Response to Biological Therapy with Different Attack Points among 40 Hungarian Patients Treated for Behcet’s Disease
- 1. Department of Clinical Immunology, Adult and Paediatric Rheumatology, National Institute of Locomotor System Disorders and Disabilities, Hungary
- 2. Department of Internal Medicine and Haematology, Semmelweis University, Hungary
Abstract
Behcet’s disease is a systemic vasculitis with heterogeneous clinical manifestations. In the absence of a disease-specific criteria system, the diagnosis can be made after exclusion of diseases with similar symptoms. Its targeted treatment is hampered by the lack of complete knowledge of the molecular pathomechanism, therefore the selection of the most appropriate therapy for the patient is based on international recommendations and on empirical basis. More and more often, good therapeutic results are reported with biological preparations with various mechanisms of action including TNF-alpha inhibitors. Based on a retrospective review of the written documentation, 3 therapeutic uses of biologics with different points of attack occurred among patients treated at the Clinical Immunology Outpatient Clinic of our Institute. We examine the therapeutic response to the used biological preparations among the patients under care, summing up the involvement of organ systems, previous treatments, and the duration of biological therapies. We collected the available data based on the electronic documentation of patients diagnosed based on the 2006 The International Criteria of Behcet’s disease (ICBD) criteria system between January 2009 and May 2022, and then performed a statistical analysis. In the examined period, Behcet’s disease was confirmed in 40 patients based on the classification criteria system. During the follow-up biological therapy was started in 21 patients. The patients had been under biological therapy for an average of 3.82 years. It took 2 years from the diagnosis to the start of the first biologic. In all cases, the first biological drug of choice was a TNF-alpha inhibitor. In 8 cases, it became necessary to change biological therapy due to loss of efficacy. The first line therapy was switched to another TNF-alpha inhibitor in 6 patients, IL-17A inhibitor and IL-6 receptor inhibitor, both in one case. Complete remission lasting more than 6 months was achieved in 13 cases due to the first or the second chosen biological therapy in monotherapy or in combination with corticosteroid or csDMARD in all. To maintain remission, 10 patients currently require biological treatment, 5 of them receive the first preparation.
Keywords
• Behcet’s Disease
• TNF-Alpha Inhibitor
• IL-17A Inhibitor
• IL6 Receptor Inhibitor
CITATION
Vincze A, Bazsó A, Szabó NA, Kiss EV (2023) Response to Biological Therapy with Different Attack Points among 40 Hungarian Patients Treated for Behcet’s Disease. Ann Vasc Med Res 10(4): 1172.
ABBREVIATIONS
ADA: Adalimumab; AZA: Azathioprine; bDMARD - biological Disease-Modifying Anti-Rheumatic Drug; CSA: Cyclosporine-A; CTS: Corticosteroid; csDMARD - conventional synthetic Disease-Modifying Anti-Rheumatic Drug; EET – Etanercept; GOL – Golimumab; ICBD - The International Criteria of Behcet’s Disease; IFX – Infliximab; MPED – Methylprednisolone; MTX – Methotrexate; NSAID – non-steroidal anti-inflammatory drug; PED – Prednisolone; SEC – Secukinumab; TNF – Tumor Necrosis Factor; TOZ – Tocilizumab
INTRODUCTION
Behcet’s disease is a rare systemic vasculitis with no vessel size preference, and may involve the arterial, venous, and even the capillary system. In addition to the cardiovascular system, the disease most often affects mucocutaneous tissues, eyes, joints, the central nervous system and the gastrointestinal tract. The pathomechanism responsible for the development of the diseaseis not well understood. The possibility of an autoimmune disease has arisen, in which auto-antibodies produced against endothelial cells trigger inflammation of the vessel walls [1]. However, this hypothesis is not supported by the male dominance observed in endemic areas. According to another thesis, it belongs to the family of autoinflammatory pathologies, even though Behcet’s disease that starts in childhood is very rare [2]. According to the MHC-I-opathy theory of Dennis McGonagol and his colleagues, interactions between HLAB alleles and certain tissue factors are responsible for the similarities between HLAB-associated symptoms, such as seronegative spondyloarthropathies and Behcet’s disease [3]. Based on this, it is conceivable that the immune pathological processes involved in the development of the two groups of diseases overlap, explaining the effectiveness of the therapies used in the treatment of spondyloarthropathies in Behcet’s disease. It is a multifactorial disease, the genetic predisposition represented by carrying the HLA-B51 or HLA-B52 allele among others [4,5], and most likely certain infections (e.g.: ParvoB19 virus) play a role in its development [6]. The incidence
