Intentional Occlusion of the Brachial Artery in Arteriovenous Fistula Ruptured Treated with Merit Wrapsody Covered Stent: A Case Report
- 1. Hospital do Servidor Público Estadual de São Paulo, IAMSPE, Brazil
Abstract
We present a case report on the off-label use of the Merit Wrapsody® covered stent for the treatment of an Intentional occlusion of the brachial artery in arteriovenous fistula. A female patient with a previous brachiobasilic arteriovenous fistula was admitted with a cut and bruise wound on the left arm (bathroom stall), which evolved into basilic vein laceration and acute hemorrhagic shock. The patient was then subjected to an arm tourniquet to prevent acute bleeding, which led to acute brachial artery occlusion. The patient was submitted to an endovascular approach with thrombectomy with Indigo System and then an implant of a Merit Wrapsody 8x125mm covered stent within the brachial artery and concomitant resection of the venous segment and skin containing the cut-bruise wound + venous reconstruction. The patient evolved very well with complete resolution of the acute trauma and complete arteriovenous fistula salvage in a 3-year follow-up. This device typically indicated for use in hemodialysis access, was successfully employed to correct an occluded artery. This case highlights the feasibility and potential advantages of this endoprosthesis in arterial settings beyond its original indication.
Keywords
• Intentional Occlusion
• Brachial Artery
• Arteriovenous Fistula
• Merit Wrapsody Covered Stent
Citation
Nishinari K, Centofanti G, de Athayde Soares R (2025) Intentional Occlusion of the Brachial Artery in Arteriovenous Fistula Ruptured Treated with Merit Wrapsody Covered Stent: A Case Report. Ann Vasc Med Res 12(2): 1190.
INTRODUCTION
Hemodialysis (HD) treatment using vascular access is the most commonly used renal replacement therapy for patients with chronic kidney disease. Repeated access to the circulation is essential to perform adequate maintenance HD [1]. HD fistulas are surgically created communications between the native artery and vein in an extremity. Arteriovenous fistula (AVF), is an autologous arteriovenous access created by a connection of a vein to an artery (e.g. cephalic vein joined to radial artery) where the vein serves as the accessible conduit [2]. The arteriovenous access s routinely used for HD 2-5 times per week. However, the overall complication rates of AVFs are low typically ranging from 0–16%. Complications include arterial emboli (1-7%); post–percutaneous transluminal angioplasty (PTA) flow-compromising ruptures (2-5%; the rate can be higher in native fistulas of upper arm [15%]); fluid overload or pulmonary edema; reactions to the contrast agent; extravasation hematomas at puncture sites of previous dialysis procedures; infection; and death (very rare). Death may result from cardiac arrhythmia, pulmonary edema, or a reaction to the contrast medium. Although clots may migrate into the pulmonary circulation, clinically evident pulmonary embolism has been reported in only six cases; however, pulmonary embolism may occur with native fistulas [3,4]. The Primary fistulas suggested in AVF include: (I) brachiocephalic fistula, (II) brachiocephalic fistula, and (III) basilic vein transposition (brachial-basilic) fistula. The most common trauma to AV fistulas is repeated needle cannulations during dialysis. Dialysis technicians and patients are taught to rotate needle insertion sites during each dialysis. Proper cannulation is a vital and fundamental skill that every hemodialysis nurse should master. First cannulation should be carried out by nurses who have great expertise [5]. Nevertheless, bleeding from an arteriovenous fistula (AV fistula) due to trauma, especially for dialysis patients, is a serious complication requiring immediate attention. Effective management involves controlling the bleeding with direct pressure and potentially other interventions while also addressing the underlying fistula injury and assessing for potential complications. Merit Wrapsody are flexible self-expanding endoprosthesis indicated for use in hemodialysis patients for the treatment of stenosis or occlusion within the dialysis outflow circuit of an Arteriovenous (AV) fistula or AV graft, consisting of Nitinol, ePTFE, PTFE structure. Several papers showed encouraging results regarding the target lesion primary patency at 30 days of 100% (45 of 45 patients had reached 30 days of follow-up). The target lesion primary patency for the patients who had completed 12 months of follow-up was 84.6% (33 of 39).4 Indeed, despite the massive use of Wrapsody in venous obstructions, there are already published papers showing satisfactory results of the use of Wrapsody in arterial segments, with 1-year follow-up [6-8]. In this study, we aimed to report the use of Merit Wrapsody® for the treatment of an Intentional occlusion of the brachial artery in arteriovenous fistula ruptured.
