The Expression of Ghrelın, Heat Shock Protein-70, BCL-2 and BAX Proteıns in Idıopathıc Thrombocytopenıc Purpura at Bone Marrow
- 1. Department of Child Hematology Oncology, University of Firat, Turkey
- 2. Departments of Pediatrics, University of Wisconsin-Madison, USA
- 3. Department of Radiology, University of Firat, Turkey
- 4. Department of Pathology, University of Firat, Turkey
Abstract
Purpose: The pathogenesis of immune thrombocytopenia (ITP) have not yet been clarified. Bone marrow is an important sample source for studying the pathogenic mechanisms of ITP. In ITP bone marrow biopsy samples; it was desired to evaluate the effects of oxidative stress, apoptosis and antiapoptosis, which are accused in the etiology, by performing ghrelin, heat shock protein (Hsp)-70, Bcl-2 and Bax staining. The role of these parameters in the pathogenesis of ITP was investigated. The answer to the question of whether ITP can be predicted to be Newly Diagnosed (ndITP) or Chronic (cITP) was investigated with ghrelin, Hsp-70, Bcl-2 and Bax dye.
Methods: Bone marrow biopsy samples of 45 retrospectively evaluated cases with ndITP (n: 23) and cITP (n: 22) were included in the study. The healthy Control Group (C) was consisted of 20 cases. Bone marrow examination samples taken on paraffin blocks were immunohistochemically; stained with ghrelin, Hsp-70, Bcl-2 and Bax.
Results: Among the antioxidant parameters (ghrelin, Bcl-2, Hsp-70), only low ghrelin was detected. Ghrelin staining prevalence and score in cITP was lower than in ndITP (p<0.05). Ghrelin staining intensity was lower than ndITP and Control (p<0.05). Bax staining intensity was lower in all ITP cases compared to Control (p<0.05). Ghrelin/Bax staining score ratio was lower in cITP compared to ndITP (p<0.05).
Conclusion: Antioxidant levels were found to be lower in ITP. Antioxidant level was lowest in cITP. Low levels of antioxidants in the bone marrow may indicate the diagnosis of ITP and the possibility of its chronicity.
KEYWORDS
- Immune thrombocytopenia (ITP)
- Platelets
- Pathogenesis
- Ghrelin
- Hsp-70
- Apoptosis
- Bcl-2; Bax
CITATION
AKARSU S, YILDIRIM M, POYRAZ AK, ÖZERCAN IJ (2025) The Expression of Ghrel?n, Heat Shock Protein-70, BCL-2 and BAX Prote?ns in Id?opath?c Thrombocytopen?c Purpura at Bone Marrow. Pediatr Child Health 15(1): 1348.
INTRODUCTION
Immune thrombocytopenia (ITP) is an acquired and heterogeneous autoimmune-mediated hematological disease typically characterized by a low platelet count [1,2]. The aetiology and pathogenesis of ITP have not yet been clarified [3]. Antiplatelet antibodies cannot be detected in at least 20% of patients with ITP [4].
Ghrelin plays an important role in both physiological and pathological processes, such as inhibition of inflammation, and regulation of immune function. Several studies have shown that ghrelin has anti-apoptotic and protective effects on various cell types [5].
Heat shock protein (Hsp)s prevent the aggregation and denaturation of many proteins [5]. Ghrelin prevents cells from undergoing apoptosis by inhibiting the activation of the nuclear factor-κB pathway. Ghrelin suppresses apoptosis by stimulating Hsp-70 expression. The positive correlation has been shown between Hsp- 70 concentration and platelet counts. Hsp-70 can play an immunosuppressive role [6,7].
Emerging evidence over the past several years suggests that platelet biogenesis and ageing are regulated, at least in part, by apoptotic mechanisms [1,2]. Apoptosis; it is regulated by the balance between antiapoptotic and proapoptotic proteins. The control and regulation of these apoptotic mitochondrial events occurs through members of the Bcl-2 family of proteins. The platelet life span is governed by the interplay between Bcl-2 family proteins. These proteins have special significance since they can determine if the cell commits to apoptosis process. Ghrelin exerts a protective effect by regulating the ratio of Bcl-2 and Bax. The Bcl-2/Bax ratio is considered the key of death [1,5,8-10].
Bone marrow is an important sample source for studying the pathogenic mechanisms of ITP [11). Different mechanisms may play a role in the pathogenesis of ITP. In ITP, plasma samples; apoptosis and antiapoptosis with oxidant and antioxidant mechanism have also been evaluated [1,10,12]. However, evaluation at the tissue level (bone marrow biopsy samples) has not yet been performed. In ITP bone marrow biopsy samples; it was desired to evaluate the effects of oxidative stress, apoptosis and antiapoptosis, which are accused in the etiology, by performing ghrelin, Hsp-70, Bcl-2 and Bax staining. The role of these parameters in the pathogenesis of ITP was investigated. The answer to the question of whether ITP can be predicted to be Newly Diagnosed (ndITP) or Chronic (cITP) was investigated with ghrelin, Hsp-70, Bcl- 2 and Bax dye.
