Integrated Embolic Protection for Reduction of Microembolization during Carotid Artery Stenting: A DW MRI Proof of Principle Study
- 1. Medizinisches Versorgungs zentrum (MVZ), Prof. Schofer, Hamburg, Germany
- 2. Asklepios Klinik, Institute for Diagnostic and Interventional Radiology, Asklepiosklinik St. Georg, Hamburg, Germany
Abstract
Background: Carotid artery stenting (CAS) has shown equivalent effectiveness and safety from major adverse events compared to carotid endarterectomy. However, the risk of minor stroke remains higher with CAS. This may be related to the microemboli created during the CAS procedure itself, particularly during post stent dilation. To date, traditional distal filter-based embolic protection strategies have not been able to completely mitigate the risk of minor stroke. The Paladin® balloon system with integrated embolic protection (IEP) featuring a 40 μm pore filter was developed to address procedural microembolization.
Methods: A total of 33 patients underwent CAS. Paladin® IEP was used for post dilation per its instructions for use in all cases. To identify new ischemic lesions, diffusion weighted magnetic resonance imaging (DW-MRI) was performed the same day pre-procedure (baseline) and post procedurally within 48 hours.
Results: Among 33 CAS patients, the mean age was 69.7 ± 8.4 years and 84.8% were symptomatic. Mean baseline stenosis was 90.3% and mean lesion length was 16.8 mm. CAS was performed on the internal carotid artery (63.6% right, 36.4% left) with carotid stents from 3 manufacturers. Procedure technical success was 100%. DW-MRI identified new ischemic lesions in 7 (21.2%) patients (6 symptomatic at baseline) with a mean lesion volume of 0.044 ± 0.09 cm3.
Conclusions: Despite 84.8% of patients being symptomatic, the incidence of new cerebral lesions post-CAS detected by DW-MRI in this study was lower than has been previously reported, suggesting that the Paladin® IEP System’s 40 μm pore size successfully captures smaller microemboli when compared to current filters, preventing these particles from entering the cerebral circulation.
Keywords: Carotid Artery Stenting; Cerebral Embolic Protection; Distal Protection Device; Periprocedural Stroke; Diffusion-Weighted Magnetic Resonance Imaging ;Minor Stroke
Abbreviations: CAS: Carotid Artery Stenting; CEA: Carotid Endarterectomy; DW-MRI: Diffusion-Weighted Magnetic Resonance imaging; EPD: Embolic Protection Device; MI: Myocardial Infarction; NIHSS: National Institutes Of Health Stroke Scale;
Introduction
Carotid artery stenting (CAS) is an effective treatment for carotid artery revascularization on par with carotid endarterectomy (CEA) in preventing major stroke, as evidenced by key randomized trials [1-3]. However, the risk of minor stroke during CAS, although numerically low, remains higher in CAS vs. CEA [4-9]. This higher risk is believed to be largely associated with the procedural aspect of endovascular intervention, specifically during the post dilation stage immediately following stent deployment and expansion [10-14]. Studies have shown that stent post dilation is associated with a higher risk of peri-procedural stroke and mortality [15]. Post dilation consists of a percutaneous transluminal angioplasty (PTA) balloon inflating into the stent covering the atherosclerotic plaque with high outward radial pressure in an attempt to maximize luminal gain. However, the process of balloon deflation and plaque extrusion through the stent struts may inadvertently create atheromatous particles that can be distally embolized to the brain. Currently, risk mitigation is managed by the use of embolic protection devices (EPDs), but all currently available distal filter based embolic protection devices have pore sizes ≥ 100 μm and several studies have shown that 72% to 90% of embolic debris released during stent post dilation is < 90 µm [14,16,17]. Evidence from DW-MRI and transcranial doppler have revealed microemboli do indeed reach the middle cerebral artery in nearly every case even when an EPD is used, showing a causal mechanistic explanation for the higher risk for minor stroke with CAS compared to CEA [18-21]. Although traditional EPDs have demonstrated effectiveness in preventing large particles from entering the cerebral circulation, these devices may not prevent all microparticles from entering the brain [22,23]. EPD filter malapposition may be one reason for an increased risk of cerebral embolization if there is a size and shape mismatch against the arterial wall allowing emboli to evade capture at any time during the procedure, particularly in more curved or tortuous carotid anatomies. Furthermore, microparticles may travel uninhibited if they are smaller than the filter pores [22,23]. Patient or operator movements during the post-stent dilation might lead to transient malapposition with distal EPDs [24]. To reduce this risk, many practitioners have opted to obviate post-dilation altogether unless absolutely necessary and “double-down” with dual EPD ?ltration during CAS by using 2 distal filters or 1 distal filter in tandem with 1 proximal occlusion balloon [25-28]. The Paladin® Carotid Balloon System with Integrated Embolic Protection (IEP; Contego Medical, Inc., Raleigh, NC) is a seamless 2-in-1 angioplasty balloon with an integrated embolic protection filter with 40 μm pores designed to reduce the number of particles reaching the brain during the post dilation phase of CAS (Figure 1). In 2019, Langhoff et al., conducted a prospective, multicenter, nonrandomized study of 106 CAS subjects in whom the Paladin® IEP System was used and reported initial acute safety outcomes [17]. The device captured particles in every filter used, 90% of which were <100 μm in size and demonstrated greater capture efficiency of microparticles that might have otherwise traveled through filters with pore sizes of >100 μm, thus ostensibly reducing the risk of stroke. This current observational series was conducted in our center to further demonstrate the protective nature of the integrated 40 μm embolic protection filter against periprocedural minor stroke and clinically silent new ischemic lesions on DW-MRI following CAS.
