Early Diagnosis and Treatment of Infants with Ischemic/ Hemorrhagic Perinatal Brain Lesions
- 1. Department of Developmental Neurology, St. Margaret Hospital, Hungary
Abstract
Ischemic/hemorrhagic perinatal brain lesions may severely affect infants’ psychomotor and cognitive development. The ischemic/hemorrhagic lesions are frequently unilateral. 79 term infants (51 male and 28 female) from 1 week to 3.5 months were studied. All patients had unilateral lesions due to transient or permanent occlusion of the left or right middle cerebral arteries, left or right intraventricular hemorrhages, and left or right parenchymal bleeding. Except for 25 infants that did not show any neurological signs, the remaining 54 infants were treated with Katona’s neurotherapy/neurohabilitation and followed up for 18th-24th months. The follow-up ended when the ability to independent, stable walking developed. Results: Patients with lesions due to vasoconstriction/ occlusion of the cerebral arteries after treatment showed the following outcomes: 42 % were completely recovered, 21% showed latent hemiparesis, and 37% a severe hemiparesis. Treatment of infants with parenchymal bleeding showed an outcome of 52% completely recovered, 15% have latent hemiparesis, and 12% have severe hemiparesis. Conclusions: Katona’s neurotherapy/neurohabilitation treatment decreases the risk of neurological sequels in infants with ischemic/hemorrhagic perinatal brain lesions.
Keywords
• Neonatal stroke; Unilateral hemorrhagic insult; Early diagnosis; Hemiparesis; Katona’s neurotherapy (neurohabilitation)
Citation
Berényi M, Bajusz S, Csepeli BA (2025) Early Diagnosis and Treatment of Infants with Ischemic/Hemorrhagic Perinatal Brain Lesions. Pediatr Child Health 13(3): 1357.
INTRODUCTION
Perinatal unilateral insult can be caused by ischemic stroke, hemorrhagic stroke (arterial or venous), unilateral III-IV grade ventricular bleeding, and venous thrombosis. A severe hypoxic episode often accompanies these cerebral insults. Ischemic/hemorrhagic stroke is one of the most frequent cerebral insults during the perinatal period.The prevalence of perinatal ischemic/hemorrhagic episodes in infants is 6-17‰ according to the cited literature [1-5]. In the special, selected patient population of the Department of Developmental Neurology at Saint Margaret Hospital in Budapest, the prevalence is 22 ‰. The stroke usually affects one side of the brain, but sometimes both sides are damaged, with one side being more affected [6]. Hemiparesis is a common symptom caused by this damage. Hemiparesis is characterized by a difference in muscle tone and usage of the affected limb with a late appearance, typically during the 4th or 6th month of the infant’s life. This paper describes our experience with the early evaluation and treatment of infants with this pathology.
PATIENTS AND METHOD
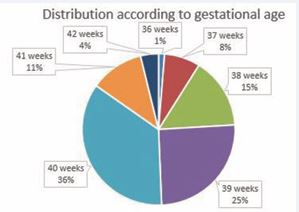
Between November 15th, 2007, and December 31st, 2018, 4020 infants were admitted to the Department of Developmental Neurology of Saint Margaret Hospital (Budapest). Among them, 91 had ischemic/hemorrhagic insult related to one hemisphere. In 12 infants, the follow up was discontinued, so the data of 79 infants were processed (among them 51 boys and 28 girls). The vast majority were born on the 39th or 40th gestational week (Figure 1).
Figure 1: Distribution according to gestational age (79 infants).
Maternal and fetal risk factors observed were: nullipara {multiple spontaneous miscarriages before the 20th gestational week}, primipara, preeclampsia, fever during childbirth, meconium in the amniotic fluid, oligohydramnion, acute Cesarea section, low Apgar score, hypoglycemia, early septic state, etc.). All these factors have been referred to as if they may increase the risk of ischemic/hemorrhagic insults [7-11]. Table 1 shows the distribution of the lesions in our sample.