METHODS
Patients and ethical statements
This study enrolled 45 ITP children (aged 1–17 years) diagnosed in the University of Firat, Faculty of Medicine, Pediatric Hematology Oncology Department from August 2015 to August 2020. Twenty healthy children between 1 and 17 years were also enrolled in the study as controls. The diagnosis was done following the International Working Group (IWG) consensus criteria based on the exclusion of other infections leading to thrombocytopenia, including autoimmune diseases. As stipulated by the IWG, patients diagnosed within the previous 3 months were regarded as new diagnosis immune thrombocytopenia (ndITP), and children with ITP diagnosed over 12 months were regarded as chronic ITP (cITP). Twentythree out of the 45 ITP children were reported as the ndITP, while 22 children were reported to be in the cITP phase. The patients’ management was done following the IWG consensus [13, 14]. Age, sex and complete blood count data were obtained during the study and control group enrolment (Table 1).
Table 1: The characteristics of the ITP children and the healthy controls
|
|
NEW DIAGNOSIS (n= 23) (mean±SD) (min-max) |
CHRONIC (n= 22) (meant±SD) (min-max) |
TOTAL (n=45) (mean±SD) (min-max) |
CONTROL (n=20) (mean±SD) (min-max) |
|
Gender (Female (n)/ Male (n)) (%) |
11/12 (47.8/52.2) |
12/10 (54.5/45.5) |
23/22 (51.11/48.88) |
7/13 (35/65) |
|
Age (Year) |
6,73±4,79 (1-16) |
10,27±5,06 (1-17) |
8.13±5.21 (1-17) |
7,37±5,32 (1-17) |
|
Platelet (x109/L) |
23,9±23,00a (1,73-84,00) |
33,4±31,07a (3,00-129,00) |
28,6±27,00a (1,73-129,00) |
460,5±200,15 (174,00- 862,00) |
|
Hemoglobin (g/dL) |
12,2±1,08 (10,6-14,9) |
13,1±1,67a (7,8-15,4) |
12,6±1,37 (7,8-15,4) |
11,6±1,24 (9,5-13,3) |
|
WBC (x109/L) |
8,66±2,62 (3,9-14,9) |
7,96±2,29 (3,91-15,1) |
8,31±2,45 (3,90-15,1) |
9,56±3,66 (4,5-17,5) |
Data were expressed as mean±standard deviation. a. p<0.05 of the current group compared to the Control Group b. p<0.05 of the current group compared to the ndITP Group
All cases were statistically evaluated in terms of prevalence, intensity and staining score separately for ghrelin, Hsp-70, Bcl-2 and bax (Table 2).
Table 2: Scoring system for cytoplasmic histochemical staining
|
STAINING PREVALENCE |
STAINING INTENSITY |
STAINING SCORE (Prevalence+Intensity) |
|
No staining: 0 <10%: 1 |
Negative: 0 Weak: 1 |
0: Negative 1 or 2: +1 |
|
10-50%: 2 <50%: 3 |
Medium: 2 Strong: 3 |
3 or 4: +2 5 or 6: +3 |
The ITP and healthy controls’ characteristics are presented in Table 1. The study was authorized by the Ethical Review Board of University of Firat, Faculty of Medicine under the ethical number TF.13.51. Written consent was obtained from the guardians of all the children included in the study. The study was done retrospectively. Bone marrow examination samples taken on paraffin blocks were immunohistochemically; stained with ghrelin, Hsp-70, Bcl- 2 and Bax.
Immunohistochemical Staining
After the H&E stained archive preparations of the cases were re-examined with light microscopy and the diagnosis was confirmed, 4 micrometer-thick sections were taken from paraffin blocks for immunohistochemical (IHC) application. Sections obtained from the blocks of the cases were taken on polylysine-coated slides. The slides were first kept in an oven at 60ºC for 10 minutes, then ghrelin (Boster, Rabbit Anti-ghrelin Polyclonal Antibody, 100 µl, 1/100, USA), Hsp-70 (Novus, Rabbit Anti-Hsp70 Polyclonal Antibody, 100 µl, 1/100, USA), Bcl-2 (Boster, Rabbit AntiBcl-2 Polyclonal antibodies, 100 µl, 1/100, USA) and Bax (Santacruz, Rabbit Anti-Bax Polyclonal Antibody, 100 µl, 1/100, USA) They were processed in an automatic stainer (Ventana Medical System, SN: 712299, REF: 750-700, Arizona, USA) for staining. After treatment with primary antibody, slides were washed in tap water and covered with ultramount. Samples of salivary gland tissue were used for Hsp-70 and ghrelin, and tonsil tissue samples were used for Bcl-2 and Bax as positive controls. Slides stained with antibodies were evaluated under an Olympus BX51 light microscope. Imaging was done with the Olympus DP71. Cytoplasmic staining was considered positive for all three stains. The extent of the positive staining areas and the intensity of the staining were recorded in all cases. For all cases, those with demonstrative properties were selected as “reference preparations” and microscopic photography was performed. The values obtained from both evaluations were summed up and the staining score was obtained from this sum [15].
Staining prevalence (No staining: 0, <10% Minimal: 1, 10-50% Moderate: 2, >50% Common: 3), intensity (Negative: 0, Weak: 1, Moderate: 2, Strong: 3) and score (Negative: 0, Weak positive [1+]: 1 or 2, Moderate positive [2++]: 3 or 4, Strong positive [3+++]: 5 or 6) were determined (Table 2) [15].
Statistical analysis
Data were expressed as mean±standard deviation. Chi-square test was used for categorical data, Kruskal- Wallis test was used for nonparametric data analysis, and Spearman Correlation analysis was used for correlation analysis to analyze the differences between the control and patient groups.