Materials and Methods
Study Device: The Paladin® IEP System is a rapid exchange catheter that uniquely consists of a semi-compliant balloon and a nitinol-based 40 μm embolic protection filter deployed in a single step aimed at intercepting debris during CAS (Figure 2). To facilitate clear identification under fluoroscopy, radiopaque markers are incorporated on to both the balloon and filter. The filter can be adjusted in vivo up to a diameter of 7 mm to customize to individual patient anatomies.
Carotid Artery Stenting : A total of 33 patients with either a symptomatic carotid artery stenosis of at least 50% or an asymptomatic carotid artery stenosis of at least 70% were included. Twenty eight (72.4%), patients were symptomatic or had experienced a neurological event within the 6 months prior to the CAS procedure. Written informed consent was obtained from all patients. DW-MRI was obtained the morning before CAS. The procedure was performed under local anesthesia with femoral artery access in all patients. Patients were administered dual antiplatelet therapy with aspirin 100 mg per day and clopidogrel 75 mg per day for at least 5 days prior to the procedure, or they had received a loading dose of aspirin 500 mg and/or clopidogrel 600 mg by mouth immediately prior to the procedure. Intravenous unfractionated heparin was administered during the CAS to a target activated clotting time (ACT) greater than 250 seconds. A 6F Flexor® Shuttle® Guiding Sheath (Cook Medical LLC, Bloomington, IN) was advanced into the common carotid artery using standard interventional techniques. An Emboshield NAV6™ filter device (Abbott Cardiovascular, Abbott Park, IL) was placed distal to the lesion in the extracranial segment of the internal carotid artery (ICA). Pre-dilation of the lesion was performed at the discretion of the operator along with the choice of carotid stent. Post-dilatation was then performed using the Paladin® IEP System. The operator was directed to place the balloon entirely within the stented segment so the filter was immediately distal to the stent while the balloon was entirely positioned within. The integrated 40 μm filter was then expanded in place and wall apposition was confirmed with fluoroscopy. After balloon dilatation and deflation, the filter was collapsed, and the Paladin® IEP System was removed. Patients were discharged with clopidogrel 75 mg per day for 30 days and aspirin 100 mg per day indefinitely.
DW-MRI: Within 48 hours post-CAS, brain DW-MRI was performed to evaluate for the presence of new ischemic lesions compared to the pre-procedure imaging. MR images included T1, T2, and DW MRI, using a 1.5-T scanner (Magnetom Sonata, Siemens, Erlangen, Germany), and were evaluated by an independent radiologist without knowledge of the patients’ symptom status. Each set of images was analyzed for the number, volume, and location of new ischemic lesions (post-procedure as compared with baseline). The location of a lesion was defined as ipsilateral if it was in the vascular territory of the target carotid artery, otherwise it was defined as contralateral. Lesion volume was calculated using a method described by Sims et al. [29].
