Heparin-Derived Oligosaccharides Inhibit VEGFInduced Angiogenesis In vitro
- 1. School of Life Science and Technology, China Pharmaceutical University, China
- 2. Suzhou Institute of Biomedical Engineering and Technology, Chinese Academy of Sciences, China
Abstract
Anti-angiogenesis treatment of tumors has become a potential anti-tumor treatment option. We found that HDO can significantly inhibit the proliferation, migration, and adhesion of HUVEC cells, and has anti-angiogenic activity. One of its mechanisms of action is to affect the formation of the VEGF/VEGFR complex by downregulating the transcription and expression of VEGFR-2, thereby regulating the activation of downstream signaling pathways.
Keywords
Heparin-derived oligosaccharide, Tumor metastasis, Angiogenesis, Vascular endothelial growth factor, Vascular endothelial growth factor receptor 2
ABBREVIATIONS
HDO: Heparin-Derived Oligosaccharide; LMWH: Low Molecular Weight Heparin; HUVEC: Human Umbilical Vein Endothelial Cells; MTT: 3-(4, 5-dimethylthiazol-2-yl) - 2, 5-diphenyl tetrazolium bromide; PCR: Polymerase Chain Reaction; SDS: Sodium Dodecyl sulfate; VEGF: Vascular Endothelial Growth Factor; VEGFR-2: Vascular Endothelial Growth Factor Receptor 2
Citation
Qin Y, Xue J, Rubayiza J, Liu ZY, He X, et al. (2022) Heparin-Derived Oligosaccharides Inhibit VEGF-Induced Angiogenesis In vitro. Ann Vasc Med Res 9(3): 1149.
INTRODUCTION
There are two main ways of forming blood vessels in the body, namely vascular genesis and angiogenesis. Vascular genesis refers to the formation of new blood vessels from the differentiation of endothelial precursor cells or hemangioblasts of the mesoderm, and the subsequent formation of primitive and uniform vascular structures, which eventually develop into hierarchical structures including arteries, veins, and capillaries [1,2]. Angiogenesis refers to the formation of new blood vessels by endothelial cells in primitive blood vessels. It is a highly integrated and complex dynamic process. It is closely related to the activation, proliferation, extracellular matrix degradation, adhesion, and migration of endothelial cells. A variety of physiological and pathological processes are strictly controlled, such as wound healing and cancer [3]. Therefore, in normal adult tissues, angiogenesis is usually rare. Angiogenesis requires the conversion of endothelial cells from a resting state to an activated state [4].
As early as 1971, Judah Folkman first put forward the important argument that tumor growth and metastasis must depend on blood vessels, and speculated that tumors can be treated by anti-angiogenesis [5]. Since then, anti-angiogenesis strategies as a potential anti- tumor treatment option have become a research hotspot in recent years [6]. Under normal physiological conditions of the body, endothelial cells are often in a highly resting state to maintain the body’s vascular homeostasis [7,8]. When tumor growth reaches a certain limit, hypoxia, low pH, etc. will increase the secretion of some cytokines such as VEGF in the Tumor microenvironment. These cytokines bind to vascular endothelial cell surface receptors to stimulate cell activation and activate downstream signal transduction pathways. Thereby promoting the formation of new blood vessels, further causing oxygen and nutrients to reach the tumor microenvironment, supporting tumor growth and distant spread [9,10].
The balance between pro-angiogenesis factors and antiangiogenesis factors in the tumor microenvironment regulates tumor angiogenesis. VEGF is the most important pro- angiogenic factor among the pro-angiogenic factors, and it is a key factor in many diseases such as tumors, inflammations, and vascular diseases. The combination of VEGF and VEGFR activates endothelial cells, triggers the proliferation and migration of endothelial cells, and promotes angiogenesis and tumor growth [11-13]. Due to the overexpression of VEGF in tumor tissues and its important role in tumor angiogenesis, it has become the main target of anti-angiogenic drugs in cancer treatment. However, most anti-angiogenic drugs currently have vascular complications, such as thromboembolism and bleeding [14]. The migration of endothelial cells plays a key role in the process of angiogenesis. Cell migration involves dynamic coordinated changes in cell adhesion, cytoskeleton organization, and signal transduction [15,16]. Three main mechanisms stimulate endothelial cell migration: chemical tropism, contact tropism, and mechanical tropism [17]. These effects activate the downstream signaling cascade through VEGF/VEGFR-2 mediated downstream signaling pathways (MAPKs pathway, PI3K/Akt pathway, JAK/STA T pathway), leading to increased activation of transcription factors [18-23]. Transcription factors such as S TAT 3, c-Jun and NF-κB p65 bind to receptors and promoters of angiogenation-related genes after entering the nucleus, regulate gene transcription and ultimately increase endothelial cell proliferation. Migration and Angiogenesis.