All bone marrow biopsy specimens were simultaneously stained with ghrelin, Hsp-70, Bcl-2 and Bax and evaluated by the same person.
RESULTS
Demographic characteristics of the groups are given in Table 1. There was no statistically significant difference between the groups in terms of age and gender (p>0.05).
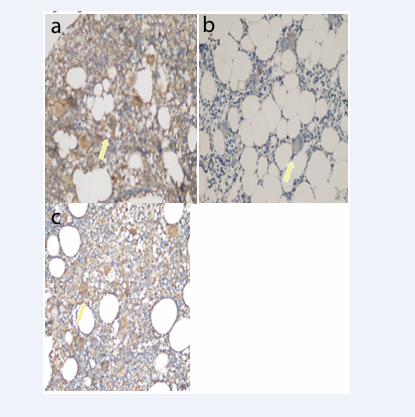
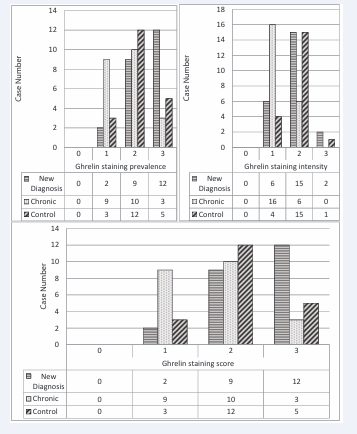
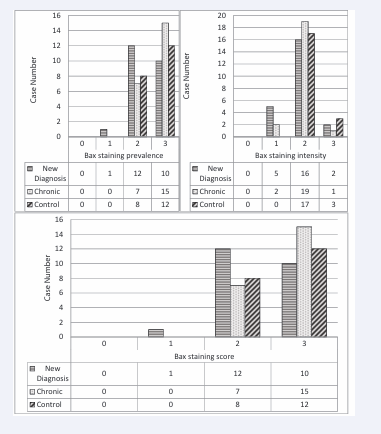
In cITP, ghrelin staining prevalence and score were statistically significantly lower than ndITP. Ghrelin staining intensity was 8.69% in ndITP and 5% (+++) in the control group. In cases with cITP (+++), there was no intense staining. Ghrelin staining intensity, in cases with cITP; were lower than ndITP and control groups (p<0.05) (Table 3, Figure 1 and 2).
Table 3: Ghrelin, Hsp-70, Bax and Bcl-2 staining evaluation in ITP andControl Groups
|
Ghrelin |
NEW DIAGNOSIS |
CHRONIC |
TOTAL |
CONTROL |
|
(n=23) |
(n=22) |
(n=45 ) |
(n=20) |
|
|
(mean±SD) |
(mean±SD) |
(mean±SD) |
(mean±SD) |
|
|
(min-max) |
(min-max) |
(min-max) |
(min-max) |
|
|
Prevalence |
2,43±0,66 |
1,72±0,70b |
2,08±0,76 |
2,10±0,64 |
|
(1-3) |
(1-3) |
(1-3) |
(1-3) |
|
|
Intensity |
1,82±0,57 |
1,27±0,45ab |
1,55±0,58a |
1,85±0,48 |
|
(1-3) |
(1-2) |
(1-3) |
(1-3) |
|
|
Score |
2,43±0,66 |
1,72±0,70b |
2,08±0,76 |
2,10±0,64 |
|
(1-3) |
(1-3) |
(1-3) |
(1-3) |
|
|
Hsp-70 |
|
|||
|
Prevalence |
2,60±0,49 |
2,72±0,45 |
2,66±0,47 |
2,45±0,60 |
|
(2-3) |
(2-3) |
(2-3) |
(1-3) |
|
|
Intensity |
2,17±0,57 |
2,18±0,45 |
2,17±0,49 |
2,20±0,69 |
|
(1-3) |
(2-3) |
(2-3) |
(1-3) |
|
|
Score |
2,60±0,49 |
2,72±0,45 |
2,66±0,47 |
2,45±0,60 |
|
(2-3) |
(2-3) |
(2-3) |
(1-3) |
|
|
Bax |
|
|||
|
Prevalence |
2,39±0,58 |
2,68±0,47 |
2,53±0,54 |
2,60±0,50 |
|
(1-3) |
(2-3) |
(1-3) |
(2-3) |
|
|
Intensity |
1,86±0,54 |
1,95±0,37 |
1,91±0,46a |
2,15±0,36 |
|
(1-3) |
(1-3) |
(1-3) |
(2-3) |
|
|
Score |
2,39±0,58 |
2,68±0,47 |
2,53±0,54 |
2,60±0,50 |
|
(1-3) |
(2-3) |
(1-3) |
(2-3) |
|
|
Bcl-2 |
|
|
|
|
|
Prevalence |
0,13±0,45 |
0,18±0,58 |
0,15±0,52 |
0,25±0,63 |
|
(0-2) |
(0-2) |
(0-2) |
(0-2) |
|
|
Intensity |
1,00±0,00 |
1,04±0,21 |
1,02±0,14 |
1,00±0,00 |
|
(1-1) |
(1-2) |
(1-2) |
(1-1) |
|
|
Score |
1,04±0,20 |
1,09±0,29 |
1,06±0,25 |
1,10±0,30 |
|
(1-2) |
(1-2) |
(1-2) |
(1-2) |
|
Figure 1: Ghrelin staining in a) ndITP (++), b) cITP (+) and c) Control Group (++) staining intensity (Immunperoxidase, X200)
Figure 2: Ghrelin staining prevalence, intensity and score
Ghrelin staining prevalence and score were 52.1% in ndITP, 13.6% in cITP, and 25% (+++) in the control group (p<0.05).