Table 1
Table 1: Demographic and lesion characteristics (n=33).
|
Characteristic |
Mean ± SD or % (n) |
|
Age, years |
69.7 ± 8.4 |
|
Male |
60.6 (20) |
|
Symptomatic |
84.8 (28) |
|
Current smoker |
9.1 (3) |
|
Contralateral stenosis |
18.2 (6) |
|
Dyslipidemia requiring medication |
90.9 (30) |
|
Hypertension requiring medication |
93.9 (31) |
|
Diabetes requiring medication |
27.3 (9) |
|
Target vessel was ICA |
100 (33) |
|
Right ICA |
63.6 (21) |
|
Left ICA |
36.4 (12) |
|
Stenosis (%) |
90.3 ± 4.6 |
|
Lesion length (mm) |
16.8 ± 5.1 |
|
Calcification |
30.3 (10) |
|
Ulceration |
15.2 (5) |
|
Thrombi |
0.03 (1) |
Values are % (n), n, or mean ± SD; Internal carotid artery (ICA)
Table 2
Table 2: Procedure characteristics.
|
Characteristic (N=33) |
% (n) or mean ± SD |
|
Stent type Cristallo Ideale™ (Medtronic, Santa Rosa, CA) Protégé™ RX (Medtronic, Santa Rosa, CA) Xact® (Abbott, Abbott Park, IL) |
6.1 (2) 6.1 (2) 87.8 (29) |
|
Treatment technical success |
100 (33) |
|
Fluoroscopy (n=32) Time (minutes ± SD) Dose (mean ± SD) |
7.3 ± 2.8 10.6 ± 5.1 |
Values are % (n) or mean ± SD.
Table 3
Table 3: DW-MRI analysis (n=33).
|
All Lesions |
|
|
Subjects with new ischemic lesions |
21.2% (7) |
|
Total number of new ischemic lesions |
9 |
|
Mean lesion volume, cm3 |
0.044 ± 0.091 |
|
Lesion volume characteristics, cm3 Minimum lesion volume Median lesion volume Maximum lesion volume |
0.1 0.22 0.29 |
Values are % (n), n, or mean ± SD.
Figure 1
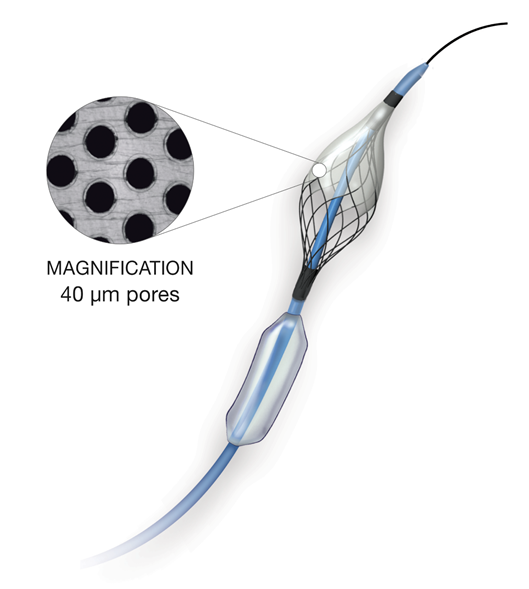
Figure 1: The Paladin® IEP System 2-in-1 (semi-compliant balloon with nitinol-based 40 μm embolic protection filter).
Figure 2
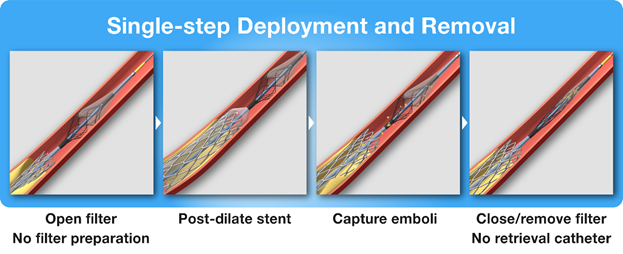
Figure 2: Deployment and removal of the Paladin® IEP System.
Results
Baseline patient demographic and lesion characteristics are reported in Table 1. All patients included in this imaging study were identified with de novo atherosclerotic carotid arteries requiring CAS intervention. The mean age was 69.7 ± 8.4 years. The majority of patients were male (60.6%), 84.8% were symptomatic, and more than 90% of all patients had hyperlipidemia (90.9%) and/or hypertension (93.9%) that required pharmacotherapy. Procedure characteristics are reported in Table 2. All target lesions were located in the internal carotid artery (ICA; 63.6% right, 36.4% left), which was accessed by a 6F guiding sheath into the CCA in all cases. 6 (18.2%) patients presented with severe contralateral carotid stenosis. Mean lesion length was 16.8 ± 5.1 mm. Pre procedure angiography revealed mean vessel stenosis of 90.3% ± 4.6%. Three different carotid stent systems were used (Table 2). Pre dilation was performed in 4 cases (12.1%). The Paladin® IEP System was used for post-dilation in 100% of patients. The balloon was inflated at a maximum pressure of roughly 9 atmospheres (atm) averaging 8.8 ± 3.8 seconds. Technical success was achieved in all cases with a mean time under fluoroscopy (FT) of 7.3 ± 2.8 minutes at a mean radiation dose of 10.6 ± 5.1 cumulative air kerma (CAK).