CASE DESCRIPTION
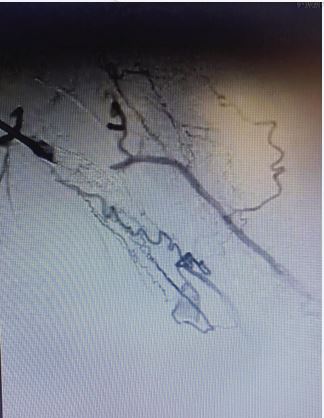
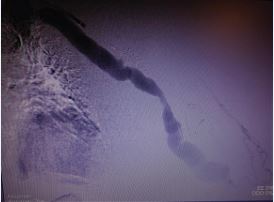
A female patient, 48 years, with a previous history of two unsuccessful kidney transplants, with a basilic vein transposition (brachial-basilic) arteriovenous fistula and a Viabahn 8x100 implanted at brachial artery due to an aneurysm, was admitted at the emergency room with an important AVF bleeding. The patient was admitted with a cut and bruise wound left arm (bathroom stall), which evolved into a basilic vein laceration and acute hemorrhagic shock. Due to the important bleeding, an arm tourniquet was applied, which provoked an AVF and brachial artery acute thrombosis. The patient was urgently submitted to an endovascular treatment of the AVF and brachial artery acute thrombosis. Under general anesthesia, a left common femoral artery was retrograde punctured guided with a duplex ultrasound to perform the whole intervention percutaneously with a proper sheat. Then, upper left arm arteriography showed: brachial artery occlusion immediately before stenting, with brachial artery refilling before anastomosis focal venous stenoses. (Figures 1 and 2).
Figure 1 Intraoperative image showing brachial-basilic arteriovenous fistula and a Viabahn occlusion.
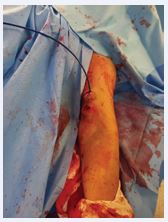
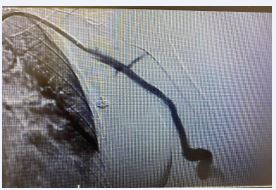
The patient was heparinized with a 5000UI intravenous bolus. The brachial artery was properly catheterized and we performed a thrombectomy with Indigo 8F, with a successful arterial recanalization followed by a proper angioplasty. After proper evaluation, we performed an implant of a Merit Wrapsody 8x125mm covered stent within the brachial artery and a concomitant resection of the venous segment and skin containing the cut-bruise wound + venous reconstruction (Figures 3 and 4).
Figure 3 Intraoperative image central vein stenosis after successfully thrombetomy.
Figure 4 Basilic vein puncture.
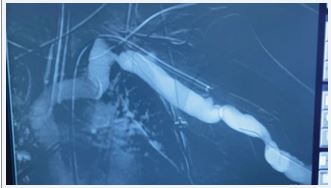
The Wrapsody stent was implanted through a basilic vein puncture, then a guidewire placement past the arterial stent femoral artery loop with a clothesline construction. Final arteriography demonstrated stent patency with excellent outflow (Figures 5 and 6).
Figure 5 Postoperative image showing covered self-expandable Merit Wrapsody 8x125mm Merit Wrapsody.
Figure 6 Postoperative image showing Arteriovenous fistula patency.
All the materials were removed, and we completed the final step of the perclose Proglide® puncture closing. The patient was transferred to the intensive unit care, being discharged from the hospital two days after the surgery with no complications, taking Clopidogrel 75mg/day. After 1 month the patient was evaluated, presenting no further symptoms, and was submitted to a Duplex Ultrasound, that showed brachial artery patency, with no stenosis. Furthermore, three years after follow-up, the patient remained asymptomatic, with Duplex Ultrasound showing stent patency and AVF patency and continually used at HD.