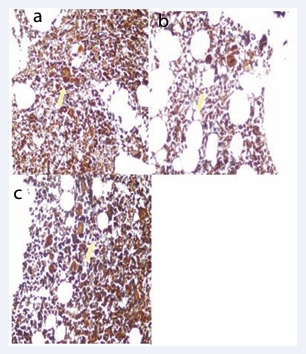
Hsp-70 staining prevalence, intensity and score were not different in the groups (Table 3, Figure 3).
Figure 3: Hsp-70 staining intensity in a) ndITP (+++), b) cITP (+++) and c) Control (+++) Group (Immunperoxidase, X200)
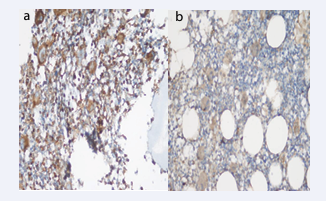
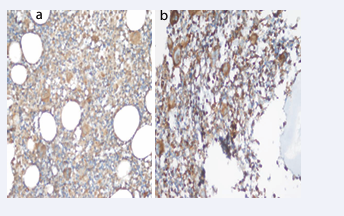
Bax staining intensity was 8.69% in ndITP, 4.54% in cITP and 15% in the control group (+++). Bax staining intensity was statistically significantly lower in all ITP cases compared to the control group (p<0.05). Bax staining prevalence was 43.4% in ndITP, 68.1% in cITP and 60% in the control group (+++). There was no difference in the extent and score of Bax staining (Table 3, Figure 4-6).
Figure 4: Bax staining in a) ndITP (+++) and b) (++) staining intensity (Immunperoxidase, X200).
Figure 5: Bax staining intensity in a) cITP (++) and b) Control (+++) Group (Immunperoxidase, X200).
Figure 6: Bax staining prevalence, intensity and score
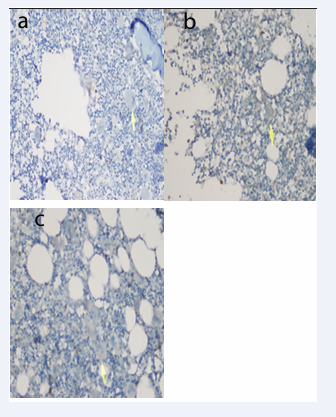
Bcl-2 staining prevalence, intensity and score were not different between the groups (Table 3, Figure 7).
Figure 7: Bcl-2 staining intensity (+) in a) ndITP, b) cITP and c) Control Group (Immunperoxidase, X200).
In the evaluation of oxidant/antioxidant ratios, Bax/ Ghrelin staining score ratio was statistically significantly higher in cases with cITP than in cases with ndITP (p<0.05). In the evaluation of antioxidant/oxidant ratios, Ghrelin/Bax staining score ratio was lower in cITP compared to ndITP (p<0.05). In the evaluation of antioxidant/antioxidant ratios, Bcl-2/Ghrelin staining score ratio was statistically significantly higher in patients with cITP than in patients with ndITP (p<0.05). The Hsp-70/Ghrelin staining score ratio was significantly higher in patients with cITP than in both ndITP and control groups (p<0.05) (Table 4).
Table 4: Staining score ratios
|
Oxidant / Antioxdant |
TOTAL |
NEW DIAGNOSIS |
CHRONIC |
CONTROL |
|
(n= 45) |
(n= 23) |
(n= 22) |
(n=20) |
|
|
(mean±SD) |
(mean±SD) |
(mean±SD) |
(mean±SD) |
|
|
(min-max) |
(min-max) |
(min-max) |
(min-max) |
|
|
Bax/Bcl-2 |
2,43±0,58 |
2,32±0,59 |
2,54±0,57 |
2,50±0,68 |
|
(1-3) |
(1-3) |
(1,5-3) |
(1-3) |
|
|
Bax/Hsp-70 |
0,97±0,26 |
0,94±0,28 |
1,00±0,23 |
1,13±0,36 |
|
(0,33-1,5) |
(0,33-1,5) |
(0,67-1,5) |
(0,67-2) |
|
|
Bax /Ghrelin |
1,44±0,76 |
1,07±0,51 |
1,82±0,81b |
1,40±0,65 |
|
(0,33-3) |
(0,33-3) |
(0,67-3) |
(0,67-3) |
|
|
Antioksidant/ Oksidant |
|
|
|
|
|
Bcl-2/Bax |
0,46±0,15 |
0,41±0,11 |
0,44±0,13 |
0,45±0,20 |
|
(0,33-1) |
(0,33-0,67) |
(0,33-1) |
(0,33-1) |
|
|
Hsp-70/Bax |
1,17±0,48 |
1,04±0,24 |
1,11±0,38 |
0,97±0,31 |
|
(0,67-3) |
(0,67-1,5) |
(0,67-3) |
(0,50-1,5) |
|
|
Ghrelin/Bax |
1,08±0,49 |
0,66±0,30b |
0,88±0,46 |
0,85±0,35 |
|
(0,33-3) |
(0,33-1,5) |
(0,33-3) |
(0,33-1,5) |
|
|
Antioksidant/ Antioksidant |
|
|
|
|
|
Bcl-2/Hsp-70 |
0,41±0,09 |
0,40±0,11 |
0,41±0,10 |
0,47±0,16 |
|
(0,33-0,67) |
(0,33-0,67) |
(0,33-0,67) |
(0,33-1) |
|
|
Bcl-2/Ghrelin |
0,47±0,19 |
0,74±0,38b |
0,60±0,32 |
0,56±0,20 |
|
(0,33-1) |
(0,33-2) |
(0,33-2) |
(0,33-1) |
|
|
Hsp-70/ Ghrelin |
1,18±0,51 |
1,82±0,73ab |
1,49±0,70 |
1,30±0,58 |
|
(0,67-3) |
(0,67-3) |
(0,67-3) |
(0,5-3) |
|
a. p<0.05 of the current group compared to the Control Group b. p<0.05 of the current group compared to the ndITP Group
DISCUSSION
In children, ITP mostly presents itself in a self-limiting manner for a maximum of 12 months in the presence or absence of treatment, and the platelet counts may eventually return to normalcy. Nevertheless, in about 20% of the newly diagnosed childhood cases, the ITP develops to the chronic form defined based on the standardization criteria [7].