MRI-identified New Ischemic Lesions
DW-MRI was performed within 48 hours post procedure as compared to baseline (Table 3). New ischemic lesions were identified in 7 (21.2%) patients. All new lesions were identified in patients who underwent CAS without contralateral disease. A total of 5 of those 7 patients were identified with 1 new ischemic lesion; 2 of the 7 had 2 new ischemic lesions. Of the 28 symptomatic patients, imaging revealed new ischemic lesions in 6 of these, whereas the other 2 patients with new ischemic lesions were asymptomatic at baseline. Mean lesion volume per patient was assessed to be 0.044 ± 0.09 cm3. Clinical symptoms were not present in any of the 7 patients with new lesions post-CAS.
Discussion
Although the clinical outcomes of carotid revascularization are excellent, procedure-related sub clinical embolization is common, ranging up to 87.1% [30-32]. The embolic risk during CAS is highest during the stent deployment and post-dilation phases of the procedure, with post dilation associated with the highest risk [10-14]. The embolization during post-dilation can be detected by transcranial Doppler or DW-MRI sequencing. Prior studies show 72% to 90% of particles released during the CAS procedure are <100 µm and are smaller than pores sizes of all currently available distal filters and cell sizes of all stent types [14,16,17]. It is thus not surprising that all previous randomized trials comparing CAS to CEA have demonstrated a higher risk of minor stroke during CAS, regardless of the combination of distal filter and stent used [1,2,7,33]. Available filter-based embolic protection devices (EPDs) used during CAS procedures are beneficial in preventing large embolic particles from reaching the brain, but they do not capture all microemboli [22,23]. Highly sensitive transcranial Doppler has shown that embolic particles reach the middle cerebral artery during CAS despite the use of filter-based embolic protection [20]. Embolic particles that are smaller than 100 µm might be most likely to reach the cerebral circulation despite the use of distal filters (as a result of malapposition or through the pores of the filter) and may contribute to the higher risk of procedural minor stroke seen with CAS [17]. This is supported by Langhoff et al. in 2019, who reported their initial experience of utilizing integrated embolic protection with the Paladin® IEP System 40 µm pores that demonstrated a 30 day death, stroke, or MI rate of 1% [17]. By design, the Paladin® IEP System 40 µm filter captures microemboli less than 100 µm in size, as validated by histopathology analysis performed by Langhoff et al. [17]. Furthermore, the filter size can be dynamically adjusted to an individual patient’s anatomy to ensure a complete sealing against the carotid artery lumen, effectively maximizing capture efficiency and preventing subsequent embolization. Despite 84.8% of patients being symptomatic, DW-MRI data in the present study indicate the incidence of new lesions when CAS was performed with the 40 µm integrated filter occurred in 21.2% of subjects (7 of 33), which is comparable with CEA (17%) [34], and significantly lower when compared to a single distal filter protection (87.1%) [32], proximal protection (45.2%) [39], or mesh covered stents (48%) [35]. The mean lesion volume per patient of 0.044 ± 0.09 cm3 was 10-fold lower compared to patients with single distal filter protection (0.47 cm3) [32]. In addition to enhanced procedural embolic protection, the integrated semi-compliant angioplasty balloon reduces the number of catheter exchanges, further reducing procedural time and risk. In 2023, safety and effectiveness data were reported in the PERFORMANCE II study that evaluated the Neuroguard IEP® 3-in-1 System (Contego Medical, Inc., Raleigh, NC). Neuroguard IEP® is composed of a carotid stent, post-dilation balloon, and an integrated EPD, the same 40µm filter featured in Paladin® IEP System [36]. This prospective, multicenter, single arm study evaluated outcomes in 305 patients who were at elevated risk for adverse events (composite of death, stroke, and MI) enrolled across 32 centers in the US and Europe. The study found the rate of all stroke to 30 days plus ipsilateral stroke from day 31 to 12 months post-index procedure was 1.8%, which is the lowest stroke rate ever reported among any type of revascularization (CAS, TCAR, or CEA) [36]. There is a growing body of evidence suggesting that microembolization during CAS may have a negative impact on neurocognitive function. To this end, Hitchner et al. reported in 2016 that microembolization predicted short term transient cognitive decline in a population of both CAS and CEA patients (40 patients per group) [31]. The impact on longer-term cognitive and executive functioning has been unclear. To address this, Zhou et al. [37], conducted a study to determine if silent brain infarct (SBI) volume was associated with long-term cognitive changes. This study prospectively recruited 119 patients who underwent clinically indicated carotid revascularization for severe asymptomatic and symptomatic stenosis and evaluated them with rigorous brain DW-MRI pre and postoperatively, along with cognitive battery tests at pre-op, 1-, 6-, and 12-month post-CAS. They found that when the size of SBIs was correlated to memory function, patients with medium and high infarct volumes showed significant memory deterioration, whereas those with lower SBI volume did not experience the same extent of cognitive impairment [37].