A large number of preclinical studies also found that heparin and LMWH LMWH can inhibit tumor metastasis in animals, and its anti-angiogenesis effect is considered to be the main mechanism [24,25]. Both heparin and LMWH have been shown to inhibit VEGF- induced proliferation of vascular endothelial cells, and inhibit angiogenesis in a human in vitro angiogenesis model [26]. The effects of different heparins and LMWH on angiogenesis are closely related to their molecular weight and structure. Norrby et al., studied the effects of heparin components with average molecular weights of 2.5, 5.0, and 16.4 kD on VEGF-mediated blood-brain barrier angiogenesis in rats. The 2.5 and 5.0 kD heparin components significantly inhibited angiogenesis; however, 16.4 kD heparin components enhances angiogenesis [27]. However, because heparin has many side effects, the application of heparin in the field of anti-tumor is limited [28].
The heparin oligosaccharide used in this study is composed of 12 sugar units obtained by separation and purification of heparin after degradation of nitrous acid, with a molecular weight of about 3200 D [29]. Preliminary laboratory studies have found that homemade HDO can inhibit VEGF-induced proliferation of vascular smooth muscle cells and human umbilical vein endothelial cells [30], inhibit the combination of VEGF and VEGFR-2, and reduce the expression of intracellular VEGF [31]. Therefore, it is speculated that HDO has anti-angiogenic activity targeting VEGF/VEGFR. Moreover, the HDO reduces the anticoagulant activity based on retaining the antithrombotic activity and has important value in improving the bleeding complications of anti-angiogenic drugs.
MATERIALS AND METHODS
Cell lines and reagents
Human umbilical vein endothelial cells (HUVEC) were purchased from ATCC (VA, USA). HUVEC was cultured in DMEM medium (Thermo Fisher Scientific, MA, USA) containing 10% fetal bovine serum (FBS) (Thermo Fisher Scientific, MA, USA), and 100 U/mL penicillin and 0.1 mg were added to the medium /mL Streptomycin. All cells are cultured in a 37°C, 5% CO2 incubator.
HDO are composed of 12 sugar units, which are separated and purified after heparin is degraded by nitrous acid, and have a molecular weight of about 3200 D.Vorolanib (CM082) was purchased from Sigma (Germany). Recombinant human VEGFA-165 was purchased from Peprotech (USA).
E-selectin, ICAM-1, VCAM-1, VEGFR2, FAK, p38, ERK1/2, AKT, JNK1/2/3, and STAT3 antibodies were purchased from Zhengneng Bio (Chengdu).CD31 antibody was purchased from Biorbyt (UK). P-FAK, P-ERK1/2, P-AKT, P-p38, P-JNK, and P-STAT3 antibodies were purchased from Abmart (Shanghai, China).GAPDH antibody was purchased from Abways (USA). Horseradish peroxidase-conjugated anti-rabbit/mouse IgG antibody was purchased from Zhengneng Biotech (Chengdu, China).
Cell viability assay: Use MTT reagent to detect cell viability
Tube formation and migration of endothelial cells
Use the matrigel angiogenesis test to detect cell angiogenesis ability. Inoculate Matrigel (50 μL per well) (Corning, New York, USA) in a pre-chilled 96-well plate, and then incubate in a 37 ? incubator for 1 h to form a gel. HUVEC (1 × 104 per well) and medicated medium are placed in a 96-well plate. After incubating in a 37°C incubato r for 4-6 hours, use a microscope to image the microvessels.
Use the cell scratch test to detect cell migration ability. According to the picture, measure the width of the scratch position before and after the administration, calculate the healing rate of the scratch, the change in the width of the cell scratch before and after the administration/the width of the cell scratch before the administration × 100%.
Use the Transwell cell invasion test to detect cell invasion ability. Observe the cells under a microscope and take pictures. After taking pictures, add 500 μL of 10% acetic acid solution to each well to extract crystal violet, shake for 10 min, add 200 μL to a 96-well plate, and measure the absorbance at 600 nm with a microplate reader.
Western blot
Extract protein using RIPA lysis buffer containing protease and phosphatase inhibitors. The extracted protein was separated by SDS-PAGE electrophoresis, transferredTo the PVDF membrane, and incubated with the primary antibody and the horseradish peroxidase coupled secondary antibody. ECL chemiluminescent substrate (Tanon, Shanghai, China) was used to detect protein expression.
