Phenotypes of Asthma, Asthma Severity and Hospitalization in Children Attending a Respiratory Clinic at a Tertiary Hospital
- 1. Department of Child and Adolescent Health Unit, University of Zimbabwe, Zimbabwe
- 2. Department of Family Medicine, Global and Public Health Unit, University of Zimbabwe, Zimbabwe
- 3. Asthma, Allergy and Immune Dysfunction Clinic, Zimbabwe
Abstract
Objective: To describe the phenotypes of asthma, asthma severity and hospitalization in children presenting to the children’s respiratory clinic at a tertiary hospital
Methods: A hospital based, 6month longitudinal study was conducted among asthmatic children aged 3 months to 18 years attending a respiratory out-patients clinic at Parirenyatwa, a tertiary hospital in Zimbabwe. A structured pretested questionnaire was used to collect demographic data, phenotypes, asthma control and frequency of hospitalization. Data was analysed using Statistical Software Package for Social Sciences (SPSS-16.0)
Results: A total of 102 asthmatic patients were enrolled, of these 56(55%) were males. The age of the study participants ranged from 3 months to 18 years, with a median of 4years (Q1=2.75; Q3=6). Of the study participants 90 (88%) had early onset asthma, 19(19%) had cough variant asthma, 34(33%) had peripheral blood eosinophilia while 17(17%) had neutrophilia. Most of the study participants had allergic comorbidities: 61(60%) had allergic rhinitis, 56(55%) had atopic dermatitis, 22(22%) had allergic conjunctivitis and some had a combination of these. HIV infection was present in 3 patients and 4 of the study participants were ex pre-term. Sixty patients (59%) were sensitized to aeroallergens of these 48 were polysensitized. The most common sensitizations were to D. pteronyssinus 44(43%), D. farinae 42(41%), Bermuda grass 26(26%), Timothy grass 23(23%) and A.siro 20(20%). Eighty-six (84%) of the study population were hospitalised at least once. There was a significant association between peripheral blood eosinophilia or neutrophilia with poor asthma control and hospitalization.
Conclusion: Most of the study participants had early onset asthma. Sensitization to aeroallergens and allergic comorbidities were common among the study participants. The presence of peripheral neutrophilia or eosinophilia were independently associated with poor asthma control and hospitalization. Asthmatic children should have their phenotypes characterized to guide patient tailored management.
Keywords
Asthma; Asthma phenotypes; Sensitization; Asthma control; Hospitalization
Citation
Magwenzi P, Rusakaniko S, Gumbo FZ, Sibanda EN. Phenotypes of Asthma, Asthma Severity and Hospitalization in Children Attending a Respiratory Clinic at a Tertiary Hospital. Ann Pediatr Child Health 2023; 11(1): 1297.
INTRODUCTION
Asthma is the most common chronic respiratory tract disease in children below 18 years of age. An estimated 262 million people were affected by asthma in 2019, with 455 000 deaths. Most of these deaths occur in low- and lower-middle income countries where underdiagnosis and undertreatment are a problem [1]. According to the Global Asthma Report of 2022, the overall prevalence of reported asthma symptoms was 9.1% in children, 11% in adolescents and 6.6% in adults [2]. Phase 111 ISAAC data from Africa suggest that the prevalence of childhood asthma as defined by symptoms in the previous one year, ranges from 4 to 21.5% in children aged 13-14 years in the various African countries [3]. The highest prevalence of asthma in Sub Saharan Africa (SSA); (21.3%) as measured by self-reported current wheeze has been reported in South African adolescents [4]. Time trends in the prevalence of reported asthma symptoms in children below 15 years of age in low to middle income countries (LMIC) have shown an increase from 12.1% in 1990 to 13.9% in 2010 [5]. The main cause of this increase is increased urbanization with the associated pollution [6]. The prevalence of reversible airway obstruction in rural and urban Zimbabwean school children in 1991 was reported to be 0.1 % and 5.8% respectively [7].