Study Limitations
There are several limitations worth noting. This was a single-arm, nonrandomized study with no active control group. While the Paladin filter and a primary filter were used in all cases, a variety of stents were employed from different manufacturers (all nitinol bare stents), hence an analysis of stent-specific differences was not undertaken. Radiographs were read by a radiologist blinded to patient and surgical details, and was later reviewed by the surgeon, however interrater agreement was not implemented in the study protocol. Cognitive impairment and other neurofunctional testing were not implemented in the protocol. DW MRI has been the imaging modality of choice to detect ischemia in patients undergoing carotid procedures and demonstrated sensitivity and specificity in identifying procedure-related microemboli, even if used intraoperatively, however, its limitations have been documented [38-40].
Conclusion
In this study, the use of the novel Paladin® IEP System demonstrated a low number of new ischemic cerebral lesions post-CAS on DW MRI and compres favorably to historic TCAR and CEA MRI studies [30,32,41,42]. These findings suggests that the Paladin® IEP System with its integrated 40 μm filter reduces the number of microembolic particles reaching the brain during CAS.
Acknowledgements
The authors wish to thank Matt Groesbeck, MPH and Elizabeth Saylors, MSc for their assistance in data analysis and the preparation of this manuscript.
References
- Mantese VA, Timaran CH, Chiu D, Begg RJ, Brott TG, CREST Investigators. The Carotid Revascularization Endarterectomy versus Stenting Trial (CREST): stenting versus carotid endarterectomy for carotid disease. Stroke. 2010; 41: S31-S34.
- Rosenfield K, Matsumura JS, Chaturvedi S, Riles T, Ansel GM, Metzger DC, et al. Randomized Trial of Stent versus Surgery for Asymptomatic Carotid Stenosis. N Engl J Med. 2016; 374: 1011-1020.
- Yadav JS, Wholey MH, Kuntz RE, Fayad P, Katzen BT, Mishkel GJ, et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004; 351: 1493-1501.
- SPACE CG, Ringleb PA, Allenberg J, Brückmann H, Eckstein HH, Fraedrich G, et al. 30-day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet. 2006; 368: 1239-1247.
- Mas JL, Chatellier G, Beyssen B, Branchereau A, Moulin T, Becquemin JP, et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006; 355: 1660-1671.
- Fairman R, Gray WA, Scicli AP, Wilburn O, Verta P, Atkinson R, et al. The CAPTURE registry: analysis of strokes resulting from carotid artery stenting in the post approval setting: timing, location, severity, and type. Ann Surg. 2007; 246: 551-556.
- Massop D, Dave R, Metzger C, Bachinsky W, Solis M, Shah R, et al. Stenting and angioplasty with protection in patients at high-risk for endarterectomy: SAPPHIRE Worldwide Registry first 2,001 patients. Catheter Cardiovasc Interv. 2009; 73: 129-136.
- Brott TG, Hobson RW 2nd, Howard G, Roubin GS, Clark WM, Brooks W, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010; 363: 11-23.
- Sardar P, Chatterjee S, Aronow HD, Kundu A, Ramchand P, Mukherjee D, et al. Carotid Artery Stenting Versus Endarterectomy for Stroke Prevention: A Meta-Analysis of Clinical Trials. J Am Coll Cardiol. 2017; 69: 2266-2275.