of the disease shows a geographical distribution. It is considered endemic in Middle and Far Eastern countries located along the ancient Silk Road [7]. In addition to the male predominance observed in endemic areas, the first symptoms appear in the 20s, while in Europe and North America the disease occurs more often among women and the symptoms appear later in the 30s [8]. The diagnosis is made difficult by the lack of disease-specific genetic, biochemical, radiological and histological markers. In the absence of controlled, randomized clinical trials and professional guidelines, its treatment is based on clinical experience and international recommendations [9]. Among biological drugs, only tumour necrosis factor-alpha (TNF-alpha) inhibitors appear in these recommendations. Among the TNF-alpha inhibitors, infliximab has proven to be effective in treating the ophthalmic manifestation of Behcet’s disease in more than 300 cases. In addition, several gastrointestinal and central nervous system manifestations were successfully treated with infliximab or adalimumab [10]. Etanercept was found successful in sustaining remission for mucocutaneous findings in significantly more patients than placebo [11]. Golimumab also effectively reduced intraocular inflammation in patients previously treated with infliximab or adalimumab [12]. However, the effectiveness of biological disease-modifying anti-rheumatic drug (bDMARD) with different points of attack is controversial. Partial improvement [13] and worsening of mucocutaneous symptoms[14] have also been reported in connection with IL-17A inhibitor treatment. While the improvement of arterial lesions resistant to previous therapy [15] and the worsening of mucocutaneous symptoms [16,17] were also described in connection with IL-6 receptor inhibitor. The purpose of this study is to present the first Hungarian Behcet’s disease patient registry and to examine the therapeutic response to bDMARD with different attack points.
MATERIALS AND METHODS
Between 2009 and 2022, altogether 40 patients with Behcet’s disease were identified and followed up in Clinical Immunology Department of National Institute of Locomotor System Disorders and Disabilities in Hungary. The diagnostic criteria of ICBD was used for the diagnosis. Prevalence and the time of onset of each clinical manifestation was assessed, then routine, immunological and microbiological tests were performed. Oral and genital aphthae, erythema nodosum, as well as papulopustular eruptions were evaluated clinically. Findings were applicable if no other clinical explanation was present. To evaluate ocular involvement, slit-lamp examinations and confirmation tests of retinal vasculitis were performed by ophthalmologist. To evaluate joint involvement, X-rays and MRIs examination were performed and erosive arthritis was excluded, as the arthritis of Behcet’s disease is usually non-erosive type. Based on a retrospective review of the written documentation we examine the therapeutic response to the used biological preparations among the patients under care, considering the involvement of organ systems, previous treatments, and the duration of biological therapy. The statistical analysis was performed with the Statistic 10.0 program.
RESULTS
At our tertiary centre 40 patients (median age: 45.1 years, minimum-maximum age: 20-74 years; 47.5 % of patients are male, 52.5% of patients are female) were diagnosed with Behcet’s disease based on ICBD criteria during the study period. The mean age at the onset of complaints was 31.8 years (minimum- maximum age: 4-70 years), compared with an average age of 35.6 years (minimum-maximum age: 17-72 years) at the time of diagnosis. The mean time between the onset of complaints and the diagnosis was 5, 0 years. (minimum-maximum years: 0-31 years). The reason for the significant dispersion of the data is a patient who has been treated for juvenile idiopathic arthritis since the age of 4, and in whom the appearance of extra-articular manifestations almost 30 years later made it possible to establish a diagnosis of Behcet’s disease based on the classification criteria.
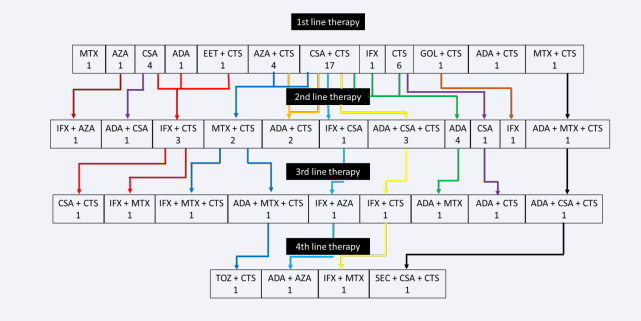
Biological therapy was administrated in 21 patients. In the case of remaining 18 patients, the disease showed no or only low activity next to systemic corticosteroid or csDMARD mono- or combined therapy, while in 1 patient the main complaint (panuveitis) responded well to local ophthalmic treatment (Figure 1).