The Global Initiative for Asthma (GINA) 2019 guidelines define asthma as a “heterogeneous disease characterized by chronic airway inflammation with a history of respiratory symptoms such as wheeze, shortness of breath, chest tightness and cough that vary over time and in intensity, together with variable expiratory airflow limitation.”. Historically all patients with asthma have been described under one umbrella diagnosis as ‘asthma’. Under this definition, asthma is known as an atopic disease, involving allergen exposure, IgE-mediated sensitization and eosinophilic airways inflammation, resulting in clinical symptoms [8]. This definition is increasingly being challenged and disproved. The past two decades have seen major strides in describing the various clinical presentations of asthma, these are called phenotypes with underlying immune-patho-genetic mechanisms called endotypes [9].
Children in LMIC including Zimbabwe tend to have severe asthma symptoms [10,11]. The reason for this is not well described and may be due to use of solid fuel, asthma underdiagnosis and strained health systems with inadequate asthma care [12]. The phenotypes and endotypes of these children remain largely unknown. Knowledge of these is important since they may explain the disease severity that has been documented in children in LMIC. Targeted therapy can then be informed by the underlying phenotypes and endotypes.
This study describes the phenotypes of the asthma, asthma severity and hospitalization over a six-month period in children attending a respiratory clinic.
MATERIALS AND METHODS
Study type and study setting
An observational longitudinal study over 6 months was conducted at Parirenyatwa Hospital Children’s Respiratory Clinic in Harare, Zimbabwe. Study participants were enrolled for one year commencing July 2020. The respiratory clinic is a once weekly out-patients clinic run by paediatricians. It provides tertiary level care to children referred for respiratory symptoms from over 17 primary care clinics in urban Harare and from district and provincial hospitals.
Study Population, eligibility
Asthmatic patients aged from 3 months to 18 years presenting to the children’s respiratory clinic who gave assent and whose parents or caregivers gave informed written consent were enrolled into the study. The diagnosis of asthma was based on the Asthma Predictive Index (API) 2000 [13,14], for children less than 5 years and the Global Initiative for Asthma (GINA) 2020 guidelines for children above 5years [15,16]. Patients presenting to the respiratory clinic commonly present with a variety of respiratory symptoms including persistent, recurrent, or intermittent cough, noisy breathing, wheezing, and difficulty breathing. Patients with chronic heart disease, and chest, abdominal or skeletal surgery in the past one year were excluded from the study because of the confounding effect of these on respiratory disease and lung function assessments.
Data Collection tool
The data was collected using an investigator administered electronic pre-defined structured questionnaire and laboratory data collection tool, REDCap. The ISAAC symptom-based questionnaire was adapted into this tool to identify participants with atopy (allergic conjunctivitis, rhinitis, and atopic dermatitis/ eczema).
Study procedures
Study participants were followed up for 6 months. The schedule of follow-up visits (at enrolment, 2 weeks, 1 month, 3 months and 6 months) were explained to the study participants and their caregivers. Study participants who failed to visit the clinic on a scheduled date of follow up were reminded on the day through a telephone call and were rescheduled the next week and reminded again on future scheduled visits. Asthmatic children were given standard treatment according to the step wise management of asthma (GINA 2020 guidelines) including asthma education and asthma action plan. Inhaler-spacer-facemask technique was taught, assessed, and reinforced. Adherence was checked for and reinforced at each visit. Children needing hospitalization were admitted to the children’s medical wards and their follow-up while in hospital was recorded.
The study procedures during each visit are summarised in Table 1.
| Visit | Clinical assessment | Laboratory assessment |
| Screening visit |
History: presenting symptoms Adherence checked Asthma Control Test (ACT) Examination: (oxygen saturation (SpO2), respiratory rate, temperature pulse, weight, height, chest auscultation, cardiovascular exam, abdomen, skin examination/atopy, skeletal, genitourinary system) Asthma education Inhaler technique emphasized Asthma Action plan |
Phenotype markers Full Blood count (Including total eosinophil and neutrophil count and percentages), Serum specific IgE for inhalant allergens Spirometry: FVC, FEV1, and reversibility testing for children above 5 years Comorbidities screening as guided by history Biomarker profile (IL-2, IL-4, IL-6, IL-10, IL-17A, IFN γ and TNF-alpha) |
| Follow-up (2 weeks, 1 month and 3 months) |
History ACT Adherence checked Hospitalization checked Examination Asthma education Inhaler technique emphasized Asthma Action plan |
|
| Exit visit (6 months) |
History ACT Adherence checked Hospitalization checked Examination Asthma education Inhaler technique emphasized Asthma Action plan |
Biomarker profile (IL-2, IL-4, IL-6, IL-10, IL-17A, IFN γ and TNF-alpha) |
Collection of blood
At enrolment into the study, blood was drawn from study participants and added to an ethylene diamine tetra-acetic acid (EDTA) tube for full blood count (including eosinophil and neutrophil count and percentages) as well a standard serum separation tube to identify sensitization to inhaled allergens (aeroallergens). Blood for full blood count was analysed and reported on the day it was drawn, while serum for allergen specific Immunoglobulin E (sIgE) and cytokine analysis was stored at -50o C for batched analysis.