Real-time qPCR
RNA was extracted using an RNA-easy Isolation Reagent (Vazyme, Nanjing, China). Use HiScript®Q RT SuperMix for the qPCR kit (Vazyme, Nanjing, China) for reverse transcription, and then use ChamQ Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China) to detect gene expression.
Cell-based ELISA
After HUVEC cells are routinely digested, adjust the cell density and spread a 96-well plate. When the cells adhere to the wall and grow to 70%-80% confluence, the cells are grouped and administered. After the administration, they are placed in an incubator and cultured for 24 hours, and then the 96-well plate is taken out. The plate was washed twice with PBS solution, and the cells were fixed at room temperature with 4% paraformaldehyde after drying at room temperature. Block with 10% BSA blocking solution at 37°C for 90 min. Incubate the primary antibody overnight. After incubating the secondary antibody at 37°C for 60 minutes, incubate the TMB color solution at 37°C for 30 minutes in the dark. Stop the reaction with 1 M H2SO4 stop solution, and measure the absorbance at 450 nm with a microplate reader.
Tumor xenograft and immunohistochemistry
The animal experiment was approved by the Ethics Committee of China Pharmaceutical University. Male C57BL/6J mice aged 5-6 weeks were purchased from the Comparative Medicine Center of Yangzhou University (license number SCXK(Su)2017- 0007, quality certificate number NO.202016478). The mice were fed with basic feed, and they were free to eat and drink. Next, 1×105 melanoma B16F10 cells were inoculated into the tail vein of the mouse. 42 mice were randomly divided into 7 groups, namely: Control group (normal saline), Model group (normal saline), HDO low-dose administration group (10 mg/kg·d), HDO medium -dose administration group ( 20 mg/kg·d), HDO high -dose administration group (40 mg/kg·d), 20 mg/kg·d LMWH and 1 mg/kg·d Voro lanib control group, intraperitoneally administered once a day for continuous After 10 days of treatment, blood was taken to measure VEGF expression using ELISA, and lung tissue was taken to measure Ki-67 and CD31 expression using IHC.
Statistical Analysis
The experimental data were statistically analyzed using GraphPad Prism 9.0 software, all data were expressed as Mean ± SD, and statistically significant differences were analyzed by t-test. * Means P < 0.05, ** means P < 0.01, *** means P < 0.001.
RESULTS AND DISCUSSION
Heparin oligosaccharides inhibit the lung metastasis of melanoma in mice
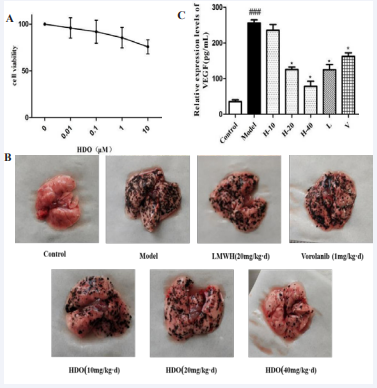
The most destructive aspect of cancer is metastasis. Through the mouse melanoma lung metastasis model, to study whether self-made HDO has an anti-tumor metastasis effect in vivo. In vitro, HDO has weak cytotoxicity to melanoma B16F10 cells, and only about 25% cytotoxicity at high concentrations (10 μM), as shown in Figure 1A.
Ten days after injection of melanoma B16F10 cells, tumor nodules were seen on the lung surface of mice in each group compared with the Control group. There were significant tumor nodules in the Model group, and no tumor nodules were seen in other tissues such as the liver. After intraperitoneal injection of HDO treatment, the number and size of lung surface tumor nodules were significantly reduced and dose-dependent, while the number of lung surface tumor nodules also decreased after LMWH and Vorolanib treatment.
These results indicate that HDO can inhibit melanoma lung in vivo the effect of metastasis, the result is shown in Figure 1B.
Figure 1: The effect of HDO on melanoma lung metastasis In vivo.
(A): Cytotoxicity of heparin oligosaccharide on B16F10 cells; (B):The effect of HDO on melanoma lung metastasis in vivo; (C): The effect of HDO on the expression of VEGF in the serum of mice with melanin lung metastasis. mean±SD, n=6; *P<0.05, **P<0.01 vs Control
Most tumor tissues highly express VEGF, which plays an important role in regulating tumor angiogenesis. This experiment tested the effect of drug treatment on the expression of serum VEGF in mouse melanoma lung metastasis. The result is shown in Figure 1C. Compared with the Control group, in the melanoma lung metastasis model, the expression of VEGF in the serum of mice was significantly increased. After adding different doses of drug treatment, the content of VEGF in the serum was significantly decreased, and HDO was dose-dependent. It indicates that inhibiting tumor tissues to secrete VEGF and inhibiting angiogenesis in tumor tissues may be one of the mechanisms of HDO’s anti-tumor metastasis.