Table 1: Distribution of pathology according to gender.
|
Lesion |
Number |
Male |
Female |
|
Right Middle Cerebral Artery |
9 |
6 |
3 |
|
Left Middle Cerebral Artery |
16 |
11 |
5 |
|
Right intraventricular hemorrhage |
3 |
2 |
1 |
|
Left intraverticular hemorrhage |
1 |
1 |
1 |
|
Right parenchymal bleeding |
16 |
9 |
7 |
|
Left parenchymal bleeding |
23 |
13 |
10 |
|
Bilateral parenchymal hemorrhages |
11 |
7 |
4 |
|
Total |
79 |
49 |
30 |
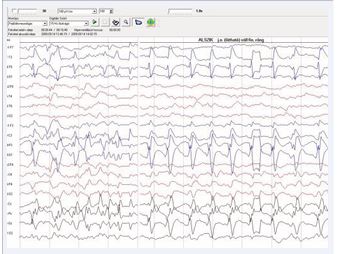
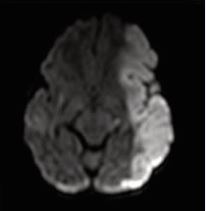
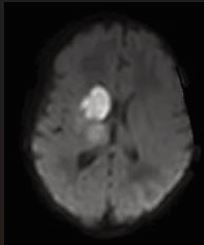
The presence of abnormal neurologic symptoms in the examined 79 infants was observed at the following ages: 9 in the delivery room; 21 during the first 24 hours; 15 during the first or second day of life; 13 at the first or second week; 21 infants showed their first symptoms during the 3rd or 4th month. Examining the Apgar score, 31 out of 79 infants had 9/10 or 10/10, 20 had 8/9 or 9/9, and 28 had an extremely low as 0/0/4 up to 6/7.In 25 patients no abnormal neurological signs were observed during the whole study, including the follow up. Seizures were observed in 50 infants as their first sign (Figure 2), but 8 also had apnea, irritability, and opisthotonic spasms. Neurosonography was performed in all infants, MRI [10], in 77 infants, CAT scan in 1, and no MRI/CAT in 1. Figures 3a, 3b, and 4 show some examples of the lesions.
Figure 2: Epileptic seizure with left side involvement.
Figure 3a MCA stroke on the left (a) and right side (b)
Figure 3b MCA stroke on the left (a) and right side (b).
Phenobarbital (PHB) is the first recommended drug for perinatal seizures [12], and was administered on the sample examined by us. Fifty of our infants during the first days of life were treated with loaded PHB without having seizures because of pathological results detected with neuroimaging and/or laboratory tests such as elevated lactate level, low pH, etc. Five infants were treated with other anticonvulsants {carbamazepine (CBZ), phenytoin (PHT), valproic acid (VPA), vigabatrin (VGA), diazepam (DZP), levetiracetam (LEV) fentanyl (FEN)}. According to their case history, no medication was given in 5 cases despite the presence of seizures. Hypothermia was applied in 13 newborns; 12 were treated at the 1st Department of Pediatrics, Semmelweis University.
TREATMENT
The necessity of treating infants suffering from cerebral insult is decided after thorough examinations or during the follow-up according to the initial or modified diagnosis [13 15]. No therapy was initiated when functional deviation was not found. Infants who “only” had motor symptoms were given treatment that facilitated bilateral symmetrical movements. Patients with ischemic/hemorrhagic insult and severe hypoxia received the complete Katona’s neurohabilitation/ neurotherapy supplemented -if necessary- with anticonvulsive drugs. The treatment used was Katona’s neurotherapy/ neurohabilitation [15]. This procedure is diagnostic and therapeutic, based on the analyses of the “elementary sensorimotor patterns” that are chains of movements triggered by different head positions to stimulate the vestibular receptors (Figures 5 and 6).
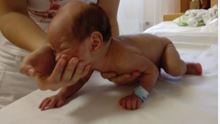
Figure 5: The elementary sensorimotor pattern of “assisted crawling”. The infant is held in the prone position by the examiner/parent. The head is elevated (nose in the midline, chin in horizontal), trunk supported (one palm of the examiner is at the epigastric level), and limbs contact the surface. The patient is pulled horizontally at an even pace while his/her limbs are touching the surface. This “stimulus” position elicits alternating, symmetric flexion-extension movements in all extremities in a physiologic situation. At the same time, due to a right side lesion, flexion is increased on the left upper extremity.
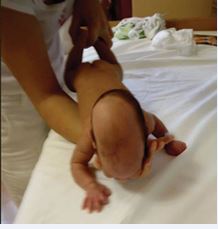
Figure 6: The elementary sensorimotor pattern of “wheelbarrow reaction” (the position is similar to “assisted crawling”, without lower limb contact). Observe finger’s extension on the right side, while the left side remains in fist due to the right side lesion!
Signs of abnormal sensorimotor development were detected in 31 infants, and in the other 3 infants, early symptoms of epilepsy, together with sensorimotor defects were detected, and 20 infants presented motor signs and needed therapy to promote bilateral symmetric movements. In 29 patients, the treatment began during the first month, and the remaining infants during the first 3 months of life. The follow-up of the neonates and infants was continued until the 18th-24th month. The follow-up ended when the ability of independent, stable walking developed.