Sensitization
The Mediterranean Inhalation (IgE) EUROLINE test kit was used for semiquantitative determination of sIgE to any of 20 aeroallergens Bermuda grass, Timothy grass, birch, hazel, olive, plane tree, cypress, privet, Common (C) ragweed, mugwort, English (E.) plantain, wall pellitory, Dermatophaigodes pteronyssinus (D.pteronyssinus), Dermatophaigodes farinae (D.farinae), Acarus (A) siro, cat, dog, horse, Cladosporium (C.) herbarum and Alternaria (A.) alternata. This kit tests for sIgE to whole allergen extracts of region-specific profile of 20 inhalant allergens grouped into tree and grass pollens, house-dust-mite and flour-mite proteins, animal epithelium/dander and yeast/ mould.
Spirometry
Spirometry was done on participants who had no contraindications and could reliably perform this test ~5 years and above according to American Thoracic Society /European Respiratory Society 2005 guidelines and predicted values were based on Global Lung function Initiative (GLI) ethnicity adjusted references. Portable ultrasonic spirometers were used. A calibration check was done before testing. The participants were tested in the sitting position and for each participant 3 reproducible forced expirations that met the acceptability and repeatability criteria were recorded. The best test was used in the analysis. Forced Expiratory Volume in 1 second (FEV1 ), Forced Vital Capacity (FVC), Forced Expiratory Flow between the 25% and 75% of FVC (FEF25-75) were recorded for each participant and adjusted for age, sex, height, and ethnicity. Reversibility was tested using 200 micrograms of salbutamol via a metered dose inhaler (MDI) and spacer device with breath holding for 5-10 seconds and retesting after 30 minutes and was scored as positive if there is an improvement in FEV1 by more than 12%.
Definitions
An asthmatic meant a study participant whose diagnosis of asthma was based on the Asthma Predictive Index (API) 2000 for children less than 5 years and the Global Initiative for Asthma (GINA) 2020 guidelines for children above 5years.
Newly diagnosed asthmatic: A study participant who had their asthma diagnosed during the study period and was not on treatment at enrolment.
Known asthmatic: A study participant who had their asthma diagnosed prior to enrolment into the study and was already on treatment at enrolment. For these participants the diagnosis of asthma was confirmed using API 2000 and GINA 2020 guidelines.
Age of onset of asthma Study participants were divided into two groups based on the age of onset of asthma symptoms.
i) Those whose onset of asthma symptoms was at or before 5 years of age.
ii) Those whose onset of asthma symptoms was after 5 years of age.
Adherence
The adherence levels were assessed during each visit and scored on a scale of 1 to 4 starting at week 2 of enrolment. A score of 1 meant the patient had only 1 out of the four visits of adherence greater than 95% while a score of 4 meant the patient had been adherent throughout the four visits.
Asthma severity was measured using the 6-point asthma control test (ACT) for patients who had been on treatment for more than 2 weeks.
Sensitization A patient was said to be sensitized to a particular allergen if the serum specific Immunoglobulin class E (sIgE) was ≥ 0.35kU/L. Monosensitization meant sensitization (as confirmed by sIgE) to one aeroallergen while polysensitization meant sensitization to two or more aeroallergens [17].