- Rapp JH, Pan XM, Sharp FR, Shah DM, Wille GA, Velez PM, et al. Atheroemboli to the brain: size threshold for causing acute neuronal cell death. J Vasc Surg. 2000; 32: 68-76.
- Antonius Carotid Endaterectomy, Angioplasty, and Stenting Study Group. Transcranial Doppler monitoring in angioplasty and stenting of the carotid bifurcation. J Endovasc Ther. 2003; 10: 702-710.
- Ackerstaff RG, Suttorp MJ, van den Berg JC, Overtoom TT, Vos JA, Bal ET, et al. Prediction of early cerebral outcome by transcranial Doppler monitoring in carotid bifurcation angioplasty and stenting. J Vasc Surg. 2005; 41: 618-624.
- Hellings WE, Moll FL, De Vries JP, Ackerstaff RG, Seldenrijk KA, Met R, et al. Atherosclerotic plaque composition and occurrence of restenosis after carotid endarterectomy. JAMA. 2008; 299: 547-554.
- Petkoska D, Zafirovska B, Vasilev I, Saylors E, Sachar R, Kedev S. Transradial carotid artery stenting using double layer micromesh stent and novel post-dilation balloon with integrated embolic protection. Cardiovasc Revasc Med. 2024; 63: 43-51.
- Bosiers M, de Donato G, Deloose K, Verbist J, Peeters P, Castriota F, et al. Does free cell area influence the outcome in carotid artery stenting? Eur J Vasc Endovasc Surg. 2007; 33: 135-141.
- Langhoff R, Petrov I, Kedev S, Milosevic Z, Schmidt A, Scheinert D, et al. PERFORMANCE 1 study: Novel carotid stent system with integrated post-dilation balloon and embolic protection device. Catheter Cardiovasc Interv. 2022; 100: 1090-1099.
- Langhoff R, Schofer J, Scheinert D, Schmidt A, Sedgewick G, Saylors E, et al. Double Filtration During Carotid Artery Stenting Using a Novel Post-Dilation Balloon With Integrated Embolic Protection. JACC Cardiovasc Interv. 2019; 12: 395-403.
- Gargiulo G, Sannino A, Stabile E. New cerebral lesions at magnetic resonance imaging after carotid artery stenting versus endarterectomy: an updated meta-analysis. PLoS One. 2015; 10: e0129209.
- Montorsi P, Caputi L, Galli S. Microembolization During Carotid Artery Stenting in Patients With High-Risk, Lipid-Rich Plaque: A Randomized Trial of Proximal Vs Distal Cerebral Protection. Journal of Vascular Surgery. 2012; 55: 1834-1835.
- Chen CI, Iguchi Y, Garami Z, Malkoff MD, Smalling RW, Campbell MS, et al. Analysis of emboli during carotid stenting with distal protection device. Cerebrovasc Dis. 2006; 21: 223-228.
- Gossetti B, Gattuso R, Irace L, Faccenna F, Venosi S, Bozzao L, et al. Embolism to the brain during carotid stenting and surgery. Acta Chir Belg. 2007; 107: 151-154.
- Müller-Hülsbeck S, Jahnke T, Liess C, Glass C, Paulsen F, Grimm J, et al. In vitro comparison of four cerebral protection filters for preventing human plaque embolization during carotid interventions. J Endovasc Ther. 2002; 9: 793-802.
- Order BM, Glass C, Liess C, Heller M, Muller-Hulsbeck S. Comparison of 4 cerebral protection filters for carotid angioplasty: an in vitro experiment focusing on carotid anatomy. J Endovasc Ther. 2004; 11: 211-218.
- Vuruskan E, Saracoglu E, Ergun U, Poyraz F, Duzen IV. Carotid artery stenting with double cerebral embolic protection in asymptomatic patients - a diffusion-weighted MRI controlled study. Vasa. 2017; 46: 29-35.
- Maynar M, Baldi S, Rostagno R, Zander T, Rabellino M, Llorens R, et al. Carotid stenting without use of balloon angioplasty and distal protection devices: preliminary experience in 100 cases. AJNR Am J Neuroradiol. 2007; 28: 1378-1383.
- Spacek M, Zimolova P, Veselka J. Carotid artery stenting without post-dilation. J Interv Cardiol. 2012; 25: 190-196.
- Saw J, Bajzer C, Casserly IP, Exaire E, Haery C, Sachar R, et al. Evaluating the optimal activated clotting time during carotid artery stenting. Am J Cardiol. 2006; 97: 1657-1660.
