Success of High-Flow Nasal Cannula (HFNC) as Primary Support for Acute Viral Bronchiolitis: A Longitudinal Study
- 1. Rio de Janeiro Hospital, Brazil
- 2. D’or Institute for Research and Education, Brazil
- 3. National institute of health for women, children and adolescents Fernandes Figueira / Fiocruz, Brazil
- 4. Unimed Hospital, Brazil
Abstract
Acute viral bronchiolitis (AVB) is the most common lower respiratory tract infection in the pediatric population, characterized by self-limited evolution and variable severity. Among the support alternatives, high-flow nasal cannula (HFNC) is indicated for mild to moderate cases due to its physiological effects.
Objectives: To evaluate the success of HFNC in infants diagnosed with AVB and describe the evolution of vital signs after support installation.
Methods: Retrospective longitudinal study. Infants diagnosed with AVB who used HFNC as primary support over a 30-month period were selected. Data related to HFNC, vital signs, and clinical outcomes were collected. HFNC success was defined as no replacement of therapy by mechanical ventilation. Data were analyzed by male/female patient groups and HFNC success/failure.
Results: 151 cases of AVB using HFNC during the period. Of these, 57% were male; median age was 6 months; weight 8kg; incidence of RSV infection 35%, and associated pneumonia 23%. Vital signs improved from the first reassessment, and within 24 hours, they were normalized for age. The median duration of HFNC use was 4 days, and the hospitalization period was 8 days. HFNC success rate was 75%.
Conclusion: HFNC achieved a high success rate for ventilatory rescue in moderate bronchiolitis, with a positive clinical response observed within the first few hours of therapy. Moreover, within 48 hours of support, patients showed parameters considered readiness for weaning.
Keywords
• Bronchiolitis
• Oxygen therapy
• Respiratory failure
CITATION
Cássio Daniel Silva A, Roberta Monteiro B, Larissa Guarany S, Rebeca Costa F, Guilherme de Souza CB, et al. (2024) Success of High-Flow Nasal Cannula (HFNC) as Primary Support for Acute Viral Bronchiolitis: A Longitudinal Study. Ann Pediatr Child Health 12(3): 1340
INTRODUCTION
Acute viral bronchiolitis (AVB) is the most common lower respiratory tract infection in the pediatric population up to two years of age, primarily caused by respiratory syncytial virus (RSV), with a self-limited evolution of symptoms and a median duration of 7 to 14 days [1-3]. Respiratory dysfunction in AVB results from cellular destruction of the ciliated epithelium of the lower airways, leading to hypersecretion and bronchiolar obstruction with microatelectasis formation; in severe cases, dynamic pulmonary hyperinflation further exacerbates ventilation disturbance, potentially causing hypoxemia and increasing ventilatory effort requiring escalated ventilatory support [1,4,5].
Generally, treatment consists of supportive therapy aimed at hydration, airway care, and rigorous monitoring [1-3]. Among the support alternatives for these patients, high-flow nasal cannula (HFNC) is described as a possible first-line option for mild to moderate cases of AVB, being a non-invasive and easily manageable therapy that allows for feeding and reduces metabolic expenditure, with ample evidence of its efficacy in obstructive respiratory conditions in general [1,2,6,7]. More specifically, it is postulated that the increment of heated and humidified high gas flow is capable of “washing out” the anatomical dead space of the upper airways, thereby reducing resistance and optimizing mucociliary function by maintaining adequate epithelial temperature, in addition to generating some improvement in functional residual capacity [6-8]. Thus, the objective of the present study was to describe the use of HFNC in a population of infants diagnosed with acute viral bronchiolitis, evaluating its success and the evolution of vital signs after support installation.
MATERIALS AND METHODS
Study Design and Population
This was a retrospective longitudinal study approved by the Human Research Ethics Committee of the D’Or Institute for Research and Education (approval number: 6.067.468), conducted in the pediatric units (Emergency, Intermediate Care, and Intensive Care Unit) of a private tertiary hospital in the West Zone of Rio de Janeiro (RJ), Brazil. Initially, all infants aged 0 to 24 months diagnosed with acute viral bronchiolitis who used high-flow nasal cannula (HFNC) as primary ventilatory support (ventilatory rescue aiming to avoid escalation to positive pressure therapy) during hospitalization from January 2021 to July 2023 were selected. Patients who did not adapt to the support within 30 minutes, making therapy impossible due to psychomotor agitation or interface refusal (transitioned to low- flow oxygen therapy or non-invasive ventilation as indicated and tolerated), were subsequently excluded. Patients with incomplete or unavailable vital sign reassessment protocols and those who were discharged against medical advice or transferred externally were also excluded.