Figure 1: Therapeutic agents used in mono- or combination therapy. Each row indicates the level of entry of agent into therapy. Arrows indicate therapeutic shifts. Abbreviations: ADA: Adalimumab; AZA: Azathioprine; CTS: Corticosteroid; CSA: Cyclosporine-A; EET: Etanercept; GOL: Golimumab; IFX: Infliximab; MTX: Methotrexate; SEC: Secukinumab; TOZ: Tocilizumab
The average age of the two groups was the same (bDMARD group: 44.5 years; minimum-maximum age: 23-74 years vs. non- bDMARD group: 45.8 years; minimum-maximum age: 20-72 years), while the proportion of men was higher among patients receiving biological therapy (bDMARD group: 52.4%; n=11 vs. non-bDMARD group: 42,1%; n=8). The time elapsed between the appearance of the first complaints and the establishment of the diagnosis was 3 times longer in the bDMARD group compared to the non-bDMARD group (6.8 years; minimum-maximum time: 0-31 years vs. 2.6 years; minimum-maximum time: 0-9 years).
In all cases the first chosen biological preparation was a TNF-alpha inhibitor: in 14 cases adalimumab, in 5 cases infliximab, and golimumab and etanercept in 1-1 cases. The first bDMARD was started in the first line of treatment in 5 cases, 2 of 5 patients were corticosteroid. and conventional synthetic disease-modifying anti-rheumatic drug (csDMARD) naive, while the other 3 patients received the first bDMARD in combination with systemic corticosteroid. In all 5 cases, non-steroidal anti- inflammatory drug (NSAID)-refractory sacroiliitis was the basis for the introduction of bDMARD. The first bDMARD was started as second line therapy in 15 cases, before those 2 patients received systemic corticosteroid, 3 patients csDMARD monotherapies (in 2 cases cyclosporine, in 1 case azathioprine), while 10 patients received systemic corticosteroid and csDMARD combination induction (in 7 cases cyclosporine, in 2 cases azathioprine and in 1 case methotrexate). The switch from the first bDMARD therapy to a second biological preparation belonging to the same efficacy group occurred in the second line in 4 out of 5 cases in patients who had already received biological treatment in the first line of therapy. (in 1 case from golimumab to infliximab; in 1 case from adalimumab to infliximab; in 1 case from etanercept to infliximab; in 1 cases from infliximab to adalimumab). In third line the first bDMARD was started after a systemic corticosteroid followed by a cyclosporin monotherapy in 1 patient. In the third line of therapy the first biological agent was changed to a second bDMARD belonging to the same group of effects in 1 case, from adalimumab to infliximab. In the case of 4 patients, a fourth-line therapeutic modification was also necessary, of which the first bDMARD was changed to a second biological preparation in 3 cases (in 1 case from infliximab to adalimumab; in 1 case from adalimumab to secukinumab and in 1 case from adalimumab to tocilizumab) (Figure 1).
The required maximum daily steroid dose of patients who later received biological therapy was twice that of patients who did not receive bDMARD (mean dose of daily steroid in bDMARD group: 123.7 mg/day; minimum-maximum dose of daily steroid in bDMARD group: 16-1000 mg/day vs. mean dose of daily steroid in non-bDMARD group: 51.6 mg/day; minimum- maximum dose of daily steroid in non-bDMARD group: 32-125 mg/day), while the duration of steroid treatment was halved when biological treatment was used (average length of steroid treatment in bDMARD group: 35.9 months; minimum-maximum length of steroid treatment in bDMARD group: 1-97 months vs. average length of steroid treatment in non-bDMARD group: 76.1 months; minimum-maximum length of steroid treatment in non- bDMARD group: 1-408 months).
Due to the heterogeneity of the disease, during an acute phase, several organ systems may be affected at the same time. Number of organ systems affected by Behcet’s disease in the bDMARD group before initiation of biological therapy: in 6-6 cases one or two organ systems were affected, while in 4 cases three and in 5 cases four organ systems were affected. The initiation of biological therapy was necessary due to sacroiliitis in 66.7%, mucocutaneous symptoms in 61.9%, ocular inflammation in 38.1%, involvement of the gastrointestinal tract in 33.3%, arthritis in 28.6%, central nervous system disease in 14.3% and cardiovascular manifestations in 4.7% of cases, in which the previously used corticosteroid, NSAD and csDMARDs proved to be ineffective. It took an average of 2 years from the establishment of the diagnosis to the initiation of biological therapy (minimum- maximum time: 0-12 years). Biological treatment took an average of 45.8 months (minimum-maximum time: 1-160 months).