Ghrelin suppresses apoptosis by stimulating Hsp-70 expression. Ghrelin exerts a protective effect by regulating the ratio of Bcl-2 and Bax [1,5,6,8-10]. Neutrophils play a role in tissue injury by releasing various inflammatory mediators (neutrophil elastase, oxygen-free radicals). Ghrelin treatment suppresses neutrophil-dominated inflammation. The Bcl-2 family of enzymes maintains mitochondrial stabilization to regulate apoptosis and Bcl-2 (anti-apoptosis)/Bax (proapoptosis) balance, thereby determining whether cells undergo apoptosis. Ghrelin exerts a protective effect by regulating the ratio of Bcl-2 and Bax. Ghrelin treatment has been decreased the Bcl-2:Bax ratio [5]. In our study, among the antioxidant parameters (ghrelin, Bcl-2, Hsp-70), only ghrelin was found at low levels. Ghrelin staining prevalence and score in cITP was lower than in ndITP (p<0.05). Ghrelin staining intensity was lower than ndITP and C (p<0.05). Antioxidant defense is very active in ndITP. Antioxidant defense is lower in cITP than in the control group (Table 3, Figure 1 and 2).
Hsp-70, a stress-inducible molecular chaperone, has an intracellular protective function. This is explained by the antiapoptotic property of Hsp. Hsp-70 release is stimulated by factors such as increased temperature, nutritional deprivation, heavy metals, oxidative stress, and viral infections [16,17]. Hsp members play essential roles in the change of platelet shape and spreading. Hsp-70 has been indicated intracellular and extracellular functions and associated chaperones in trafficking, assembly of protein and platelets regulation. Hsps have been regarded as toxic autoantigens and have an association between autoimmunity and infection. Hsps that have anti- inflammatory properties, such as Hsp-70, specifically affect the presence or the functional activities of these immune- regulatory cells [7].
Hsps prevent the aggregation and denaturation of many proteins [5]. The role of Hsp-70 in the development of childhood ITP has been reported in several articles. In one study, Hsp-70 was found to be elevated in childhood ITP in the blood. The limitation of this study is that it did not investigate the Hsp-70 expression in ITP patients’ tissues and lacked in vivo studies to investigate the association of Hsp-70 with other important inflammatory factors, such as TNF-α and IL-4. Another limitation is the possibility that thrombocytopenia itself leads to higher levels of Hsp-70 in circulation. As an immunosuppressant, Hsp-70 has been shown to pass through a lipid bilayer [7]. With our study, this risk will be eliminated by evaluating tissue level ghrelin, Hsp-70, Bcl-2 and Bax in ndITP and cITP. Bone marrow is an important sample source for studying the pathogenic mechanisms of ITP [11]. This is the first investigation to report the intracellular Hsp-70 level in childhood primary ITP. Hsp-70 has a direct role in the inflammation and establishment of childhood ITP. This study provides a new insight into the use of Hsp-70 as a biomarker to determine the establishment and progression of ITP in children [7]. Hsp-70 blocks Bax translocation [16,17]. In our study, Bax staining intensity was found to be lower than normal in all ITP cases (Table 3). The Hsp-70/Ghrelin staining score ratio was significantly higher in patients with cITP than in both ndITP and control groups (p<0.05). If there is a low level of antioxidant in ITP cases, it can be thought that it will progress in the form of cITP (Table 4).
Emerging evidence over the past several years suggests that platelet biogenesis and ageing are regulated, at least in part, by apoptotic mechanisms. However, the association between decreased platelets and apoptosis in ITP patients is poorly understood [1,2]. Radiation, toxins, hypoxia, hyperthermia, viral infections and free radicals act as positive signals that trigger apoptosis [6,7]. Electron microscopy studies have shown membrane abnormalities in 50-70% of ITP megakaryocytes and abnormalities related to apoptosis in approximately 78% [4]. Apoptosis- related proteins have been significantly decreased in ITP patients, which further confirmed the important effect of apoptosis on ITP pathogenesis [11]. Although platelets are anucleate, they contain at least some of the machinery necessary for apoptosis [1].