DISCUSSION
This present report demonstrates a clinical case of the off-label use of the Merit Wrapsody® covered stent for the treatment of an Intentional occlusion of the brachial artery in arteriovenous fistula ruptured. Trauma to hemodialysis (HD) arteriovenous (AV) fistula can lead to acute thrombosis, especially in this present case, where an arm tourniquet was necessary to stop the massive bleeding. Dember et al [9]. defined fistula suitability as the ability to use the fistula for dialysis with 2 needles and maintain a dialysis machine blood flow rate adequate for optimal dialysis (≥300 mL/min) during 8 of 12 dialysis sessions occurring during a 30-day suitability ascertainment period. Indeed, approximately 430,000 patients in the United States are dependent on hemodialysis (HD) [10], and AV acute thrombosis occurs approximately 0.5-2.0 times per year and AVF thrombosis occurs 0.1 to 0.5 times per year [11]. Conversely, in 2023, Brazil had an estimated 157,357 patients on dialysis, with a prevalence rate of 771 per million population. Of these, the vast majority (around 95%) were on hemodialysis. The number of dialysis patients and the prevalence rate have been steadily increasing in recent years [12]. Ideally, both fistula and graft thrombosis should be treated within 48 hours in order to avoid the placement of a catheter. Furthermore, older thrombi are adherent to the wall and very difficult to remove. Grafts can be salvaged up to 1 week after thrombosis, but fistulas have a shorter window of opportunity, typically 48 hours. An endovascular approach involves pharmacologically or mechanically disrupting and removing the thrombus, and then correcting the underlying lesion. This typically involves infusion of a thrombolytic such as tissue plasminogen activator, tPA in conjunction with using a lacerating device or a balloon catheter to remove the clot. Following mechanical or pharmacologic thrombolysis, an angioplasty is done to correct any underlying stenosis. An endovascular approach to thrombosed fistulas is associated with an 80% to 90% success rate and improved primary patency rates of 34% to 50% at 12 months [13]. Especially in this case report, the indigo system was performed for AVF acute thrombosis thrombectomy. A recent report evaluated twenty-six mechanical thrombectomy procedures for decluttering arteriovenous fistula thrombosis, using the Indigo System, performed in 22 patients. Technical and clinical success was achieved in 23/26 cases (88%). The mean follow-up was 9 months (range 11-539 days). The 6-month primary, primary assisted, and secondary patency rates were 71%, 86%, and 93% and the 12-month primary, primary assisted, and secondary patency rates were 71%, 72%, and 80%, respectively. The authors concluded that vacuum-assisted thrombectomy of acutely thrombosed arteriovenous fistulas and grafts with the Indigo System is safe and effective, providing good short-term patency rates [14]. Moreover, another paper published evaluated 35 patients, who underwent 44 percutaneous thrombectomy aspiration for arteriovenous fistula thrombosis with Indigo System. The authors demonstrated an average thrombectomy time of 20.2 min and a median of 15 min, with a difference between the AV fistula and synthetic graft (Graft) (p = 0.001), being shorter in the Graft. The technical success rate was 86.4% and the clinical success rate was 84.1%. Once again, the device demonstrated feasibility and efficacy in the salvage of the fistula for hemodialysis access (HD) with acute thrombosis [15]. Merit Wrapsody® are flexible self-expanding endoprosthesis indicated for use in hemodialysis patients for the treatment of stenosis or occlusion within the dialysis outflow circuit of an arteriovenous (AV) fistula or AV graft, consisting of Nitinol, ePTFE, PTFE structure. Several papers showed encouraging results regarding the target lesion primary patency at 30 days of 100% (45 of 45 patients had reached 30 days of follow-up). The target lesion primary patency for the patients who had completed 12 months of follow-up was 84.6% (33 of 39).15 The WAVE trial evaluated 245 patients with stenosis in their venous outflow circuit randomized to treatment: 122 receiving Wrapsody (CIE) and 123 receiving balloon angioplasty (PTA) across 43 international centers. The results are promising, showing that at six-month target lesion primary patency (TLPP) and Access circuit primary patency (ACPP) were significantly higher for the Wrapsody cohort versus PTA (TLPP: 89.6% vs. 62.3%; ACPP: 72.2% vs. 57.0%). Thirty days post-index procedure, there was no statistically significant difference in the freedom from safety events for the CIE versus PTA (96.6 vs. 95.0%). The authors concluded that among patients with stenosis in their AVF, the CIE was superior to PTA concerning six month TLPP and ACPP with no observed difference in 30 day primary safety events [16]. Another important fact that should be mentioned is that this present case report demonstrates acute bleeding of the AVF due to trauma. The tourniquet applied upon the arm was an important decision to stop the bleeding and prevent hemorrhagic shock and consequently death, although it has caused the brachiobasilic AV occlusion. A standard part of patient teaching should be on managing interdialytic bleeding including compression with a clean cloth and calling the dialysis facility for instructions. They should also be instructed to go to the Emergency Room or call an emergency support ambulance for a more severe, uncontrollable bleed. All patients should be taught to compress bleeding access, wash skin over access with soap and water daily and before dialysis, and also recognize signs and symptoms of infection. All patients should know to avoid carrying heavy items draped over the access arm, avoid wearing occlusive clothing, and wear protective clothing over the exposed fistula vein if working around machinery or sharp tools [17]. This case report has some limitations since it is a single case report. Larger studies should be performed in order to evaluate the safety and efficacy of endovascular treatment with Merit Wrapsody® for arterial occlusion and AVF acute thrombosis salvage.