The hospital where the study was conducted had Airvo® and Optiflow® HFNC equipment, with the choice between them based solely on availability. Additionally, the primary indication for ventilatory rescue with HFNC was based on the Wood-Downes score (WDS) [9], indicating moderate respiratory crisis (4-7 points), with this objective assessment performed at hospital admission and daily by physiotherapy and medical teams. The evaluation for HFNC was protocol-driven, only after initial rescue measures (airway clearance + bronchodilation according to medical prescription) and physiotherapy intervention. The flow rate calculation in the service was as follows: up to 10kg of actual weight, 2 liters of flow per kg; above 10kg, the first 10kg followed the previous formula, while the excess weight was multiplied by 0.5 liters. Finally, therapy began with the maximum flow calculated based on the patient’s weight and the minimum oxygen delivery necessary to maintain peripheral saturation >96%. Weaning from HFNC started with gradual reductions in FiO2 to 21%, followed by halving the flow rate and suspending therapy 12 hours later.
Data Collection
The data used in the study were secondary, extracted from medical records and the institutional protocol for ventilatory support management (Appendix 1). Clinical reassessment was protocol-driven, conducted just before support installation, and subsequently at 30 minutes, 60 minutes, 2 hours, 4 hours, 12 hours, 24 hours, and 48 hours after initiation. Therapy was considered successful in cases where HFNC was not replaced by non-invasive rescue ventilation (NIV), or in cases where NIV was used only preventively, in conjunction with HFNC (intermittently) for a maximum of 6 hours daily. HFNC failure was identified based on clinical criteria such as worsening respiratory pattern associated with deteriorating vital signs and Wood-Downes score corresponding to severe respiratory crisis (>7), in addition to chest radiography showing signs of hypoventilation/atelectasis.
Statistical Analysis
The data were processed using Epi Info® software version 7.2. Descriptive analysis was performed by presenting variables as mean values and standard deviations for normally distributed continuous variables or as median, minimum, and maximum values for non-normally distributed continuous variables. Categorical variables were described using absolute frequencies and percentages.
Unpaired Student’s t-test and Mann-Whitney test were used for comparison between two groups, and ANOVA variance test for three or more groups, based on data distribution (parametric or non-parametric). Exploratory association testing of some variables was conducted using the Mantel-Haenszel Chi-square test, with a significance level set at less than 0.05.
RESULTS
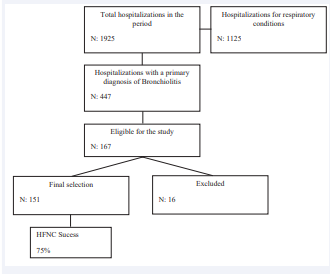
During the study period, there were 1925 admissions to the unit, of which 447 were diagnosed with AVB (Figure 1).
Figure 1: Outlines the steps of population selection for the study.
From the 151 cases of HFNC included in the study, 57% were male patients; median age was 6 months; incidence of RSV infection was 35%, pneumonia was 23%, and viral co-infection was 15%. The success rate of HFNC was 75%, with no statistical difference between males and females. When stratifying cases into success and failure, there was a significant difference in the Wood-Downes score at admission, length of hospital stay, and days on HFNC. Table 1
Table 1: Demographic profile and clinical characterization of the study population stratified in between male/female and success/fail of HFNC.M: male; F: female; S: success; F: fail; RSV: Respiratory Syncytial Virus; Kg: kilograms; Co-infection: presence of two or more simultaneous viruses screened in a viral panel; HFNC: high flow nasal cannula; IQR: interquatile range.