Ethical considerations
Ethical approval was obtained from the Parirenyatwa Hospital Institutional Review Board (IRB), Joint Research Ethical Committee (JREC) and Medical Research Council of Zimbabwe (MRCZ) number MRCZ/A/2562. Study participants provided informed assent and informed written consent was obtained from the primary caregiver. Patient confidentiality was observed by coding the data collection tool.
Safety
The investigators captured any adverse events attributable to the study procedures such as pain following blood draw or feeling faint following spirometry.
Data Analysis
Data was uploaded onto a REDCap server and exported into excel and then analysed using Statistical Software Package for Social Sciences (SPSS-16.0) for descriptive statistics. Bivariate analysis (Pearson Chi Square test and Fishers exact test) was used to test for associations between phenotype and asthma control and phenotype and hospitalisation. Multivariate logistic regression was used to test for association between phenotype and asthma control and phenotype and hospitalisation after factoring in confounding variables.
RESULTS
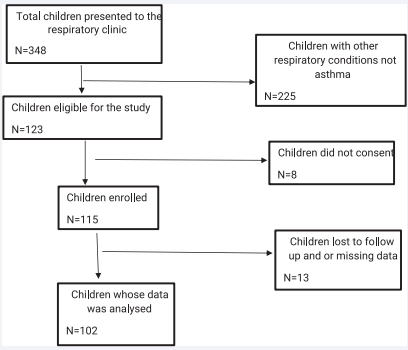
A total of 348 children presented to the respiratory clinic in the period July 2020 to June 2021. Figure 1 summarises the flow of patients in the study.
Figure 1: Flow diagram of patient flow
Demography
The age of the study participants ranged from 3 months to 18 years, with a median of 4 years (Q1=2.75; Q3=6). There were 56(55%) males and 46(45%) females.
Clinical Profiles (Phenotypes)
Age at onset of asthma symptoms: Of the study population 90 (88%) had early onset of asthma symptoms (at or before five years) and 12 (12%) had onset asthma symptoms after 5 years of age. There was no association between the age of onset of asthma symptoms and asthma control or hospitalization.
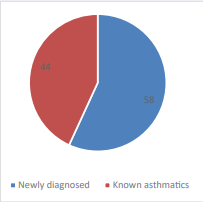
Age at asthma diagnosis: The median age at asthma diagnosis was 3 years (Q1=0, Q3=6). Figure 2 illustrates the proportion of the study population that were newly diagnosed to those that were known asthmatics and already on treatment at enrolment into the study.
Of the patients that were newly diagnosed during the study period, 17(29%) had intermittent asthma while 41 (71%) had persistent asthma. There was no significant association between the asthma severity at diagnosis and asthma control or hospitalization.
Figure 2: Proportion of newly diagnosed to known asthmatics.
Of the patients that were known asthmatics and already on treatment at entry into the study, 2 were well controlled and 42 were not well controlled. Forty-one (93%) of the 44 known asthmatics were on the correct step of asthma management according to GINA guidelines. However only 27(61%) had continuous supply of inhaled corticosteroid in the previous month and 19(43%) were using spacer/facemask device. There was a significant association between the asthma control at entry into the study and hospitalization. (p-value 0.000 OR 10.50). Patients who were not well controlled at entry into the study were 10 times more likely to be hospitalized at least once during the six-month follow-up.
| Asthma control for known asthmatics (n=44) | Frequency (Percentage) |
| Well controlled | 2 |
| Partly controlled | 23 (52%) |
| Poorly controlled | 19 (43%) |
Asthma control of the patients who were known asthmatics at enrolment is shown in Table 2.
Predominant symptoms
Of the study population; 83(81%) were predominantly wheezers while 19(19%) had cough variant asthma. There was no association between the predominant symptoms with either asthma control or hospitalization
Comorbidities
The co-morbidities that were present in the study participants are shown Table 3. Of note is some had a combination of these. There was no association between any of the comorbidities with asthma control or hospitalization.
| Co-morbidity | Frequency (Percentage) |
| Allergic rhinitis | 61 (60%) |
| Atopic dermatitis | 56 (55%) |
| Allergic conjunctivitis | 22 (22%) |
| Ex prematurity | 4 |
| HIV Infection | 3 |
| Obstructive sleep apnoea | 2 |
| Obesity | 2 |
Nutritional status
Using the WHO Z score for weight-for-height (WHZ), 100 of the study population were normal (WHZ between -2SD and +2SD). However, 2 patients had weight for age z-score (WAZ) above +3SD suggesting obesity.