CONCLUSION
Endovascular treatment with Merit Wrapsody covered stents for Intentional Occlusion of the brachial artery in arteriovenous fistula ruptured seems to be a safe and effective alternative for treating minimally invasive this condition, leading to an adequate flow sealing and proper aneurysms exclusion. Further and more robust studies are needed to validate these preliminary results.
INFORMED CONSENT
Proper informed consent has been obtained from the patient for publication of the case report and accompanying images.
REFERENCES
- Vascular Access Work Group. Clinical practice guidelines for vascular access. Am J Kidney Dis. 2006; 48: S248-S273.
- Lee T, Mokrzycki M, Moist L, Maya I, Vazquez M, Lok CE. North American Vascular Access Consortium. Standardized definitions for hemodialysis vascular access. Semin Dial. 2011; 24: 515-524.
- Maya ID, Oser R, Saddekni S, Barker J, Allon M. Vascular access stenosis: comparison of arteriovenous grafts and fistulas. Am J Kidney Dis. 2004; 44: 859-865.
- Gomes A, Schmidt R, Wish J. Re-envisioning Fistula First in a patient- centered culture. Clin J Am Soc Nephrol. 2013; 8: 1791-1797.
- Gilmore J. KDOQI clinical practice guidelines and clinical practice recommendations--2006 updates. Nephrol Nurs J. 2006; 33: 487-488.
- Guilherme Centofanti, Nicole Inforsato, Mariana Krutman, Kenji Nishinari and Rafael de Athayde Soares, et al. The Experience of off- the-shelf use of Merit Wrapsody® Covered stent for Arterial Disease, Int J Cardiovasc Med. 2025; 4.
- de Matos Luz CD, Horn AM, Dias VR, Fornari V, de Athayde Soares R. The Experience of Merit Wrapsody® Covered Stent for the Treatment of Popliteal Artery Aneurysms: an Alternative Device off the Shelf in a Case Series Report. J Cardiol Cardiovasc Med. 2025; 10: 093-097.
- Pedro H.M.Nunes, Paulo CG, Câmara Fábio HR. de Souza, Rafael de A.Soares. The Experience of Merit Wrapsody® Covered Stent for Aortoiliac Occlusive Disease: a Case Series Report. Int J Cardiovasc Med. 2025; 4.
- Dember LM, Beck GJ, Allon M, Delmez JA, Dixon BS, Greenberg A, et al. Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis: a randomized controlled trial. JAMA. 2008; 299: 2164-2171.
- Saran R, Robinson B, Abbott KC, Agodoa LY, Albertus P, Ayanian J, et al. US Renal Data System 2016 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2017; 69: A7-A8.
- Quencer KB, Friedman T. Declotting the Thrombosed Access. Tech Vasc Interv Radiol. 2017; 20: 38-47.
- Nerbass FB, Lima HDN, Moura-Neto JA, Lugon JR, Sesso R. Brazilian Dialysis Survey 2022. J Bras Nefrol. 2024; 46: e20230062.
- Turmel-Rodrigues L, Pengloan J, Baudin S, Testou D, Abaza M, Dahdah G, et al. Treatment of stenosis and thrombosis in haemodialysis fistulas and grafts by interventional radiology. Nephrol Dial Transplant. 2000; 15: 2029-2036.
- Cavalcante RN, Nishinari K, Centofanti G, Krutman M, De Fina B, Sato VH, et al. The role of vacuum-assisted mechanical thrombectomy in the management of acutely thrombosed arteriovenous fistulas and grafts. J Vasc Access. 2024; 25: 113-118.
- Pires GLO, Moreira Lutterbach ACT, Rivera MACP, Cavalcanti D, Ayub JP, Appolonio F, et al. Retrospective study of vacuum aspiration mechanical thrombectomy device in the treatment of arteriovenous fistula thrombosis and patient is on hemodialysis. J Vasc Access. 2025.
- Razavi MK, Balamuthusamy S, Makris AN, Hoggard JG, Harduin LO, Roy-Chaudhury P, et al. Six-month safety and efficacy outcomes from the randomized-controlled arm of the WRAPSODY Arteriovenous Access Efficacy (WAVE) trial. Kidney Int. 2025; 107: 740-750.
- Gilmore J. KDOQI clinical practice guidelines and clinical practice recommendations--2006 updates. Nephrol Nurs J. 2006; 33: 487-488.