There are two distinct and convergent pathways for apoptosis: the extrinsic and the intrinsic pathways. The intrinsic pathway is governed by the Bcl-2 family of proteins, which consists of both anti-apoptotic (Bcl-2, Bcl- xL, and Mcl-1) and pro-apoptotic (Bax and Bak) members. Total of 25 genes have been identified in the Bcl-2 family. The tumor suppressor protein p53 has a critical role in regulation of the Bcl-2 family of proteins. Platelets absolutely depend on the pro-survival protein Bcl-xL, which functions to restrain the activity of pro-apoptotic Bak and Bax. The research on the mutations of Bcl-xL in mice that cause dose-dependent reductions in platelet life span further support that Bcl-xL is the critical regulator of platelet survival [1,8-10]. Bcl-2 and Bax are in equilibrium under normal conditions, but when cells are exposed to external stimuli and damage, the expressions of the two are unbalanced, which causes either excessive or inhibited apoptosis [3]. The abnormal apoptosis of megakaryocytes exacerbates the development of ITP. Bcl-xL and Bax play an opposite role in the regulation of apoptotic process with Bcl-xL for cell survival and Bax for cell apoptosis. Given the critical roles in the regulation of platelet apoptosis, whether Bcl-xL or Bax was involved in the pathogenesis of ITP remains unknown [12]. The expression levels of Bcl- xL in platelets from chronic ITP patients have significantly been lower than those in controls, while the expression of Bax and Bak have been found to be significantly higher [1]. At the molecular level, the expression of Bax has been silenced by inhibitor, on the contrary, the expressions of Bcl-2 has been upregulated, in ITP [3].
Apoptosis is an important process whereby cells are intentionally marked for clearance from the body and is the main mechanism that regulates the life span of the cell and the elimination of damaged or infected nucleated cells. Platelets contain the necessary components and the intrinsic program for apoptosis. The process of platelet activation and senescence in different models have been associated with processes that resemble programed cell death, such as depolarization of mitochondrial membrane potentials, microparticle formation, caspase activation, phosphatidylserine (PS) exposure on the platelet surface and Bcl-2 family protein expression [1]. Apoptosis is characterized by a variety of morphological features. One of the most important phenomena is the translocation of the membrane PS from the inner to the outer leaflet of the platelet membrane. This is recognized as early-to- middle stages of apoptosis. Externalization of PS onto the surface of cells undergoing apoptosis represents the most universal and best characterized ‘eat me’ signal, leads to subsequent clearance of the apoptotic cells by phagocytes and causes or aggravates prevailing thrombocytopenia [1]. The causes of abnormal apoptosis in the megakaryocytes in ITP patients remain unclear. ITP plasma exhibits decreased rates of apoptosis. In patients with ITP, the presence of apoptotic abnormalities in megakaryocytes is the current diagnostic marker. It is also accepted that megakaryocyte apoptosis in ITP patients is dependent on caspase-3 activation and Bcl-2 [18].
Apoptosis is a main mechanism responsible for the regulation of life span and elimination of infected or damaged nucleated cells [12]. As enucleated cells, platelets have also been observed to undergo apoptotic-like events as demonstrated by activation and translocation of Bcl- 2 family members, cytochrome c release, activation of caspase-9 and caspase-3. The development and survival of megakaryocytes depend on the inhibition of the intrinsic apoptotic signalling pathway by the Bcl-2 protein family. Activation of the intrinsic apoptosis pathway contributes to the clearance of megakaryocytes after platelet shedding and limits platelet longevity in circulation. Thrombopoiesis requires activation of the intrinsic apoptotic signalling pathway in megakaryocytes. Megakaryocytes may inhibit apoptosis through platelet formation and platelet shedding to survive and progress safely. Megakaryocyte apoptosis is thought to contribute to thrombocytopenia and ITP. A study has been showned that megakaryocyte apoptosis was increased in ITP patients, and megakaryocytes from ITP patients exhibited ultrastructural features associated with apoptosis, possibly due to the presence of factors in plasma. Intravenous immunoglobulin therapy is associated with a decrease in the number of apoptotic megakaryocytes in the bone marrow of ITP patients. However, the inhibition of megakaryocyte apoptosis can inhibit platelet release in vitro. In some studies, the number of megakaryocytes in the bone marrow of ITP patients was decreased and the megakaryocytes exhibited characteristics of apoptosis resistance. Defects in the intrinsic blockade of megakaryocytes, a lack of proapoptotic signals, or increased anti-apoptotic signals can lead to impaired platelet release and thrombocytopenia. The maturation of apoptotic megakaryocytes may also be important for platelet production because immature megakaryocytes produce fewer platelets than mature cells. These results suggest an association between megakaryocyte apoptosis and ITP [11].
In consistent with this, mice studies showed that the degradation of anti-apoptotic Bcl-xL represents a molecular clock to regulate platelet life span through controlling the pro-death activity of proapoptotic Bak, leading to platelet apoptosis. Furthermore, inhibition of Bcl-xL by BH3 mimetics can induce a transient thrombocytopathy that undermines the hemostatic function of platelets. Given shorter platelet life span and increased platelet turnover in ITP, whether Bcl-xL is involved in the pathogenesis of ITP remains poorly understood [12]. ITP plasma can induces autophagy and suppresses apoptosis [18,19].
Dimethyl fumarate (DMF) is a small molecule with immunomodulating, anti-inflammatory and antioxidative effect. DMF can prevent antiplatelet antibody-mediated platelet destruction in ITP mouse model, possibly through reducing the viability of macrophages and promoting macrophage apoptosis, indicating that it might be a novel way for the clinical treatment of ITP. However, apart from inhibition of platelet destruction, whether DMF affects platelet production in ITP remains unclear [20].