Adherence
Of the study population 94% were adherent throughout the study.
Peripheral blood white cell differential count
A total of 34 patients had peripheral blood eosinophilia while 17 patients had neutrophilia. There was a significant association between peripheral blood eosinophilia and asthma control (p value 0.049 OR 0.43). There was also a significant association between peripheral blood eosinophilia and hospitalization (p value 0.012 OR 0.11).
There was significant association between peripheral blood neutrophilia and asthma control (p value 0.001 OR 0.129). There was also a significant association between peripheral blood neutrophilia and hospitalization (p value 0.041 OR 1.23). Asthmatic patients that had peripheral blood neutrophilia were more likely to be hospitalized.
Sensitization to inhalant allergens
Of the study participants 60(59%) were sensitized to one or more of 20 common aeroallergens. Most patients were sensitized to D. pteronyssinus 44(43%) and D. farinae 42(41%), Bermuda grass 26(25.5%), Timothy grass 23(22.5%) and A.siro 20(20%). Forty-eight (47%) of the patients were polysensitized mainly to house-dust mite variants: D. pteronyssinus, D. farinae, grasses: Bermuda grass and Timothy grass. Sensitization to any one aeroallergen was significantly associated with having comorbid allergic rhinitis (p value 0.016).
Spirometry
A total of 37 patients were eligible for and had spirometry. FEV1 ranged from 62% to 83%, the median was 75 (Q1=68.5, Q3=79). 5 patients had FEV1 greater than 80%, these patients were on asthma treatment ie. Inhaled corticosteroids.
The ratio of FEV1 to FVC ranged from 63 to 90%, with a median of 74% (Q1=72, Q3=78). All 37 patients who performed spirometry had >12% reversibility defining their diagnosis of asthma.
Level of asthma control
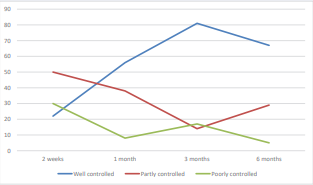
Figure 3: Asthma control of the study participants over the six month follow up
The level of asthma control of the study participants at 2 weeks, 1 month, 3 months and 6 months of follow up is shown in Figure 3. There was a significant association between asthma control at 1 month and hospitalization (p value 0.004 OR 7.33). Patients who were not well controlled were 7 times more likely to be hospitalized at least once during the study period.
Hospitalization
| Number of hospitalizations over 6-month follow-up period | Frequency |
| None | 16(16) |
| 1 | 42(41) |
| 2 | 31(30) |
| 3 and more | 13(13) |
Of the study population (86)84% of the study population were hospitalized at least once during the follow-up period as shown in Table 4.
| Variable | Category | Well controlled asthma | Not well controlled | O.R(95%CI) | p-value |
| N=56(55%) | N=46(45%) | ||||
| Gender | Male | 29(52) | 27(59) | 0.76(0.34-1.66) | 0.485 |
| Female | 27(48) | 19(41) | |||
| Age at onset of symptoms | -- | 50(89) | 40(87) | 1.25(0.37-4.17) | 0.716 |
| >5 years | 6(11) | 6(13) | |||
| Asthma severity at diagnosis | Intermittent | 12(40) | 5(18) | 3.07(0.91-10.30) | 0.064 |
| Persistent | 18(60) | 23(82) | |||
| Asthma control at study entry | Well controlled | 1(4) | 1(6) | 0.68(0.04-11.63) | 0.789 |
| Not well controlled | 25(96) | 17(94) | |||
| Allergic Rhinitis | Yes | 33(59) | 28(61) | 0.92(0.42-2.05) | 0.842 |
| No | 23(41) | 18(39) | |||
| Atopic Dermatitis | Yes | 31(55) | 25(54) | 1.04(0.48-2.28) | 0.919 |
| No | 25(45) | 21(46) | |||
| Allergic Conjunctivitis | Yes | 12(21) | 10(22) | 0.98(0.38-2.53) | 0.970 |
| No | 44(79) | 36(78) | |||
| Peripheral Neutrophilia | Yes | 3(5) | 14(30) | 0.13(0.03-0.49) | 0.001* |
| No | 53(95) | 32(70) | |||
| Peripheral eosinophilia | Yes | 14(25) | 20(44) | 0.43(0.19-1.00) | 0.049* |
| No | 42(75) | 26(56) | |||
| Serum specific IgE(sensitized) | Yes | 34(61) | 26(57) | 1.189(0.54-2.63) | 0.669 |
| No | 22(39) | 20(43) |
The relation between clinical characteristics and asthma control at 1 month into the study and hospitalization are shown in Table 5 and 6.