HE staining and immunohistochemical staining
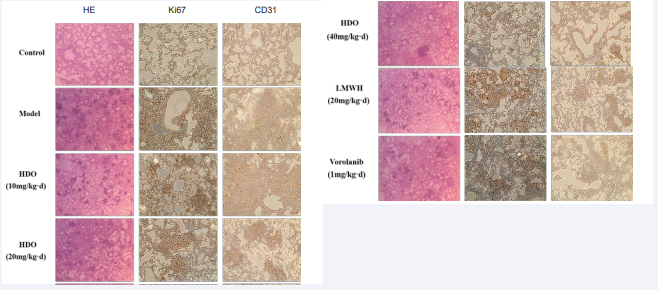
HE staining was used to observe the pathological changes of lung tissue after lung metastasis of melanoma. As shown in Figure 2. HE staining, the alveolar structure of the lung tissue of mice in the Control group was clear, and there was no thickening or congestion in the alveolar wall. In Model group, the alveoli were infiltrated by tumor cells, the alveolar wall was thickened and congested, and there were many large tumor nodules in the lung tissue. The number and size of metastatic nodules of melanoma lung were significantly reduced after drug treatment, which was consistent with morphological observation. These results indicated that HDO could effectively alleviate lung metastasis of melanoma cells, and the effect was best in the high-dose HDO group.
Figure 2: HE staining and immunohistochemical staining of mouse lung tissue In HE staining, blue is cell nucleus, red is cytoplasm and extracellular matrix, magnified 40×; in immunohistochemical staining, brown is positive signal, blue is cell nucleus, magnified 200×.
Ki67 was highly expressed in tumor nodules, indicating that tumor cells in tumor nodules were in a state of high proliferation. However, drug treatment did not affect the proliferation of tumor cells, but only affected the distribution of tumor cells by affecting the metastasis of tumor cells. After drug treatment, the expression of Ki67 in lung tissue decreased, indicating that tumor cell infiltration was inhibited, which the same as the results of HIM was staining. In addition, CD31 was also highly expressed in tumor nodules and surrounding tissues, indicating that there was more neovascularization in these locations, and this neovascularization facilitated tumor growth and metastasis.
Heparin oligosaccharides inhibit the growth of HUVECs In vitro
The proliferation of endothelial cells is a basic condition for endothelial cell angiogenesis and tumor growth and development [32]. Our previous research results and molecular docking simulation results indicate that heparin oligosaccharides can bind to the VEGF-VEGFR complex, thereby affecting receptor dimerization and phosphorylation to play a biological role [30].
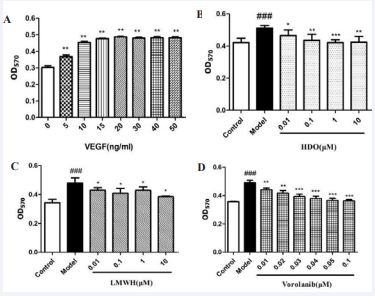
Figure 3: The effect of HDO on HUVEC cell proliferation detected by MTT
(A) : Determination of the optimal concentration of VEGF-induced HUVEC cell proliferation; (B) : Effect of HDO on HUVEC cell proliferation; (C) : Effect of LMWH on HUVEC cell proliferation; (D) : Effect of Vorolanib on HUVEC cell proliferation. mean±SD, n=6; A: ** P?0.01 vs Control group; B,C,D: ## P?0.01 vs Control group, *P ?0.05, ** P?0.01, *** P?0.001 vs Model group
Studies have found that as the molecular weight of heparin decreases, its anticoagulant activity gradually decreases, but it still retains its anti-tumor metastasis activity. Therefore, to initially compare whether there are differences in the antivascular activity of different low molecular weight heparins, this study selected low molecular weight heparin (dalteparin) prepared by the same method (nitrous acid degradation method) as self-made heparin oligosaccharides (MW: 3200 Da). Sodium, MW: 5000 Da) was used as a control. In addition, Vorolanib is a new type of tyrosine kinase inhibitor, which has a significant effect on targeting VEGFR and PDGFR against angiogenesis.
Kun Zhang et al. found that Vorolanib (CM082) can inhibit the proliferation of endothelial cells induced by VEGF with an IC50 of 0.031 ± 0.005 μM, and is more selective for VEGFR-2 than sunitinib [33].
First, the effects of heparin oligosaccharides, LMWH, and Vorolanib on the proliferation of HUVEC cells induced by VEGF were detected by the MTT method.