Apoptosis is the active process of cell death regulated by genes through which aging and abnormal cells are cleared. Helicobacter pylori (HP) infection may promote the development of ITP, possibly by inducing apoptosis of human megakaryocyte [10]. An enhancement of apoptotic processes in the platelets of pediatric patients with acute ITP have been ameliorated by intravenous administration of immunoglobulin [1]. Apoptosis is an essential physiological process for the selective elimination of cells, which is involved in a variety of biological events. The Bcl- 2 family is the best characterized protein family involved in the regulation of apoptotic cell death, consisting of anti- apoptotic and pro-apoptotic members. The anti-apoptotic members of this family, such as Bcl-2 and Bcl-XL, prevent apoptosis. In contrast, pro-apoptotic members of this family, such as Bax and Bak, trigger the release of caspases. Thus, the Bcl-2 family of proteins acts as a critical life-death decision point within the common pathway of apoptosis [19]. There was no difference between the groups in Bcl-2 evaluation.
The apoptotic pathways of megakaryocytes are intrinsic and extrinsic, and both require Bcl-2 involvement. Furthermore, recent studies have reported that Bcl-2 is a cross-over point between autophagy and apoptosis in autoimmune disease, tumor, or injury. Numerous studies have demonstrated the involvement of Bcl-2 in both apoptosis and autophagy in diverse diseases. Under conditions of cellular stress, the reduction in Bcl-2 results in a decrease in binding to Beclin-1, leading to an increased free Beclin-1, which promotes autophagy. Alternatively, phosphorylation of Beclin-1 increases Beclin-1-Bcl-2 complexes, displacing Bax from Bcl-2 and resulting in apoptosis. The existence of such a change in ITP patients was investigated by evaluation of alterations in cell morphology, apoptosis, autophagy, and protein expression.
The present results demonstrated that Bax and Beclin-1 were downregulated, whereas Bcl-2 was upregulated in ITP plasma compared with normal plasma. The ITP plasma induces autophagy and suppresses apoptosis [18]. The expressions of Bcl-2 mRNA in ITP children have been increased significantly, but the expressions of bax mRNA have decreased, the ratios of Bcl-2/bax mRNA were improved obviously [21].
Flow cytometry has showed the expression levels of Bak (39.86% vs. 20.82%), Bax (36.85% vs. 6.69%), and reactive oxygen species (ROS) (19.98% vs. 1.29%) were all elevated. In addition, pro-survival Bcl-xL (5.38% vs. 21.20%) was decreased markedly in platelets from adult patients with chronic ITP (adult patients with chronic ITP compared with healthy volunteers p<0.05). The platelets in adults with chronic ITP display a proapoptotic gene expression phenotype, based on the enhanced expression of Bak, Bax, and ROS, and attenuated expression of Bcl-xL, suggesting increased sensitivity toward apoptosis [22].
ITP is a heterogeneous autoimmune disease, characterized by accelerated platelet destruction and impaired platelet production. Bcl-xL and Bax play an opposite role in the regulation of apoptotic process with Bcl-xL for cell survival and Bax for cell apoptosis. Given the critical roles in the regulation of platelet apoptosis, whether Bcl-xL or Bax was involved in the pathogenesis of ITP remains unknown [12]. In our study, in all ITP cases; Bax staining intensity was lower than the healthy controls (p<0.05) (Table 3, Figure 4-6).
Abnormal expressions of Bcl-2/Bax may play an important role in ITP immunopathogenesis [21]. In our study, Bcl-2/Bax ratio was not found to be different between the groups. Ghrelin/Bax staining score ratio was lower in cITP compared to ndITP (p<0.05) (Table 4). In the evaluation of oxidant/antioxidant ratios, Bax/Ghrelin staining score ratio was statistically significantly higher in cases with cITP than in cases with ndITP (p<0.05) (Table 4). The decrease in antioxidant level shows this ratio as high.
Apoptotic events might play a role in the pathogenesis of thrombocytopenia in chronic ITP. Platelet apoptosis may be involved in the development of ITP, which can be induced by pro-apoptotic agonistic antibodies and prevented by apoptosis inhibitors and anti-apoptotic antibodies. Platelets isolated from adult patients with chronic ITP had a significantly higher level of PS exposure than platelets from healthy adult volunteers. Genetic or induced dysregulation of these programed cell death pathways may lead to thrombocytopenia or ineffective thrombopoiesis in ITP. Platelet apoptosis was more heterogeneous than previously understood. Although the proportion of platelets with increased apoptosis markers was higher in ITP, this was not the case in all ITP patients, even when their platelet count was greatly decreased. Some ITP patients exhibited decreased or unchanged expression during platelet apoptosis. The possibility should be considered that the remaining platelets in circulation selected for analysis were apoptotic resistant platelets because the majority of non-resistant platelets were destroyed in chronic ITP. The percentage of platelets with positive apoptosis markers was not increased in pediatric patients with chronic ITP when compared to controls. This may be related to platelet apoptosis resistance. Furthermore, in some chronic ITP patients, peripheral tolerance may be induced by preventing platelet apoptosis. Another cause of decreased platelet apoptosis in patients with chronic ITP may be altered levels of anti-apoptotic or apoptotic molecules caused by some hematologic disorders, however the exact mechanism remains unclear [1].