- Nakazaki M, Nonaka T, Takahashi A, Yonemasu Y, Nomura T, Onda T, et al. Double balloon protection during carotid artery stenting for vulnerable carotid stenosis reduces the incidence of new brain lesions. Acta Neurochir (Wien). 2016; 158: 1377-1386.
- Sims JR, Gharai LR, Schaefer PW, Vangel M, Rosenthal ES, Lev MH, et al. ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology. 2009; 72: 2104-2110.
- Bonati LH, Jongen LM, Haller S, Flach HZ, Dobson J, Nederkoorn PJ, et al. New ischaemic brain lesions on MRI after stenting or endarterectomy for symptomatic carotid stenosis: a substudy of the International Carotid Stenting Study (ICSS). Lancet Neurol. 2010; 9: 353-362.
- Hitchner E, Baughman BD, Soman S, Long B, Rosen A, Zhou W. Microembolization is associated with transient cognitive decline in patients undergoing carotid interventions. J Vasc Surg. 2016; 64: 1719-1725.
- Bijuklic K, Wandler A, Hazizi F, Schofer J. The PROFI study (Prevention of Cerebral Embolization by Proximal Balloon Occlusion Compared to Filter Protection During Carotid Artery Stenting): a prospective randomized trial. J Am Coll Cardiol. 2012; 59: 1383-1389.
- Halliday A, Bulbulia R, Bonati LH, Chester J, Cradduck-Bamford A, Peto R, et al. Second asymptomatic carotid surgery trial (ACST-2): a randomised comparison of carotid artery stenting versus carotid endarterectomy. Lancet. 2021; 398:1065-1073.
- Bonati LH, Jongen LM, Haller S, Flach HZ, Dobson J, Nederkoorn PJ, et al. New ischaemic brain lesions on MRI after stenting or endarterectomy for symptomatic carotid stenosis: a substudy of the International Carotid Stenting Study (ICSS). Lancet Neurol. 2010; 9: 353-362.
- Karpenko A, Bugurov S, Ignatenko P, Starodubtsev V, Popova I, Malinowski K, et al. Randomized Controlled Trial of Conventional Versus MicroNet-Covered Stent in Carotid Artery Revascularization. JACC Cardiovasc Interv. 2021; 14 :2377-2387.
- Gray WA, Metzger DC, Zidar J, Kedev S, Petrov I, Soukas P, et al. The PERFORMANCE II Trial: A Prospective Multicenter Investigation of a Novel Carotid Stent System. JACC Cardiovasc Interv. 2025; 18: 367-376.
- Zhou W, Baughman BD, Soman S, Wintermark M, Lazzeroni LC, Hitchner E, et al. Volume of subclinical embolic infarct correlates to long-term cognitive changes after carotid revascularization. J Vasc Surg. 2017; 65: 686-694.
- Baliyan V, Das CJ, Sharma R, Gupta AK. Diffusion weighted imaging: Technique and applications. World J Radiol. 2016; 8: 785-798.
- Piñero P, González A, Mayol A, Martínez E, González-Marcos JR, Boza F, et al. Silent ischemia after neuroprotected percutaneous carotid stenting: a diffusion-weighted MRI study. AJNR Am J Neuroradiol. 2006; 27:1338-1345.
- Jaeger HJ, Mathias KD, Drescher R. Diffusion-weighted MR imaging after angioplasty or angioplasty plus stenting of arteries supplying the brain. AJNR Am J Neuroradiol. 2001; 22: 1251-1259.
- Alpaslan A, Wintermark M, Pintér L, Macdonald S, Ruedy R, Kolvenbach R. Transcarotid Artery Revascularization With Flow Reversal. J Endovasc Ther. 2017; 24: 265-270.
- Schofer J, Musia?ek P, Bijuklic K, Kolvenbach R, Trystula M, Siudak Z, et al. A Prospective, Multicenter Study of a Novel Mesh-Covered Carotid Stent: The CGuard CARENET Trial (Carotid Embolic Protection Using MicroNet). JACC Cardiovasc Interv. 2015; 8: 1229-1234.








































































































