RESULTS
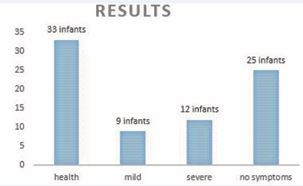
At the end of the 1.5th or 2nd years of life, the distribution of the development of the 79 infants who suffered unilateral cerebral insult and received regular follow-ups was the following: 21 infants developed hemiparesis (differing in severity), 12 cases having severe, 9 mild symptoms (latent: detected only in certain test positions, but barely noticeable in everyday life, i.e., a slight difference in hand preference, muscle tone, and walking). Despite the lesion, twenty-five infants did not show any signs of hemiparesis at first or at the closing examination, did not need or receive any therapy, and are healthy. Thirty-three infants initially with hemiparesis showed no symptoms at the final tests and were determined healthy due to successful treatment (Figure 7).
Figure 7: Results
Among the 13 patients that previously received hypothermia following the developmental neurologic diagnostic procedure, 10 were treated with neurotherapy. Due to the neurotherapy, 6 recovered completely, 2 developed mental disabilities, and 2 had motor problems. Of the 13 with hypothermia 3 did not receive neurotherapy. Out of these 3, one showed signs of mental disability at the age of 1,5, and 2 showed normal development. Out of the 11 infants that developed epilepsy during the follow-up, all received neurotherapy; 4 developed hemiparesis, 3 recovered totally, and in 4 cases, the follow-up terminated too early. Of the 50 infants with perinatal seizures, only 25 developed hemiparesis.
Appearance of hemiparesis
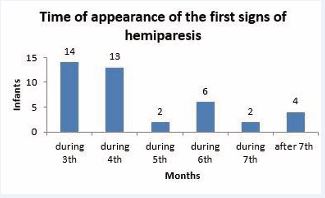
According to severity, hemiparetic symptoms were divided into two groups. One is the definite hemiparesis, where among signs, the “crossing” motion – i.e., the infant tries to reach for an object presented at one side using the hand on the opposite side – is first. During the 4th-5th months, increased muscle tone can be observed first on the upper and later on the lower limb, together with neglect of the affected limb in test situations. The characteristic position of the affected upper limb: hand in a fist, elbow flexed, shoulder rotated inside and abducted. The increased extension of the affected lower limb (plantar flexion of the feet, and hyperextension of the knee) is also prominent, and the asymmetry of walking. The other type is “latent” hemiparesis, where the tone difference is detectable only in certain test situations, and minimal asymmetry can be detected in skilled movements. Figure 8 shows the first time that hemiparesis appears in our study.
Figure 8: Time of appearance of the first signs of hemiparesis
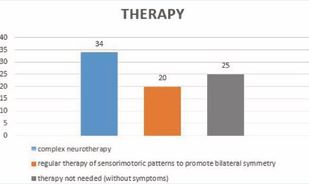
After the diagnostic period, 44 infants were considered abnormally developing, while 35 showed normal sensorimotor coordination. Of these 35, in 10 infants, the diagnosis was altered after the 1st or 2nd follow-up control (1 or 2 months after emission from the ward), so altogether 54 infants received neurotherapy. All these 54 infants received Katona’s neurotherapy/neurohabilitation; in 34 patients the complete therapeutic procedure was used, whereas in 20 infants “only” the motor development was at risk (Figure 9).
Figure 9:Types of therapies.
Neurodevelopmental changes were controlled every month after the establishment of the diagnosis. The same team executed the follow-up that made the diagnosis, including a physician, physiotherapist, psychologist/special education teacher, and technical assistant. Items of the neurotherapy are taught to parents at the emission of the ward or during outpatient examinations. They receive a written description of the treatment. It is recommended to preform items of neurotherapy six times a day, which is reinforced by our “timetable” suggestion, which helps to fit the treatments into the family’s daily schedule concerning feeding times. All items have exact duration, predefined sequence, and unique “stimulus position”. Systematic and structural characteristics of the treatment are as important as the centrally generated movements. Hence regular, repeated practice is the basis of movement learning.
Table 2: Distribution of pathology according to the results of neurotherapy.
|
Lesion |
number |
completely recovered |
latent hemiparesis |
severe hemiparesis |
no need for therapy |
|
Right Middle Cerebral Artery |
9 |
1 |
2 |
1 |
5 |
|
Left Middle Cerebral Artery |
16 |
7 |
2 |
6 |
1 |
|
Right intraventricular hemorrhage |
3 |
1 |
0 |
1 |
1 |
|
Left intraventricular hemorrhage |
1 |
0 |
0 |
0 |
1 |
|
Right parenchymal bleeding |
16 |
7 |
3 |
1 |
5 |
|
Left parenchymal bleeding |
23 |
10 |
2 |
3 |
8 |
|
Bilateral parenchymal hemorrhages |
11 |
7 |
0 |
0 |
4 |
|
Total |
79 |
33 |
9 |
12 |
25 |
The results obtained after treatment for each pathology are shown in Table 2.