|
|
Total |
M |
F |
p-value |
S |
F |
p-value |
|
Gender |
151 |
57% |
43% |
- |
M:58,5% F: 41,5% |
M: 52,5% F: 47,5% |
0,471 |
|
Age (years) (IQR) |
6 (0-24) |
6 (0-24) |
6 (0-24) |
0,538 |
7 (0-24) |
6 (0-24) |
0,192 |
|
Weight (kg) (IQR) |
8 (3-13,3) |
8,3 (3,5-13) |
7,6 (3-13,3) |
0,067 |
8 (3-13,3) |
7,2 (3,4-13) |
0,077 |
|
RSV |
35% |
38,8% |
40,6% |
0,824 |
64,4% |
35,5% |
0,056 |
|
Pneumonia |
23,1% |
24,1% |
26,5% |
0,735 |
61,4% |
31,5% |
0,429 |
|
Co-infection |
15,2% |
12,6% |
18,7% |
0,303 |
65,2% |
34,7% |
0,340 |
|
Wood-Downes Score on admission (IQR) |
5 (2-10) |
5 (3-8) |
5 (3-8) |
0,888 |
5 (2-8) |
5 (3-10) |
0,035 |
|
Days of disease progression upon admission (IQR) |
4 (1-18) |
4 (1-16) |
4 (2-18) |
- |
4 (1-18) |
3 (1-15) |
- |
|
Days of hospitalization (IQR) |
8 (1-38) |
8 (1-38) |
8 (3-28) |
0,953 |
7 (1-38) |
12 (6-35) |
0,000 |
|
Days of use HFNC (IQR) |
4 (1-24) |
4 (1-15) |
4 (1-12) |
0,209 |
4 (1-12) |
1 (1-15) |
0,000 |
|
HFNC Success |
75% |
75,5% |
70% |
- |
- |
- |
|
presents the demographic profile and clinical characterization of the study population.
The radiological pattern at admission showed typical signs of airway obstruction disease, such as significant pulmonary hyperinflation associated with peri-hilar infiltration. When comparing pre-HFNC and post-24-48 hours HFNC images, a reduction in hyperinflation level and improvement in lung aeration pattern were observed, as illustrated in examples in Appendix 1.
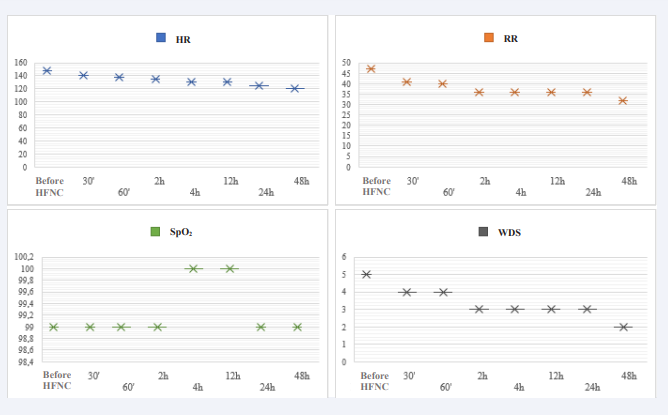
In 6% of cases, intermittent NIV was used, while in 25% of total events, there was HFNC failure and therapy replacement with rescue NIV. The period in which failures occurred after support installation was unspecified: 17% of failures within 2 hours of therapy; 20% between 2 to 6 hours; 20% between 6 to 12 hours; 30% between 12 to 24 hours of therapy. There was an association of HFNC failure with hospitalization time longer than 8 days (p-value 0.000), with an odds ratio of 3.3, corresponding to a 33% increase in hospitalization time in patients where therapy failed. Additionally, the need for orotracheal intubation occurred in only 25% of HFNC failure cases. There were no reports of adverse effects from HFNC, and the mortality rate in the population was 0.6%. (Figure 2).
Figure 2: Vital signs evaluation in average values, pre and post HFNC installation. HFNC: high flow nasal cannula; HR: heart rate; RR: respiratory rate; SPO2: peripheral oxygen saturation; WDS: Wood-downes score.
DISCUSSION
Vital sign stabilization after admission to HFNC in the study population was observed within just half an hour of therapy and continued to improve progressively until therapy weaning began at 48 hours. Previous studies have demonstrated significant improvement in vital signs within 60 minutes of therapy [10], and after 12 hours - the latter showed improvement in arterial blood gas analysis; both support the high clinical efficacy and safety of HFNC in managing bronchiolitis [11]. Recently, the ROX index, used as a predictor of HFNC success initially for the adult population, was validated in its pediatric version (p-roxi) [12]. However, due to the ongoing nature of the present study, it was opted not to utilize it.
It is believed that in addition to improving respiratory discomfort, HFNC can also reduce hospitalization time, oxygen requirements, and the incidence of therapeutic failure compared to low-flow oxygen therapy [12,13]; however, since this comparison was not made in the study, we cannot corroborate these claims.
From a pathophysiological perspective, airway inflammation and obstruction due to secretion accumulation resulting from viral infection can lead to severe obstruction, increasing respiratory system resistance and air trapping, resulting in excessive respiratory muscle work and adaptive changes in ventilatory pattern [14,15]. According to Betters et al., infants are the population that benefits most from the mechanisms proposed by HFNC. This is due to their nasal breathers with less flow loss through the mouth, as well as having smaller nostrils compared to older children and adults, which would form a tighter seal with the cannula, generating higher airway pressure – albeit variable and imprecise, reducing respiratory system resistance and eliminating dead space [16]. These physiological effects support HFNC’s ability to progressively reduce ventilatory workload over the course of therapy [14-17], as observed in the present study.