| Variable | Category | No hospitalization during study period | Hospitalized at least once during study period | O.R | p-value |
| N=16(16%) | N=86(84%) | ||||
| Gender | Male | 9(56) | 47(55) | 1.07(0.36-3.13) | 0.906 |
| Female | 7(44) | 39(45) | |||
| Age at onset of symptoms | -- | 15(94) | 75(87) | 2.20(0.26-18.34) | 0.456 |
| >5 years | 1(6) | 11(13) | |||
| Asthma severity at diagnosis | Intermittent | 4(40) | 13(27) | 1.80(0.44-7.40) | 0.414 |
| Persistent | 6(60) | 35(73) | |||
| Asthma control at study entry | Well controlled | 2(33) | 0 | 10.50(4.13-26.67) | 0.000* |
| Not well controlled | 4(67) | 38(100) | |||
| Allergic Rhinitis | Yes | 7(44) | 54(63) | 0.46(0.16-1.36)) | 0.154 |
| No | 9(56) | 32(37) | |||
| Atopic Dermatitis | Yes | 8(50) | 48(56) | 0.79(0.27-2.30) | 0.668 |
| No | 8(50) | 38(44) | |||
| Allergic Conjunctivitis | Yes | 2(13) | 20(23) | 0.47(0.10-2.25) | 0.337 |
| No | 14(87) | 66(77) | |||
| Peripheral Neutrophilia | Yes | 0(0) | 17(20) | 1.23(1.11-1.37) | 0.041* |
| No | 16(100) | 69(80) | |||
| Peripheral eosinophilia | Yes | 1(6) | 33(38) | 0.11(0.01-0.85) | 0.012* |
| No | 15(94) | 53(62) | |||
| Allergen specific IgE(sensitized) | Yes | 12(75) | 48(56) | 2.38(0.71-7.96) | 0.152 |
| No | 4(25) | 38(44) | |||
| Well controlled at 1 month | Yes | 14(88) | 42(49) | 7.33(1.57-34.23) | 0.004* |
| No | 2(12) | 44(51) |
DISCUSSION
Age of onset of asthma
Most of the study participants had onset of asthma symptoms before 5 years of age with only a tenth after 5 years of age. This is in keeping with prevalence data that report that 60 to 80% of patients with asthma have symptoms before 5 years of age [18,19]. Most clinicians are faced with a diagnostic dilemma for the preschool child who presents with recurrent wheezing. Several wheezing disorders have been described in this age group including multiple trigger wheeze and viral induced wheezing. Other authors have used one umbrella term for recurrent wheezing in this age group i.e., preschool wheeze.
Research and health systems need to be strengthened to enhance diagnosis and management of patients in this age group. The use of evaluation and diagnostic algorithms with diagnostic terms such as ‘probable asthma’ should be used for asthma diagnosis in children below 5 years. The diagnostic evaluation will include the assessment for the phenotypes described in this study. In this way the patient will benefit from a therapeutic trial of inhaled steroids (preventers) and inhaled B2 agonists (relievers) while objective diagnostic re-evaluation with spirometry can then be done at 5 to 6 years.
Asthma severity
Of the 58 patients whose asthma diagnosis was made during the study period, 71% had persistent asthma. This is in keeping with studies stating that children of African ancestry tend to have severe asthma [11,20]. The reason for this has not been well described with some authors attributing this to genetics, use of solid fuel and lack of early diagnosis and care [21].