As shown in Figure 3A, VEGF promoted the proliferation of HUVEC cells in the range of 5-50 ng/mL and was dose-dependent. The proliferation effect of VEGF concentration higher than 10 ng/mL was higher than that of 10 ng/mL. VEGF did not increase significantly, so 10 ng/mL VEGF was chosen to establish a HUVEC cell abnormal proliferation model.
As shown in Figures 3 B,C and D, the cell proliferation ability of HUVEC cells was significantly increased after being stimulated by VEGF for 24 hours. After HDO was added, the cell proliferation ability was significantly inhibited and was dose-dependent.
The inhibitory effect of 1 μM HDO was the best. 0.01-10 μM LMWH and 0.01-0.1 μM Vorolanib can also inhibit the proliferation of endothelial cells induced by VEGF. After comprehensive consideration, two concentrations of 0.1 μM LMWH and 0.03 μM Vorolanib were selected as controls.
Heparin oligosaccharides inhibit the cell migration, invasion, and tube formation of HUVECs in vitro
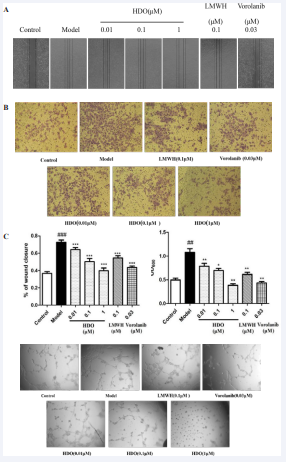
The migration of endothelial cells is the basis of endothelial cell angiogenesis. This study investigated the effect of HDO on the migration ability of HUVEC cells through a cell scratch experiment.As shown in Figures 4A and C, compared with the Control group, VEGF in the Model group significantly increased the scratch healing rate of HUVEC cells. HDO inhibited the wound healing of HUVEC cells in a dose-dependent manner, indicating that HDO can inhibit the abnormal migration of HUVEC cells. In addition, 0.1 μM LMWH and 0.03 μM Vorolanib also significantly inhibited HUVEC cell migration compared with the Model group, but the inhibitory effect of 0.1 μM.
LMWH was not as good as the concentration of HDO. The receptor inhibitor Vorolanib also significantly inhibited the migration of HUVEC cells.
Figure 4: The effect of HDO on HUVEC cell migration, invasion, and tube formation detected by Cell scratch method
(A): HUVEC cell scratch map; (B): HUVEC cell invasion crystal violet staining image; (C): HUVEC cell migration rate. (D): Acetic acid extraction after crystal violet staining to measure OD value at 600nm. (E)The effect of HDO on the angiogenesis of HUVEC in vitro detected by Tubule formation experiment Mean±SD, n=6; ### P ?0.001 vs Control group, *** P?0.001 vs Model group.
The invasion ability of endothelial cells plays a vital role in the process of endothelial cell angiogenesis. In this study, the Transwell cell invasion experiment was used to investigate the effect of HDO on the invasion ability of HUVEC cells induced by VEGF. As shown in Figures 4B and D, compared with the Control group, VEGF in the Model group significantly induced an increase in the invasion of HUVEC cells into the lower ventricle; HDO inhibited the invasion of HUVEC cells into the lower ventricle in a dose-dependent manner. In addition, 0.1 μM LMWH and 0.03 μM Vorolanib also significantly inhibited HUVEC cell invasion compared with the Model group, and the inhibitory effect of 0.1 μM LMWH on endothelial cells invasion was slightly higher than that of the same concentration of HDO. The receptor inhibitor Vorolanib also significantly inhibited the invasion of HUVEC cells.
The effect of HDO on the angiogenesis of HUVEC cells induced by VEGF was investigated by an in vitro tubule production model. As shown in Figure 4E, compared with the Control group, after HUVEC cells were induced with 10 ng/mL VEGF factor, more tubules were formed in the Model group, while HDO (0.01 μM, 0.1 μM, 1 μM) was administered. The tubular structure of endothelial cells was damaged, and 1 μM HDO damaged the tubular structure of endothelial cells extremely significantly. After administration of 0.1 μM LMWH, the structure of the small tube was slightly affected, and the effect was less than 0.1 μM HDO; after the administration of Vorolanib, the structure of the small tube was also significantly damaged, and the damaging effect was slightly higher than 0.1 μM HDO but significantly lower than 1 μM HDO, indicating HDO and Vorolanib may have similar mechanisms in anti-angiogenesis.