In 8 cases, it became necessary to change biological therapy. The background was primary ineffectiveness in 2 cases – in 1 case from golimumab to infliximab; in 1 case from adalimumab to infliximab – and secondary loss of effectiveness in 6 cases: in 2 cases from infliximab to adalimumab, in 1 case from adalimumab to infliximab, in 1 case from etanercept to infliximab, in 1 case from adalimumab to secukinumab and in 1 case from adalimumab to tocilizumab.
In addition to biological therapy, a longer or shorter period of partial or complete freedom from symptoms or complaints was available in all cases. Partial remission was defined as a significant decrease in inflammatory parameters and symptoms associated with Behcet’s disease, while complete remission was defined as normalization of inflammatory laboratory parameters and resolution of disease-specific symptoms. Complete remission was achieved in 5 cases at the end of the first six months of the therapy with the first chosen TNF-alpha inhibitor, 1 of 5 patients was corticosteroid and csDMARD naïve, who got bDMARD in the first line of induction. In the first 6 months after switching to the second bDMARD, complete remission did not develop in any patient. Complete remission lasting more than 6 months was achieved in 13 cases due to the first or the second chosen biological therapy in monotherapy or in combination with corticosteroid or csDMARD in all. 9 out of 13 patients achieved this status with the bDMARD that was started first, while 4 patients achieved this state after the change of therapy, with the bDMARD that was chosen as the second. The average duration of complete remission exceeding 6 months was 41.2 months (minimum-maximum time: 6-135 months). By the end of the first year of biological treatment, 7 patients were in complete remission due to the first and 2 patients due to the second bDMARD, while there were patients who received biological therapy for less than a year.
In addition to biological therapy, 7 out of 13 patients in complete remission were able to discontinue systemic corticosteroid therapy, which was still necessary at the start of biological therapy. The average dose of daily steroid used at the start of biological therapy was 16.7 mg/day of methylprednisolone (MPED; minimum-maximum dose of daily MPED: 4-64 mg/day). The mean dose of daily MPED required under biological therapy was 3.0 mg/day (minimum-maximum dose of daily MPED: 0-12 mg/day). The average time required to stop taking steroids or reach a prednisolone (PED) dose of <7.5 mg/day was 12.5 months (minimum-maximum time: 2-47 months). To maintain remission, 10 patients currently also require biological treatment, 5 of them receive the first chosen bDMARD.
CONCLUSION
It was previously known that male gender and the appearance of symptoms at younger age are unfavourable signs for the prognosis of Behcet’s disease, flare-ups of the disease are more frequent, and complications caused by organ manifestations are more serious. Accordingly, in our institute it became necessary to start bDMARDs much more often among young men, as well as among patients whose symptoms appeared at younger age, in their early 30s. In addition, it was observed that in case of a delay in establishing the diagnosis, biological treatment became necessary more often later. In this way, pointing out the importance of getting to know the symptoms and pathomechanism of the disease as early as possible, as well as the need for clinical examinations for the uniformity of the treatment. In all cases, TNF-alpha inhibitor was the first choice of biological preparation, with which partial or complete relief from symptoms was achieved in all cases. In addition to the biological treatment, the use of corticosteroids was significantly reduced, 7 patients became completely steroid-free, helping to reduce the risk of late complications caused by steroids. In all cases, the change of biological therapy occurred due to the loss of efficiency of the preparation, no side effects related to TNF-alpha inhibitors were observed. As expected, TNF-alpha inhibitors provided good disease control, and experiences with IL-17A and IL-6 receptor inhibitors were also encouraging. Given that the immunopathomechanism of Behcet’s disease and seronegative spondyloarthropathies may overlap, and based on literature data, spondyloarthropathies occur in a higher proportion than expected among patients with large vessel vasculitis [18,19], the IL-17A inhibitor could be a suitable choice in the treatment of therapy-refractory Behcet’s disease. With secukinumab, the symptoms necessitating the initiation of biological therapy improved, but the patient’s previously mild gastrointestinal involvement worsened with the treatment.
While the IL-6 receptor inhibitor, based on the good therapeutic response experienced during the treatment of large vessel vasculitis, was started in a patient with Behcet’s disease complicated by aortitis, following previous TNF-alpha inhibitor treatment. Tocilizumab provided good disease control, however, accumulating respiratory and gastrointestinal infections did not allow the continuous use of the preparation. Suspension of the treatment temporarily became necessary on several occasions, which led to the rapid deterioration of the patient’s condition. In summary, we can say that a better therapeutic result can be expected from biological therapies acting on cytokine signalling pathways during the treatment of a disease with heterogeneous organ involvement, such as Behcet’s disease. However, to choose the most appropriate biological preparation, a detailed understanding of the pathomechanism of Behcet’s disease is necessary. In the absence of this, we can still rely on previous experience to start biological therapy.