On the other hand, of the 44 patients who were ‘known asthmatics’ and already on treatment upon entry into the study, 42(95%) were not well controlled, these patients were 10 times more likely to be hospitalized. While most of these children had been on the correct step of asthma management, they either had no access to inhaled corticosteroids to prevent exacerbations or they were not using a spacer device with the inhaler. It is therefore essential that all asthmatics be assessed for asthma severity at diagnosis and on each follow up visit in-order to place the patients on the appropriate step of management. Availability of inhaled corticosteroid and spacer/facemasks need to be strengthened. In addition, adherence counselling, correct inhalerspacer technique needs to be emphasized at all follow-up visits.
Comorbidities
Most patients had one or more co-morbidities with the commonest being allergic rhinitis. The presence of comorbidities has been reported to worsen asthma symptoms and result in poor asthma control by several studies [22,23]. Addressing co-morbidities has been shown to improve asthma control in children [24]. However, in this study this relationship was not significant although there was a significant association between sensitization and having allergic rhinitis. The relevance of exprematurity and asthma is an area that needs further exploration.
Nutritional status
Ninety-nine percent of the study population were well nourished, 2 were obese and none were undernourished. The presence of obesity is associated with obstructive sleep apnoea as reported in a study done in children [23,25]. The presence of obesity has also been associated with severe asthma in both children and adults [26,27]. However, in this study there were only two obese patients and analysis for association with asthma severity or hospitalization could not be done.
Adherence
Adherence levels improved over the study period underscoring the importance of follow-up visits and adherence counselling. In several studies for chronic non-communicable diseases, adherence has been found to be an important factor for good outcomes [28], asthma adherence counselling has been found to increase both adherence and improve asthma control [29]. In addition asthma action planning has also been found to have similar outcomes in a systematic review done by Zemek et al. [30].
Peripheral blood neutrophilia
In this study, patients who had peripheral blood neutrophilia were more likely to have poor asthma control or to be hospitalized. The presence of bronchoalveolar lavage fluid neutrophils (neutrophilic asthma) in patients with asthma has been reported by several studies [31-33]. This has been associated with poor asthma control [31,33]. However, the relationship between peripheral blood neutrophilia and asthma severity has not been clearly defined. The cause and role of neutrophilia in severe asthma is not clear. Steroids are known to enhance neutrophil survival hence may worsen asthma. BAL neutrophilia is thought to be associated with subclinical Mycoplasma Pneumonia and Chlamydophila Pneumoniae. At present there is no treatment to target neutrophilic asthma. Some authors have reported benefit in using macrolides to manage patients with neutrophilic asthma [34]. Randomized control trials in adults; TELICAST and AZALEA showed benefit of using telithromycin (for 26 weeks at 800mg once a day) and azithromycin (for 48weeks at 250mg 3times a week) [35,36].
While the strict definition for neutrophilic asthma is based on BAL neutrophilia, peripheral blood neutrophilia was found to correlate strongly with broncho-alveolar lavage (BAL) fluid (37). In low resourced settings where BAL may not be done routinely because of cost and technical difficulty in children, peripheral blood neutrophilia therefore needs to be done in phenotyping asthmatic patients since this allows the clinician to closely monitor and step-up asthma medication accordingly.
Peripheral blood eosinophilia
As with peripheral neutrophilia, patients with eosinophilia were more likely to have poor asthma control or hospitalization. However in a study on 1475 children; eosinophilia was not found to correlate with asthma severity [39].
Sensitization
In this study two thirds of the study participants were sensitized to one or more aeroallergens which emphasizes the need for education on their avoidance as well as adherence to preventer inhaled corticosteroids. Asthmatic patients that were sensitized were more likely to have comorbid allergic rhinitis. It is important to test for sensitization to aeroallergens in patients with asthma since these trigger acute asthma and result in repeated presentation to the emergency department. In this era of precision medicine, polysensitization especially in the presence of allergic comorbidities may need to be treated with single allergen or multi-allergen immunotherapy (AIT) i.e., monoclonal antibody targeting IgE e.g. omalizumab [45].