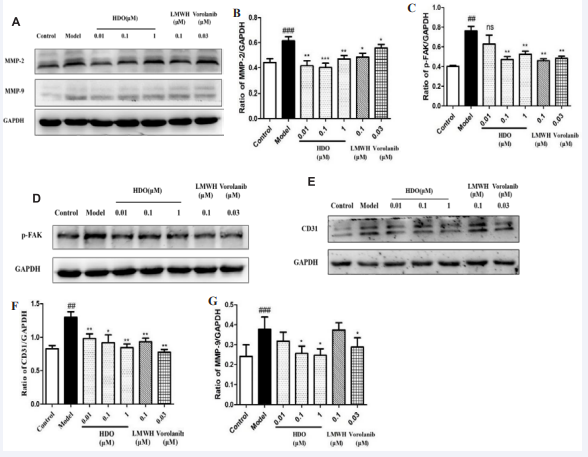
Heparin oligosaccharides inhibit VEGF-induced protein expression of MMP-2/-9, P-FAK, and CD31 in HUVEC cells
To further study the mechanism of HDO inhibiting the migration and invasion of HUVEC cells, the Western Blotting was used to investigate the protein expression of HDO on the key proteolytic enzymes MMP-2 and MMP-9 of HUVEC cells. As shown in Figures 5 A-C, the expression of MMP-2 and MMP-9 activated proteins in the Model group was significantly increased under the induction of VEGF. After HDO treatment, MMP-2 protein expression was significantly inhibited but not dose-dependent and 0.1 μM HDO showed the best inhibition effect. In addition, HDO significantly inhibited the protein expression of MMP-9 in a dose-dependent manner. After 0.1 μM LMWH treatment, MMP-2 protein expression was inhibited but MMP-9 protein expression was not inhibited, and the inhibition effect was lower than that of HDO at the same concentration.
Figure 5: The effect of HDO on the expression of MMP-2/-9, P-FAK, and CD31 in HUVEC cells by Western Blotting
(A): Western Blotting assay of MMP-2, MMP-9 in HUVEC after administration of HDO and GAPDH was used as loading control; (B,C) : Optical density value analysis results of MMP-2/GAPDH ,MMP-9/ GAPDH in HUVEC cells; (D) : Western Blotting assay of p- FAK in HUVEC after administration of heparin oligosaccharide and GAPDH was used as loading control; (E) : Western Blotting assay of CD31 in HUVEC after administration of HDO and GAPDH was used as loading control; (F) : Optical density value analysis results of p-FAK/GAPDH in HUVEC. (G) : Optical density value analysis results of CD31/GAPDH in HUVEC. Mean ± SD, n=3; ##P ?0.01 , ###P?0.001 vs Control group, *P?0.05, **P?0.01, ***P?0.001 vs Model group .
Activation of FAK can affect the formation of FA, which can then affect the cytoskeleton and structure of cell adhesion sites to regulate cell movement and lead to increased cell migration [18]. Western Blotting assay was used to detect the effect of HDO on FAK phosphorylated protein expression. As shown in Figures 5 D and F, the FAK phosphorylation level in the Model group was significantly increased compared with the Control group, while the FAK phosphorylation level was inhibited in a dose- dependent manner after the action of HDO, in which the inhibition effect of 1 μM HDO was inferior to 0.1 μM HDO. The phosphorylation of FAK was also significantly inhibited by 0.1 μM LMWH and 0.03 μM Vorolanib compared with the Model group, and the inhibition effect was similar to 0.1 μM HDO.
Western Blotting was used to detect the effect of HDO on the expression level of CD31 protein. As shown in Figures 5E and G, compared with the Control group, the expression of CD31 protein in HUVEC cells in the Model group was significantly increased after the addition of 10 ng/mL VEGF induction, and the expression of CD31 protein was inhibited to varying degrees in a dose-dependent manner after the action of different concentrations of HDO. In addition, 0.1 μM LMWH significantly inhibited the expression of CD31 protein in the control group, which was slightly lower than that in the same concentration of HDO. Vorolanib, an angiogenic inhibitor targeting VEGFR, also inhibited CD31 expression, similar to high HDO concentrations. The effect of HDO on the vascular production of VEGF-induced HUVEC cells reflected in the expression level of CD31 was consistent with the results of the tube formation experiment.