From the patients with lesions of the middle cerebral arteries (n=25), 6 (24%) of them did not present symptoms, therefore they do not need therapy. From the remaining 19 patients treated, 8 (42%), completely recovered, 4 (21%), showed latent hemiparesis and 7 (37%), severe hemiparesis. In 50 infants parenchymal bleeding was observed, 17 (34%) had no need of therapy, in the remaining 33 neurotherapy was applied, 24 (72%) recovered entirely, 5 have latent hemiparesis and only 4 had severe hemiparesis. Intraventricular hemorrhages were observed in 4 patients, 2 did not need therapy, thus therapy was given to only 2 infants, 1 completely recovered and 1 had severe hemiparesis. The overall results obtained after the treatment of the sample present that from a total of 54 patients, 33 (61%) had normal neurodevelopment, 9 (17%) developed minor sequels, and 12 (22%) developed severe sequels.
DISCUSSION
Perinatal unilateral insults can be caused by ischemic stroke, hemorrhagic stroke (arterial or venous), unilateral III-IV grade ventricular bleeding, and venous thrombosis. A severe hypoxic episode often accompanies these cerebral insults. Hemiparesis is a common symptom caused by this damage. Hemiparesis is characterized by a difference in muscle tone and usage of the affected limb with a late appearance, typically during the 4th or 6th month of the infant’s life.
The results obtained in the groups of lesions by middle cerebral arteries showed in the outcome after treatment that 37% had a severe hemiparesis. This result is better than the outcome referred by Loo et al. [16], of 76% with severe impairment, Mercuri et al. [17], reported 59% with poor outcome, and Golomb et al. [18], described 78% of infants with cerebral palsy, Lee et al. [19], described that all infants with arterial infarcts and 77% with parenchymal hemorrhages developed neurological sequels, and Vojcek et al. [20,21], describing 60 % of children with several deficiencies after a long follow-up. However, according to a meta-analysis performed by Vuillerot et al. [22], the outcome varies depending on the study.Patients treated with parenchymal bleeding also showed better outcomes than the natural outcome: only 12 % of the treated patients showed a severe outcome vs. 68% observed in the natural outcome [2].These results suggested that during the development of abnormal symptoms early, regular neurotherapy can influence the outcome. Neurotherapy can decrease the severity of hemiparesis and, in some cases, prevent their appearance. In a high percentage of cases where treatment is needed, good results can be achieved by a personalized and symptom-specific, intensive and structural neurotherapy based on an early, thorough examination, a correct diagnosis, and regular (monthly) follow-ups.The necessity of treating infants suffering from cerebral insult is decided after thorough examinations or during the follow-up according to the initial or modified diagnosis. No therapy was initiated when functional deviation was not found. Infants who “only” had motor symptoms were given treatment that facilitated bilateral symmetrical movements. Patients with ischemic/hemorrhagic insults and severe hypoxia received the complete Katona’s neurotherapy supplemented -if necessary- with anticonvulsive drugs.
CONCLUSIONS
Early diagnosis and Katona’s neurotherapy/ neurohabilitation treatment of infants with ischemic/ hemorrhagic perinatal brain lesions substantially decreased the number of infants with a poor outcome.
ACKNOWLEDGMENTS
Present and previous coworkers of the Dept. of Developmental Neurology, who participated in diagnosis and treatment: Marta Szeredai, IldikoOrbanneMaros, BorbálaTelcs MD., Maria Felkai MD., GyörgyiHajnalMD. ,EszterSzurkos, GergoKober, MRI pictures are published with the permission of Dr. G Rudas MRI Research Center,Semmelweis University. Special appreciation to Dr Thalia Harmony for her valuable advice and special thanks for editing and revising the manuscript.
REFERENCES
- Grunt S, Mazenauer L, Buerki SE, Boltshauser E, Mori AC, Datta AN, et al. Incidence and outcomes of symptomatic neonatal arterial ischemic stroke. Pediatrics. 2015; 135: e1220-1228
- Lõo S, Ilves P, Männamaa M, Laugesaar R, Loorits D, Tomberg T, et al. Long-term neurodevelopmental outcome after perinatal arterial ischemic stroke and periventricular venous infarction. Eur J Paediatr Neurol. 2018; 22: 1006-1015.