In addition, to the improvement in ventilatory workload, a longitudinal analysis of radiographic images in the study population revealed a positive therapeutic response of HFNC in patterns of images previously indicating severe pulmonary hyperinflation associated with peri-hilar infiltration. This favorable evolution aligns with the conclusions of previous studies, suggesting that HFNC not only attenuates acute respiratory patterns but also promotes progressive recovery of underlying lung changes associated with bronchiolitis, with the most prominent being hyperinflation [18-25].
Although bronchiolitis is known to evolve naturally over the course of the disease, the success rate of HFNC in the study was 75%, indicating efficacy in preventing progression to severe respiratory failure, as the study population was admitted during a significant worsening phase (4th day of disease progression). The failure rate (25%), although representing the minority of cases, may result from the heterogeneity of clinical response to HFNC in children with bronchiolitis across different studies [20]. On the other hand, Betters et al., found a considerably high success rate (94%), although they did not mention an objective classification of patient severity; in their study, individuals requiring higher FiO2 (above 50%), with a history of orotracheal intubation and/or associated cardiac comorbidities, were more likely to not respond to this treatment [21]. In our study, there was an association of therapy failure with increased hospitalization time, emphasizing the importance of adequate patient selection and monitoring through well-defined criteria and protocols for management.
Regarding therapy success, it is suggested that early initiation may be the key element, preventing airway obstruction progression and reversing some atelectasis [22]. Similarly, McKiernan et al., comparing intubation rates in a service before and after acquiring HFNC, found a 68% reduction in the need for intubation and the average ICU stay decreased from 6 to 4 days afterward. In studies comparing HFNC with low-flow oxygen therapy, high-flow efficacy was considerably better [14,24,25]; the authors argue that HFNC undeniably shows good efficacy as a rescue therapy in reducing the proportion of children requiring high-cost intensive care, although it does not appear to significantly reduce oxygen therapy duration when compared to standard therapy.
FINAL CONSIDERATIONS
The analysis of the efficacy of high-flow nasal cannula (HFNC) in stabilizing vital signs in infants with moderate respiratory crisis due to bronchiolitis revealed significant findings in this study that support its use as a first-line support in the target population. It was possible to observe improvement in respiratory pattern within the first few hours of therapy, with normalization of signs starting at 24 hours and initiation of weaning at 48 hours, achieving success rates above 70% in preventing progression to the need for mechanical ventilation. The radiological pattern also improved and supported the advantage of HFNC in achieving lung deflation objectives.
As limitations of the study, the single-center and observational design preclude more robust analyses of outcomes; the low sample size in stratified subgroups; the absence of arterial blood gas measurements before and after HFNC installation, and the lack of mortality and severity scores that could corroborate the clinical profile of the population. Further studies are needed to more deeply evaluate the observations and questions arising from the results presented here, as well as to confirm the efficacy of HFNC therapy.
FUNDING
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ACKNOWLEDGEMENTS
To the entire multidisciplinary team of the pediatric units at Rios D’or Hospital, especially Physiotherapy; To the D’or Institute for Research and Education, and to all study participants and their families.
REFERENCES
- Dalziel SR, Haskell L, O’Brien S, Borland ML, Plint AC, Babl FE, et al. Bronchiolitis. The Lancet. 2022; 400: 392-406.
- Fujiogi M, Goto T, Yasunaga H, Fujishiro J, Mansbach JM, Camargo Jr CA, et al. Trends in Bronchiolitis Hospitalizations in the United States: 2000-2016. Pediatrics. 2019;144: e20192614.
- O’Brien S, Borland ML, Cotterell E, Armstrong D, Babl F, Bauert P, et al. Australasian bronchiolitis guideline. J Paediatr Child Health. 2019; 55: 42-53.
- Dawson-Caswell M, Muncie HL Jr. Respiratory syncytial virus infection in children. Am Fam Physician. 2011; 83: 141-146.
- Hon KL, Leung AKC, Wong AHC, Dudi A, Leung KKY. Respiratory Syncytial Virus is the Most Common Causative Agent of Viral Bronchiolitis in Young Children: An Updated Review. Curr Pediatr Rev. 2023; 19: 139-149.
- Moreel L, Proesmans M. High flow nasal cannula as respiratory support in treating infant bronchiolitis: a systematic review. Eur J Pediatr. 2020;179: 711-718.