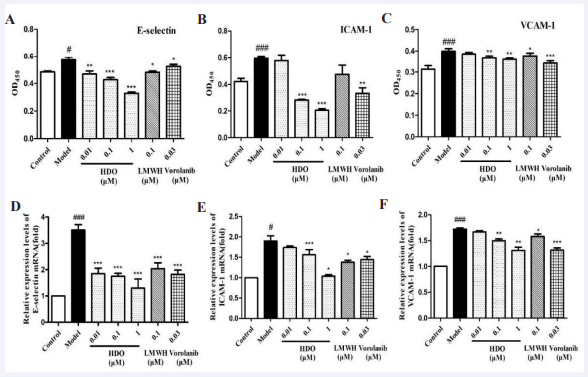
Heparin oligosaccharide inhibited VEGF-induced protein expression and gene transcription of HUVEC cell adhesion molecules
Adhesion molecules can mediate intercellular interactions of homologous or heterologous molecules and mediate intercellular adhesion. The cell-based ELISA has been used to detect the effects of HDO on the protein expression of VEGF-induced adhesion molecules E-selectin, ICAM-1, and VCAM-1 in HUVEC cells. As shown in Figure 6, compared with the Control group, the protein expression and gene transcription of adhesive molecules (E-Selectin, ICAM-1, and VCAM-1) in the Model group were significantly increased after VEGF stimulation, and the effect on ICAM-1 was the largest, followed by E-Selectin. HDO inhibited the expression and transcription of these adhesive molecules in a concentration-dependent manner. Compared with the Model group, 0.1 μM LMWH and 0.03 μM Vorolanib also significantly inhibited the expression and transcription of these adhesive molecules. In general, the inhibitory effect of 0.1 μM LMWH and 0.03 μM Vorolanib were not as good as that of HDO, which may affect the effect of Vorolanib on endothelial cell adhesion. In conclusion, HDO can regulate the transcription and expression of adhesive molecules (E-selectin, ICAM-1, VCAM-1) and inhibit the adhesion and migration of HUVEC cells through related signaling pathways.
Figure 6: The effect of HDO on the expression of adhesion molecule proteins in HUVEC detected by Cell-based ELISA and qPCR
(A) : The effect of HDO on the expression of E-selectin in HUVEC; (B) : The effect of HDO on the transcription of ICAM-1 in HUVEC; (C) : The effect of HDO on the expression of VCAM-1 in HUVEC; (D) : The effect of HDO on the transcription of E- selectin in HUVEC; (E) : The effect of HDO on the transcription of ICAM-1 in HUVEC; (F) : The effect of HDO on the transcription of VCAM-1 in HUVEC. Mean±SD, n=6; # P ?0.05, ### P?0.001 vs Control group, *P?0.05, ** P?0.01, *** P?0.001 vs Model gro
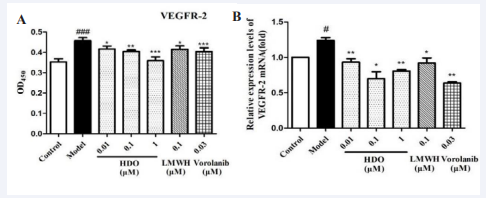
Heparin oligosaccharide inhibits VEGFR-2 protein expression and gene transcription induced by VEGF in HUVEC cells
This study investigated the effect of HDO on VEGFR-2 protein expression and gene transcription induced by VEGF in HUVEC cells by cell-based ELISA and qPCR, to explore the antiangiogenesis mechanism of HDO targeting VEGF/VEGFR-2. As shown in Figure 7A, VEGFR-2 protein levels were significantly increased after 10 ng/ mL VEGF stimulation, while the expression levels were significantly decreased in a dose-Dependent manner after different concentrations of HDO. As shown in Figure 7B, HDO also significantly inhibited VEGFR-2 mRNA levels, with 0.1 μM HDO showing the best inhibition. VEGFR-2 protein expression and gene transcription were also inhibited by 0.1 μM LMWH, but the inhibition was not as effective as HDO concentration. VEGFR inhibitor Vorolanib also significantly inhibited VEGFR-2 protein and mRNA expression levels, with similar effects compared to HDO. It is concluded that HDO may inhibit the formation of the VEGF/VEGFR-2 complex and inhibit the proliferation and migration of HUVEC cells by inhibiting the expression of VEGF and the transcription and expression of VEGFR-2. It is speculated that HDO may inhibit the activation of VEGFR-2 downstream signaling molecules and thus inhibit signal transduction.
Figure 7: The effect of HDO on VEGFR-2 of HUVEC detected by Cell- based ELISA and qPCR
(A) : Effect of HDO on the expression of VEGFR-2 in HUVEC; (B) : Effect of HDO on the transcription of VEGFR-2 in HUVEC. Mean ± SD ,n=6 ?#P?0.05,###P?0.001 vs Control group, *P?0.05, **P?0.01, ***P?0.001 vs Model group.
