dispensable for NADPH oxidase activation, Rac1 inhibition prevented neuronal degeneration caused by oxidative stress [38], reduced reactive oxygen species (ROS) generation, and oxidative stress in the hippocampal CA1 region, and delayed neuronal cell death after ischemic injury [39]. Rac1 inhibition also increased long term potentiation in the CA1 region [40]. Furthermore, comparing Rac1 and Rho A inhibition [41-43], Rac1 blockade appears to have a protective role in preventing brain damage, positively affecting cognitive function, and therefore, is a potential therapeutic target to prevent diseases caused chronic oxidative stress.
DISCUSSION
The pathophysiological roles of MR in cognitive function have been studied. We could step forward to translate these findings to diagnosis and therapy in clinical settings. Although there are no data delineating which antihypertensive drug is the most effective to prevent cognitive dysfunction, considering the action of MR in the brain, antihypertensive drugs that are also MR blockers could be possible therapeutics for dementia. Further, Rac1 inhibitors might be additional therapeutic candidates because they inhibit chronic ROS generation in the brain in diabetes, hypertension, and mental illness disease models. The further study of this field is expected to contribute to improve mental and physical health of aged people.
ACKNOWLEDGEMENTS
The study of ref [25] was supported by grants of 24790834 from KAKEN in Japan.
REFERENCES
3. Umegaki H, Iimuro S, Shinozaki T, Araki A, Sakurai T, Iijima K, et al. Risk factors associated with cognitive decline in the elderly with type 2 diabetes: pooled logistic analysis of a 6-year observation in the Japanese Elderly Diabetes Intervention Trial. Geriatr Gerontol Int. 2012; 12 Suppl 1:110-116.
4. Peters R, Beckett N, Forette F, Tuomilehto J, Clarke R, Ritchie C, et al. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lancet Neurol. 2008; 7 :683- 689.
6. Gottesman RF, Schneider AL, Albert M, Alonso A, Bandeen-Roche K, Coker L, et al. Midlife Hypertension and 20-Year Cognitive Change: The Atherosclerosis Risk in Communities Neurocognitive Study. JAMA Neurol. 2014;71:1218-1287.
8. Coirini H, Magarinos AM, De Nicola AF, Rainbow TC, McEwen BS. Further studies of brain aldosterone binding sites employing new mineralocorticoid and glucocorticoid receptor markers in vitro. Brain Res. 1985;361:212-216.
10.Geerling JC, Loewy AD. Aldosterone in the brain. Am J Physiol Renal Physiol. 2009; 297: F559-576.
12.Rogalska J. Mineralocorticoid and glucocorticoid receptors in hippocampus: their impact on neurons survival and behavioral impairment after neonatal brain injury. Vitam Horm. 2010;82: 391- 419.
14.Rybnikova E, Glushchenko T, Churilova A, Pivina S, Samoilov M. Expression of glucocorticoid and mineralocorticoid receptors in hippocampus of rats exposed to various modes of hypobaric hypoxia: Putative role in hypoxic preconditioning. Brain Res. 2011;1381:66-77.
16. Chameau P, Qin Y, Spijker S, Smit AB, Smit G, Joëls M. Glucocorticoids specifically enhance L-type calcium current amplitude and affect calcium channel subunit expression in the mouse hippocampus. J Neurophysiol. 2007;97: 5-14.
17.Karst H, Berger S, Turiault M, Tronche F, Schütz G, Joëls M. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci U S A. 2005;102 :19204-19207.
18.Olijslagers JE, de Kloet ER, Elgersma Y, van Woerden GM, Joëls M, Karst H. Rapid changes in hippocampal CA1 pyramidal cell function via preas well as postsynaptic membrane mineralocorticoid receptors. Eur J Neurosci. 2008;27:2542-50.
21.Hewitt KN, Walker EA, Stewart PM. Minireview: hexose-6-phosphate dehydrogenase and redox control of 11{beta}-hydroxysteroid dehydrogenase type 1 activity. Endocrinology. 2005;146:2539-2543.
22.Tsugita M, Iwasaki Y, Nishiyama M, Taguchi T, Shinahara M, Taniguchi Y, et al. Differential regulation of 11beta-hydroxysteroid dehydrogenase type-1 and -2 gene transcription by proinflammatory cytokines in vascular smooth muscle cells. Life Sci. 2008;83:426-32.
24.Shibata S, Mu S, Kawarazaki H, Muraoka K, Ishizawa K, Yoshida S, et al. Rac1 GTPase in rodent kidneys is essential for salt-sensitive hypertension via a mineralocorticoid receptor-dependent pathway. J Clin Invest. 2011; 121:3233-3243.
25.Mori FK, Shimosawa T, Wang H, Ogura S, Mu S, Yatomi Y, et al. NADPH oxidase-mediated Rac1 GTP activity is necessary for non-genomic actions of the mineralocorticoid receptor in the CA1 region of the rat hippocampus. Am J Physiol Endocrinol Metab. 2011.
27.Haller J, Halasz J, Mikics E, Kruk MR, Makara GB. Ultradian corticosterone rhythm and the propensity to behave aggressively in male rats. J Neuroendocrinol. 2000; 12:937-940.
28.Haller J, Mikics E, Makara GB. The effects of non-genomic glucocorticoid mechanisms on bodily functions and the central neural system. A critical evaluation of findings. Front Neuroendocrinol. 2008;29:273- 291.
30.Atkinson HC, Wood SA, Castrique ES, Kershaw YM, Wiles CC, Lightman SL. Corticosteroids mediate fast feedback of the rat hypothalamicpituitary-adrenal axis via the mineralocorticoid receptor. Am J Physiol Endocrinol Metab. 2008;294:E1011-1022.
32.Baudrand R, Campino C, Carvajal CA, Olivieri O, Guidi G, Faccini G, et al. High sodium intake is associated with increased glucocorticoid production, insulin resistance and metabolic syndrome. Clin Endocrinol (Oxf). 2014;80: 677-684.
35.Cornelisse S, Joels M, Smeets T. A randomized trial on mineralocorticoid receptor blockade in men: effects on stress responses, selective attention, and memory. Neuropsychopharmacology. 2011; 36:2720- 2728.
36.Otte C, Moritz S, Yassouridis A, Koop M, Madrischewski AM, Wiedemann K, et al. Blockade of the mineralocorticoid receptor in healthy men: effects on experimentally induced panic symptoms, stress hormones, and cognition. Neuropsychopharmacology. 2007; 32:232-238.
37.Mattsson C, Reynolds RM, Simonyte K, Olsson T, Walker BR. Combined receptor antagonist stimulation of the hypothalamic-pituitary-adrenal axis test identifies impaired negative feedback sensitivity to cortisol in obese men. J Clin Endocrinol Metab. 2009;94:1347-1352.
42.Huang B, Krafft PR, Ma Q, Rolland WB, Caner B, Lekic T, et al. Fibroblast growth factors preserve blood-brain barrier integrity through RhoA inhibition after intracerebral hemorrhage in mice. Neurobiol Dis. 2012;46:204-14.
43.Rochfort KD, Collins LE, Murphy RP, Cummins PM. Downregulation of blood-brain barrier phenotype by proinflammatory cytokines involves NADPH oxidase-dependent ROS generation: consequences for interendothelial adherens and tight junctions. PLoS One. 2014;9:e101815.