- Lin J, Zhang Y, Xiong L, Liu S, Gong C, Dai J. High-flow nasal cannula therapy for children with bronchiolitis: a systematic review and meta-analysis. Arch Dis Child. 2019; 104: 564-576.
- D’Cruz RF, Hart N, Kaltsakas G. High-flow therapy: physiological effects and clinical applications. Breathe (Sheff). 2020; 16: 200224.
- Ferrés J. Comparison of two nebulized treatments in wheezing infants. Eur Respir J 1988; 1 (Suppl): 306.
- Mayfield S, Bogossian F, O’Malley L, Schibler A. High-flow nasal cannula oxygen therapy for infants with bronchiolitis: pilot study. J Paediatr Child Health. 2014; 50: 373-378.
- Yidizdas D, Yontem A, Iplik G, Horoz OO, Ekinci F. Predicting nasal high-flow therapy failure by pediatric respiratory rate-oxygenation index and pediatric respiratory rate-oxygenation index variation in children. Eur J Pediatr. 2021; 180: 1099-1106.
- Roca O, Messika J, Caralt B, Garcia-de-Acilu M, Sztrymf B, Ricard JD, et al. Predicting success of high-flow nasal cannula in pneumonia patients with hypoxemic respiratory failure: the utility of the ROX index. J Crit Care. 2016; 35: 200-205.
- Chanchan JI, Aiqin S, Nianjyu Z, Zhenmei L, Enben G, Lirong S. Clinical application of heated, humidified high-flow nasal cannula in the treatment of moderate and severe bronchiolitis in infants. Chinese J Appl Clin Pediatr. 2017; 1412-1415.
- E?ki A, Öztürk GK, Turan C, Özgül S, Gülen F, Demir E. High-flow nasal cannula oxygen in children with bronchiolitis: A randomized controlled trial. Pediatr Pulmonol. 2022; 57: 1527-1534.
- Milési C, Baleine J, Matecki S, Durand S, Comes C, Cambonie G, et al. Is treatment with a high flow nasal cannula effective in acute viral bronchiolitis? A physiologic study. Intensive Care Med. 2013; 39: 1088-1094.
- Abboud PA, Roth PJ, Skiles CL, Stolfi A, Rowin ME. Predictors of failure in infants with viral bronchiolitis treated with high-flow, high- humidity nasal cannula therapy*. Pediatr Crit Care Med. 2012; 13: e343-349.
- Tortosa F, Izcovich A, Carrasco G, Varone G, Haluska P, Sanguine V. High-flow oxygen nasal cannula for treating acute bronchiolitis in infants: A systematic review and meta-analysis. Medwave. 2021; 21: e8190.
- González F, González M, Rodríguez R. Impacto clínico de la implantación de la ventilación por alto flujo de oxígeno en el tratamiento de la bronquiolitis en una planta de hospitalización pediátrica. An Pediatr (Barc) 2013; 78: 210-215.
- Pinchak C, García L, Peluffo G, Vazquez M, Halty M, Chamorro F, et al. Experiencia en la utilización de cánula nasal de alto flujo en niños con infecciones respiratorias agudas hospitalizados en un sector de internación. Arch pediatr Urug. 2019; 90: 257-269.
- Mikalsen IB, Davis P, Øymar K. High flow nasal cannula in children: a literature review. Scand J Trauma Resusc Emerg Med. 2016; 24: 93.
- Betters KA, Gillespie SE, Miller J, Kotzbauer D, Hebbar KB. High flow nasal cannula use outside of the ICU; factors associated with failure. Pediatr Pulmonol. 2017; 52: 806-812
- Spentzas T, Minarik M, Patters AB, Vinson B, Stidham G. Children with respiratory distress treated with high-flow nasal cannula. J Intensive Care Med. 2009; 24: 323-328.
- McKiernan C, Chua LC, Visintainer PF, Allen H. High flow nasal cannulae therapy in infants with bronchiolitis. J Pediatr. 2010; 156: 634-638.
- Franklin D, Babl FE, Schlapbach LJ, Oakley Ed, Craig S, Neutze J, et al. A Randomized Trial of High-Flow Oxygen Therapy in Infants with Bronchiolitis. N Engl J Med. 2018; 378: 1121-1131.
- Kepreotes E, Whitehead B, Attia J, Oldmeadow C, Collison A, Searles A, et al. High-flow warm humidified oxygen versus standard low-flow nasal cannula oxygen for moderate bronchiolitis (HFWHO RCT): an open, phase 4, randomised controlled trial. Lancet. 2017; 389: 930-939.