REFERENCES
- Lee KH, Chung HS, Kim HS, Oh SH, Ha MK, Baik JH, et al. Human alpha- enolase from endothelial cells as a target antigen of anti-endothelial cell antibody in Behçet’s disease. Arthritis Rheum. 2003; 48: 2025- 2035.
- Gül A. Behçet’s disease as an auto inflammatory disorder. Curr Drug Targets Inflamm Allergy. 2005; 4: 81-83.
- McGonagle D, Aydin SZ, Gül A, Mahr A, Direskeneli H. ‘MHC-I-opathy’- unified concept for spondyloarthritis and Behçet disease. Nat Rev Rheumatol. 2015; 11: 731-740.
- Wallace GR, Niemczyk E. Genetics in ocular inflammation--basic principles. Ocul Immunol Inflamm. 2011; 19: 10-18.
- Verity DH, Marr JE, Ohno S, Wallace GR, Stanford MR. Behçet’s disease, the Silk Road and HLA-B51: historical and geographical perspectives. Tissue Antigens. 1999; 54: 213-220.
- Galeone M, Colucci R, D’Erme AM, Moretti S, Lotti T. Potential Infectious Etiology of Behçet’s Disease. Patholog Res Int. 2012; 2012: 595380.
- Zouboulis CC. Epidemiology of Adamantiades-Behçet’s disease. Ann Med Interne (Paris). 1999; 150: 488-498.
- Cho SB, Cho S, Bang D. New insights in the clinical understanding of Behçet’s disease. Yonsei Med J. 2012; 53: 35-42.
- Hatemi G, Christensen R, Bang D, Bodaghi B, Celik AF, Fortune F, et al. 2018 update of the EULAR recommendations for the management of Behçet’s syndrome. Ann Rheum Dis. 2018; 77: 808-818.
- Greco A, De Virgilio A, Ralli M, Ciofalo A, Mancini P, Attanasio G, et al. Behçet’s disease: New insights into pathophysiology, clinical features and treatment options. Autoimmun Rev. 2018; 17: 567-575.
- Cantarini L, Tinazzi I, Caramaschi P, Bellisai F, Brogna A, Galeazzi M,et al. Safety and efficacy of etanercept in children with juvenile-onset Behcets disease. Int J Immunopathol Pharmacol. 2009; 22: 551-555.
- Leclercq M, Desbois AC, Domont F, Maalouf G, Touhami S, Cacoub P, et al. Biotherapies in Uveitis. J Clin Med. 2020; 9: 3599.
- Alibaz-Oner F, Direskeneli H. Advances in the Treatment of Behcet’s Disease. Curr Rheumatol Rep. 2021; 23: 47.
- Dick AD, Tugal-Tutkun I, Foster S, Zierhut M, Melissa Liew SH, Bezlyak V, et al. Secukinumab in the treatment of noninfectious uveitis: results of three randomized, controlled clinical trials. Ophthalmology. 2013; 120: 777-787.
- Zhong H, Liu T, Liu Y, Zhang X, Zhou Y, Su Y, et al. Efficacy and safety of tocilizumab in Behçet’s syndrome with refractory arterial lesions: a single-centre observational cohort study in China. Rheumatology (Oxford). 2022; 61: 2923-2930.
- Diamantopoulos AP, Hatemi G.: Lack of efficacy of tocilizumab in mucocutaneous Behçet’s syndrome: report of two cases. Rheumatology (Oxford). 2013; 52: 1923-1924.
- Cantarini L, Lopalco G, Vitale A, Coladonato L, Rigante D, Lucherini OM, et al. Paradoxical mucocutaneous flare in a case of Behçet’s disease treated with tocilizumab. Clin Rheumatol. 2015; 34: 1141- 1143.
- Eshed I, Druyan A, Stern M, Bordavka M, Lidar M. The prevalence of sacroiliitis on abdominal MRI examinations of patients with Takayasu arteritis. Acta Radiol. 2022; 63: 387-392.
- Bazsó A, Szabó NA, Kiss EV. Joint occurrence of large vessel vasculitis and ankylosing spondylitis. Hungarian Rheumatology 2023; 64: 91- 95.