Tibial Shaft Fracture and Symptomatic Thrombosis: Is Chemical Prophylaxis Necessary for Prevention?
- 1. Department of Orthopedic Surgery Medical City UNT/TCU Residency Program Director, USA
Abstract
The need for chemical prophylaxis against Deep Vein Thrombosis (DVT) and Pulmonary Embolism (PE), collectively referred to as Venous Thromboembolism (VTE), following certain orthopaedic surgeries has been well established. For example, the use of chemical prophylaxis agents in total hip and knee surgeries, as well as in hip fracture surgeries, is routine.
Keywords
• Tibial Shaft Fracture
• Deep Vein Thrombosis
• Pulmonary Embolism
• Venous Thromboembolism
Citation
Riehl JT (2025) Tibial Shaft Fracture and Symptomatic Thrombosis: Is Chemical Prophylaxis Necessary for Prevention?. Ann Vasc Med Res 12(1): 1189.
INTRODUCTION
The need for chemical prophylaxis against Deep Vein Thrombosis (DVT) and Pulmonary Embolism (PE), collectively referred to as Venous Thromboembolism (VTE), following certain orthopaedic surgeries has been well established. For example, the use of chemical prophylaxis agents in total hip and knee surgeries, as well as in hip fracture surgeries, is routine [1]. The need for DVT prophylaxis in isolated fracture in other body regions is less well understood. Although many physicians routinely give chemical prophylaxis for VTE following treatment of tibial fractures, there have been few studies to specifically look at the utility of such an approach. At the same time, many surgeons do not prescribe any chemical prophylaxis for patients with isolated tibial shaft fractures. Currently, there is little direct evidence to support either approach to VTE prophylaxis practiced by many orthopaedic surgeons. Prospective, retrospective, and database studies have been performed to attempt to determine the value of chemical prophylaxis in the treatment of isolated ankle fractures and have shown a low incidence of DVT (0.28%) and PE (0.21%) overall and have recommended against the routine use of chemical VTE prophylaxis [2]. Despite guidelines available recommending against the routine use of chemical VTE prophylaxis in isolated tibia fractures [3], some continue to insist upon its use which has profound legal implications. Given the need for more data to help guide decision making, and the unscrupulous use of this issue by some in the legal profession, studies designed to evaluate rates of VTE in patients with and without chemical prophylaxis following orthopaedic surgical intervention are a valuable tool in guiding treatment. The hypothesis of the current study was that the impact of chemical prophylaxis will reduce the rate of VTE in the treatment of tibial fractures.
METHODS
Study Type and Design
A retrospective review of prospectively collected data throughout a large United States based hospital system (including multiple clinical sites) was conducted in order to compare two groups of patients with fracture of the tibia (OTA type 41, 42, and 43 fractures) as an isolated orthopaedic injury. This research activity was determined to be exempt or excluded from Institutional Review Board (IRB) oversight in accordance with current regulations and institutional policy (reference number 2023-181). In Group 1, patients received inpatient and outpatient chemical prophylaxis following treatment of their tibia fracture. In Group 2, patients were prescribed inpatient chemical prophylaxis (i.e. Low Molecular Weight Heparin, Aspirin, apixaban, etc.) but no outpatient prophylaxis after hospitalization and treatment of their tibia fracture. Group 3 patients received no inpatient prophylaxis but were prescribed outpatient chemical VTE prophylaxis for any period of time. Patients in Group 4 did not receive any inpatient or outpatient chemical prophylaxis for VTE. Patients were identified from ICD-9/ ICD-10 codes from the years 2016 – 2022. Patient demographic data was analyzed such as age, race, and gender. Body mass index (BMI), medical comorbidities (using the Elixhauser Comorbidity Index, ECI), smoking status, use of birth control medication, and length of stay in the hospital were analyzed as well. Predisposing factors for clotting were noted (this included patients with a diagnosis of any of the following at the time of tibia fracture: stroke, sickle-cell disease, COVID-19, history of VTE, Factor V Leiden, antiphospholipid syndrome, or atrial fibrillation). Additionally, data relating to the fracture such as fracture mechanism, open versus closed fracture, fracture location on the tibia (upper 1/3, shaft, lower 1/3), surgical versus nonsurgical treatment, and the use of postoperative immobilization were also considered. As the administration of chemical VTE prophylaxis for isolated fractures below the knee varies among clinical locations and treating surgeons, there was no standardized protocol as to whom received outpatient chemical VTE prophylaxis in the current database study. Prophylaxis was, therefore, at the discretion of the treating physician.
Inclusion/Exclusion Criteria
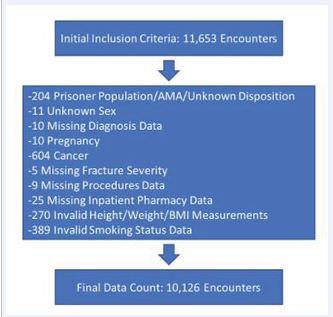
Inclusion criteria for this study were any patients over the age of 18 entered into the database with a diagnosis of a fracture of the tibia (including tibial plateau fractures, tibial shaft fractures, and tibial pilon fractures). Exclusion criteria were 1) incomplete or incorrect database entry for data points used in the regression analysis (age, gender, BMI, smoking status, etc.), 2) patients with a diagnosis of cancer, 3) patients who left the hospital against medical advice (AMA) or were part of a prisoner population, 4) those with missing inpatient pharmacy data, 5) patients with polytrauma (additional extremity fractures or those who required surgery during their hospitalization for anything other than treatment of a tibia fracture), and 6) patients who were pregnant at the time of fracture. Figure 1 depicts a flowchart of patient exclusion criteria. Endpoints were VTE diagnosis within one year from injury.