Level of asthma control
In this study, the level of asthma control one month into the study was a predictor of hospitalization. Over the study period the number of patients who were well controlled increased, this may be explained by adherence counselling and education on correct inhaler use, asthma action plan as well as assured supply of inhaled corticosteroids. In a systematic review done by Garcia et al; young age less than 5 years, lower socioeconomic status, Afro American ancestry, previous exacerbation and ED visit and coexisting allergic diseases i.e. allergic rhinitis, conjunctivitis and dermatitis were associated with poor asthma control and hospitalization [40].
Hospitalization
Ninety-four percent of the study patients were hospitalized at least once. Children with asthma should have a normal life, attend school participate in sport. In this study the independent predictors of hospitalization were poor asthma control, peripheral neutrophilia and peripheral eosinophilia. Based on these findings, it is the recommendation of this study that asthmatic patients should have their phenotype described, have an asthma control assessment at every visit as these will guide the stepwise management of asthma and hence prevent hospitalization.
Asthma phenotypes: relevance to clinical practice
Globally the shift in asthma diagnosis and care has been towards precise diagnosis with classification of patients according to their clinical and laboratory characteristics (phenotypes) and according to their immuno-patho-genetic mechanisms (endotypes) [9]. This guides patient tailored therapy including use of biologics (precision medicine). Several phenotypes have been described by different authors including allergic/nonallergic, early onset/childhood onset/adolescent onset asthma, exercise induced, preschool wheeze etc [41]. No consensus has been reached on how to standardize the classification of asthma phenotypes in-order to make clinical relevance. In addition, literature has described that the phenotypes are not static [41].
In this study 6 distinct phenotypes were described 1. According to age of onset (onset before 5 years, onset after 5 years) 2. According to peripheral white cell differential (peripheral blood neutrophilia/ peripheral blood eosinophilia), 3. According to sensitization patterns (monosensitized / polysensitized to) 4. According to the presence of co-morbidities 5. According to the level of asthma control and 6. According to the frequency of hospitalization. These phenotype groups are not mutually exclusive and can be further divided into phenotype clusters e.g. ‘A patient with early onset, persistent asthma, who has peripheral blood neutrophilia, allergic rhinitis and atopic dermatitis who is sensitized to D. farinae and is poorly controlled with 3 hospitalizations in the past six months’ describes the patient fully rather than ‘an asthma patient.’ In addition, phenotyping gives guidance on tailoring the medication accordingly.
CONCLUSIONS
Most of the study participants had early onset asthma. Allergic comorbidities and sensitization to aeroallergens and were common among the study participants. The presence of peripheral neutrophilia or eosinophilia were independently associated with poor asthma control and hospitalization. Asthmatic children should have their phenotypes characterized to guide patient tailored management. Timely follow-up of asthmatic patients improves asthma control through the asthma education offered on adherence, correct inhaler technique and asthma action plans.
FUNDING
This study was funded by TIBA which is supporting PM for her fieldwork on ‘Towards improving asthma diagnosis in children.’ PM is a TIBA fellow.
AUTHORS’ CONTRIBUTIONS
PM conceived the idea. PM drafted the protocol. Improvements to the protocol were done by FZG, SR and ENS. PM wrote the first draft of the manuscript and all authors (FZG, SR and ENS) made revisions. The final manuscript was approved for submission to the journal by all authors after reading.
ACKNOWLEDGEMENTS
We would like to acknowledge the Child and Adolescent Health Unit, College of Health Sciences, University of Zimbabwe, Dr Kandawasvika, Nurse Dingiswayo, Dr Ndarukwa, TIBA, the outpatient’s department staff at Parirenyatwa hospital, study participants and their caregivers who all made this study a success.
REFERENCES
2. The Global Asthma Report 2022. 2022.
15. GINA-2020-report_20_06_04-1-wms.pdf. 2021.
16. Ish P, Malhotra N, Gupta N. GINA 2020: what’s new and why? J Asthma. 2021; 58: 1273-1277.
27. Tashiro H, Shore SA. Obesity and severe asthma. Allergol Int. 2019; 68: 135-142.
34. At-risk children with asthma (ARC): a systematic review | Thorax [Internet]. 2021.
37. Yamasaki A, Okazaki R, Harada T. Neutrophils and Asthma. Diagnostics. 2022; 12: 1175.