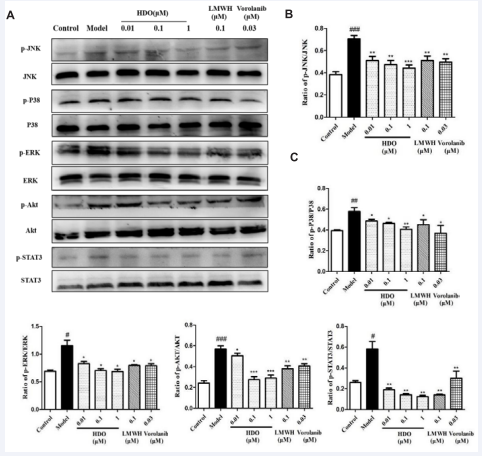
Heparin oligosaccharide inhibits VEGFR-2 - mediated activation of key downstream proteins in HUVEC cells
The binding of VEGFR-2 induces activation by oligomerization and phosphorylation of the receptor, which further activates downstream signaling pathway proteins and activates some transcriptional regulatory factors into the nucleus, thereby transducing extracellular signals into the nucleus and stimulating endothelial cell activation [13]. VEGFR-2 mediated downstream signaling pathways include the MAPKs pathway, PI3K/Akt pathway, and JAK/STAT pathway [18,19].
Figure 8: The effect of HDO on the phosphorylation level of key proteins downstream of VEGFR-2 in HUVEC detected by Western Blotting Western Blotting detection results and optical density value analysis results of p-JNK/JNK, p-P38/p38, p-ERK/ERK, p-Akt/Akt and p-STAT3/STAT3 in HUVEC after the action of HDO. Mean ± SD, n=3; #P ?0.05, ##P?0.01 , ###P?0.001vs Control group, *P?0.05,**P?0.01, ***P?0.001 vs Model group.
In this study, changes in phosphorylation levels of key protein JNK/ERK/P38 in MAPKs pathway, key protein Akt in PI3K/Akt pathway, and key protein STAT3 in JAK/STAT pathway were detected by Western Blotting. As shown in Figure 8, the phosphorylation levels of JNK, ERK, P38, Akt, and STAT3 downstream signal molecules of VEGFR-2 were significantly increased in HUVEC cells after induction of 10 ng/mL VEGF. Different concentrations of HDO significantly reduced their phosphorylation levels in a dose-dependent manner. The effect of 0.1 μM LMWH was similar to that of HDO at the same concentration. Vorolanib, a 0.03 μM VEGFR inhibitor, also showed a similar inhibitory effect, but the phosphorylation inhibition of STAT3 was weak, which is consistent with literature reports [33]. The phosphorylation inhibition of HDO on key protein JNK/ERK/P38 of VEGFR-2 mediated downstream MAPKs pathway, key protein Akt of PI3K/Akt pathway, and key protein STAT3 of JAK/STAT pathway was consistent with the expected results. These results indicate that HDO can be developed as an anti-angiogenic agent targeting VEGF/VEGFR-2, but the specific molecular regulatory mechanism of VEGF/VEGFR-2 remains to be further studied.
CONCLUSION
It can be seen from the above experimental results that VEGFR inhibitor Vorolanib can significantly destroy the tubule structure of HUVEC cells and inhibit the expression and transcription of VEGFR-2 receptor based on inhibiting the proliferation and migration of HUVEC cells induced by VEGF. It also significantly inhibited phosphorylation of JNK/ERK/P38/Akt/STAT3, a key protein in the VEGFR-2 downstream signaling pathway. HDO is similar to vegFR-2 receptor inhibitor Vorolanib, suggesting that HDO can target VEGF/VEGFR-2 and influence downstream signaling pathway activation to play an anti-angiogenesis role. However, the specific molecular regulatory mechanism of VEGFR-2 targeting remains to be further studied. In addition, we can see that VEGFR-2 expression levels are significantly decreased after HDO treatment, but the specific regulation of receptor expression needs to be further studied.
ACKNOWLEDGEMENTS
We would like to thank Mr. He Shuying for her guidance and all the laboratory staff for their effort.
REFERENCES
5. Folkman J. Proceedings: Tumor angiogenesis factor. Cancer Res. 1974; 34: 2109-2113.
6. Bagley RG. Commentary on Folkman: “Tumor Angiogenesis Factor”. Cancer Res. 2016; 76: 1673-1674.
9. Kerbel R S. Tumor angiogenesis. N Engl J Med. 2008; 358: 2039-2049.
10.Marmé, Dieter. Tumor Angiogenesis: A Key Target for Cancer Therapy. Oncol Res Treat. 2018; 41: 164.