- Klemme M, Gerstl L, Weinberger R, Olivieri M, Flemmer A, von Kries R, et al. Neonatal Arterial Ischemic Stroke - A Hospital Based Active Surveillance Study in Germany. Klin Padiatr. 2017; 229: 142-146.
- Darmency-Stamboul V, Cordier AG, Chabrier S. Neonatal arterial ischemic stroke in term or near-term newborns: prevalence and risk factors. Arch Pediatr. 2017; 24: 9S3-9S11.
- Kirton A, Armstrong-Wells J, Chang T, Deveber G, Rivkin MJ, Hernandez M, et al. Symptomatic neonatal arterial ischemic stroke: the International Pediatric Stroke Study. Pediatrics. 2011; 128: e1402-10.
- Lehman LL, Rivkin MJ. Perinatal arterial ischemic stroke: presentation, risk factors, evaluation, and outcome. Pediatr Neurol. 2014; 51: 760-768.
- Kirton A, Shroff M, Pontigon AM, de Veber G. Risk factors and presentations of periventricular venous infarction vs arterial presumed perinatal ischemic stroke. Arch Neurol. 2010; 67: 842-848
- Chalmers EA. Perinatal stroke - risk factors and management. Br J Haematol. 2005; 130: 333-343.
- Harteman JC, Groenendaal F, Kwee A, Welsing PM, Benders MJ, de Vries LS. Risk factors for perinatal arterial ischaemic stroke in full- term infants: a case-control study. Arch Dis Child Fetal Neonatal Ed. 2012; 97: F411-416.
- Lee S, Mirsky DM, Beslow LA, Amlie-Lefond C, Danehy AR, Lehman L, et al. Pathways for Neuroimaging of Neonatal Stroke. Pediatr Neurol. 2017; 69: 37-48.
- Li C, Miao JK, Xu Y, Hua YY, Ma Q, Zhou LL, et al. Prenatal, perinatal and neonatal risk factors for perinatal arterial ischaemic stroke: asystematic review and meta-analysis. Eur J Neurol. 2017; 24: 1006- 1015.
- Saliba E, Debillon T, Auvin S, Baud O, Biran V, Chabernaud JL, et al. Neonatal arterial ischemic stroke: Review of the current guidelines. Arch Pediatr. 2017; 24: 180-188.
- Katona F. Complex investigation of impaired brain function during the first postnatal months ACTA PAEDIATRICA ACADEMIAE SCIENTIARUM HUNGARICAE. 1981; 22: 147-164.
- Katona F. An orienting diagnostic system in neonatal and infantile neurology ACTA PAEDIATRICA ACADEMIAE SCIENTIARUM HUNGARICAE. 1983; 24: 299-314.
- Katona F. Developmental Clinical Neurology and Neurohabilitation in the Secondary Prevention of Pre- and Perinatal Injuries of the Brain. In Early identification of infants with developmental disabilities. Grune& Stratton. 1988; 121-144.
- Luo L, Chen D, Qu Y, Wu J, Li X, Mu D. Association between hypoxia and perinatal arterial ischemic stroke: a meta-analysis. PLoS One. 2014; 9: e90106.
- Mercuri E, Barnett A, Rutherford M, Guzzetta A, Haataja L, Cioni G, et al. Neonatal cerebral infarction and neuromotor outcome at school age. Pediatrics. 2004; 113: 95-100.
- Golomb MR, Garg BP, Saha C, Azzouz F, Williams LS. Cerebral palsy after perinatal arterial ischemic stroke. J Child Neurol. 2008; 23: 279- 286.
- Lee CC, Lin JJ, Lin KL, Lim WH, Hsu KH, Hsu JF, et al. Clinical Manifestations, Outcomes, and Etiologies of Perinatal Stroke in Taiwan: Comparisons between Ischemic, and Hemorrhagic Stroke Based on 10-year Experience in A Single Institute. Pediatr Neonatol. 2017; 58: 270-277.
- Vojcek E, Jermendy A, Laszlo A, Graf R, Rudas G, Berenyi M, et al. The role of brain territorial involvement and infection/inflammation in the long-term outcome of neonates with arterial ischemic stroke: A population-based cohort study. Early Hum Dev. 2017; 158: 105393.
- Vojcek E, Graf L, Laszlo A, Gyebnar G, Seri I. Long-term neurodevelopmental outcome of neonates born at term with perinatal haemorrhagic stroke: A population-based study. Dev Med Child Neurol. 2022; 64: 971-9788.
- Vuillerot C, Marret S, Dinomais M. Long term outcome pf perinatal stroke. Arch Pediatr. 2017; 24: 9S51-9S60.