Antioxidant levels were found to be lower in ITP. Antioxidant level was lowest in cITP. Low levels of antioxidants in the bone marrow may indicate the diagnosis of ITP and the possibility of its chronicity.
ACKNOWLEDGMENTS
Scientific Research Project Unit of our university (University of Firat Faculty of Medicine) provided financial support (Approval: TF.13.51.) for our study.
AUTHORSHIP CONTRIBUTIONS
All authors contributed to the study design and revised the manuscript which was drafted by Dr. Akarsu and Dr. Çay. All authors approved the final version submitted for publication.
REFERENCES
- Deng G, Yu S, Li Q, He Y, Liang W, Yu L, et al. Investigation of platelet apoptosis in adult patients with chronic immune thrombocytopenia. Hematology. 2017; 22: 155-161.
- Shuguang Li, Lin Wang, Chunhong Zhao, Lizhen Li, Jun Peng, Ming Hou. CD8+T cells suppress autologous megakaryocyte apoptosis in idiopathic thrombocytopenic purpura. Br J Haematol. 2007; 139: 605-611.
- Wang Y, Guo Y, Zhang X, Zhao H, Zhang B, Wu Y, et al. The role and mechanism of miR-557 in inhibiting the differentiation and maturation of megakaryocytes in immune thrombocytopenia. RNA Biol. 2021; 18: 1953-1968.
- Wickremasinghe RG, Hoffbrand AG. Biochemical and genetic control of apoptosis: Relevance to normal hematopoiesis and hematological malignancies. Blood. 1999; 93: 3587-3600.
- Zhang Q, Huang C, Meng B, Tang T, Shi Q, Yang H. Acute effect of Ghrelin on ischemia/reperfusion injury in the rat spinal cord. Int J Mol Sci. 2012; 13: 9864-9876.
- Wang Y, Cao L, Liu X. Ghrelin alleviates endoplasmic reticulum stress and inflammation-mediated reproductive dysfunction induced by stress. J Assist Reprod Genet. 2019; 36: 2357-2366.
- Ge J, Liu Y. Heat shock protein-70 is elevated in childhood primary immune thrombocytopenia. Eur J Med Res. 2022; 27: 244-251.
- Samali A, Cotter TG. Heat shock proteins increase resistance to apoptosis. Exp Cell Res. 1996; 223: 163-170.
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007; 35: 495-516.
- Lei H, Ma Y, Tan J, Liu Q. Helicobacter pylori Regulates the Apoptosis of Human Megakaryocyte Cells via NF-κB/IL-17 Signaling. Onco Targets Ther. 2021; 14: 2065-2074.
- Liu SY, Yuan D, Sun RJ, Zhu JJ, Shan NN. Significant reductions in apoptosis-related proteins (HSPA6, HSPA8, ITGB3, YWHAH, and PRDX6) are involved in immune thrombocytopenia. J Thromb Thrombolysis. 2021; 51: 905-914.
- Qiao J, Liu Y, Li D, Wu Y, Li X, Yao Y, et al. Imbalanced expression of Bcl-xL and Bax in platelets treated with plasma from immune thrombocytopenia. Immunol Res. 2016; 64: 604-609.
- Shulman NR, Marder VJ, Weinrach RS. Similarities between known antiplatelet antibodies and the factor responsible for thrombocytopenia in idiopathic purpura: physiologic, serologic and isotipic studies. Ann N Y Acad Sci. 1965; 124: 499-542.
- Neunert C, Lim W, Crowther M, Cohen A, Solberg L Jr, Crowther MA. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011; 117: 4190-4207.
- Baspinar S, Bircan S, Orhan H, Kapucuoglu N, Bozkurt KK. The relation of beclin 1 and bcl-2 expressions in high grade prostatic intraepithelial neoplasia and prostate adenocarcinoma: a tissue microarray study. Pathol Res Pract. 2014; 210: 412-418.
- Yang M, Hu S, Wu B, Miao Y, Pan H, Zhu S. Ghrelin inhibits apoptosis signal-regulating kinase 1 activity via upregulating heat-shock protein 70. Biochem Biophys Res Commun. 2007; 359: 373-378.
- Glover JR, Lindquist S. Hsp104, Hsp70, and Hsp40: a novel chaperone system that rescues previously aggregated proteins. Cell. 1998; 94: 73-82.
- Liu Z, Mei T. Immune thrombocytopenia induces autophagy and suppresses apoptosis in megakaryocytes. Mol Med Rep. 2018; 18: 4016-4022.
- Tsujimoto Y. Role of Bcl-2 family proteins in apoptosis: apoptosomes or mitochondria? Genes to Cells. 1998; 3: 697-707.
- Tong H, Ding Y, Gui X, Sun Z, Wang G, Zhang S, et al. Dimethyl fumarate inhibits antibody-induced platelet destruction in immune thrombocytopenia Mouse. Thromb J. 2021; 19: 61.
- Liu F, Wu C, Yang X, Xiao H, Zhuo X, Cheng Z, et al. Polarization and apoptosis of T cell subsets in idiopathic thrombocytopenic purpura. Cell Mol Immunol. 2005; 2: 387-392.
- Xu D, Xie L, Zhang Z, Yu W, Qiu J, Xu CW, et al. Preliminary Study on Apoptotic Proteins in Platelet from Adult Patients with Chronic Immune Thrombocytopenic Purpura. Acta Haematol. 2022; 145: 318-325.