Figure 1: Depicts a flowchart of patient exclusion criteria.
Statistical Analysis
Using the generalized logistic regression technique, we were able to perform variable and model selection in order to develop the best-predicting model of the outcome. Starting with the “full” model, which includes all of the variables indicated in the protocol, a stepwise variable deletion procedure was used to arrive at a “reduced” model that produced better fit than the full model. This was done by optimizing the AIC (or Akaike Information Criterion) by systematically testing which predictors produce the lowest value of AIC (i.e., the least complex model that produces the best fit). Critically, there was no statistical difference in the likelihood of these two models (χ²(-11) = 8.15, p = .700), suggesting that either model successfully predicts the outcome with similar fit.
RESULTS
7,685 patients were included in the final analysis. Demographic data were similar between the two groups and are depicted in Table 1.
Table 1: Coefficients and model output with full and reduced models. Variable comparisons with OR of development of VTE.
|
|
Combination of Inpatient and Discharge VTE Prophylaxis |
|
||||
|
Patient Characteristic |
Overall, N = 7,6851 |
Group 1 Both Inpatient/Discharge Prophylaxis Supplied, N = 4,3321 |
Group 2 Inpatient Prophylaxis Only, N = 2,5351 |
Group 3 Discharge Prophylaxis Only, N = 1911 |
Group 4 Neither Inpatient/Discharge Prophylaxis Supplied, N = 6271 |
p-value2 |
|
Patient Age (Binned), n (%) |
|
|
|
|
|
|
|
18-29 |
872 (11%) |
485 (11%) |
273 (11%) |
19 (9.9%) |
95 (15%) |
|
|
30-49 |
2,096 (27%) |
1,197 (28%) |
655 (26%) |
59 (31%) |
185 (30%) |
|
|
50-64 |
2,150 (28%) |
1,268 (29%) |
661 (26%) |
53 (28%) |
168 (27%) |
|
|
65-89 |
2,385 (31%) |
1,300 (30%) |
869 (34%) |
53 (28%) |
163 (26%) |
|
|
90+ |
182 (2.4%) |
82 (1.9%) |
77 (3.0%) |
7 (3.7%) |
16 (2.6%) |
|
|
Patient Sex, n (%) |
|
|
|
|
|
0.17 |
|
Male |
3,692 (48%) |
2,083 (48%) |
1,193 (47%) |
90 (47%) |
326 (52%) |
|
|
Female |
3,993 (52%) |
2,249 (52%) |
1,342 (53%) |
101 (53%) |
301 (48%) |
|
|
Patient Race, n (%) |
|
|
|
|
|
|
|
White/Caucasian |
5,508 (72%) |
3,098 (72%) |
1,810 (71%) |
138 (72%) |
462 (74%) |
|
|
Black/African-American |
884 (12%) |
546 (13%) |
262 (10%) |
21 (11%) |
55 (8.8%) |
|
|
Hispanic/Latino |
1 (<0.1%) |
1 (<0.1%) |
0 (0%) |
0 (0%) |
0 (0%) |
|
|
Asian/Asian-American |
115 (1.5%) |
64 (1.5%) |
35 (1.4%) |
5 (2.6%) |
11 (1.8%) |
|
|
Native American |
8 (0.1%) |
5 (0.1%) |
1 (<0.1%) |
1 (0.5%) |
1 (0.2%) |
|
|
Multiracial/Other |
1,169 (15%) |
618 (14%) |
427 (17%) |
26 (14%) |
98 (16%) |
|
|
Patient Ethnicity, n (%) |
|
|
|
|
|
<0.001 |
|
Hispanic/Latino |
1,273 (17%) |
636 (15%) |
493 (19%) |
27 (14%) |
117 (19%) |
|
|
Not Hispanic/Latino |
6,070 (79%) |
3,477 (80%) |
1,944 (77%) |
158 (83%) |
491 (78%) |
|
|
Unknown/Decline to Answer |
342 (4.5%) |
219 (5.1%) |
98 (3.9%) |
6 (3.1%) |
19 (3.0%) |
|
|
Patient Height in Meters, Mean (SD) |
1.70 (0.10) |
1.70 (0.10) |
1.70 (0.11) |
1.70 (0.10) |
1.70 (0.11) |
0.060 |
|
Patient Weight in Kilograms, Mean (SD) |
85.04 (24.28) |
85.72 (24.70) |
84.91 (24.18) |
82.23 (23.37) |
81.81 (21.64) |
0.002 |
|
Patient's Metric BMI, Mean (SD) |
29.30 (7.71) |
29.42 (7.73) |
29.39 (7.74) |
28.38 (7.79) |
28.35 (7.30) |
<0.001 |
|
Patient Smoking Status, n (%) |
|
|
|
|
|
0.35 |
|
Current Smoker |
2,181 (28%) |
1,230 (28%) |
723 (29%) |
49 (26%) |
179 (29%) |
|
|
Former Smoker |
1,340 (17%) |
784 (18%) |
423 (17%) |
34 (18%) |
99 (16%) |
|
|
Never Smoker |
3,885 (51%) |
2,175 (50%) |
1,287 (51%) |
104 (54%) |
319 (51%) |
|
|
Unknown Smoking Status |
279 (3.6%) |
143 (3.3%) |
102 (4.0%) |
4 (2.1%) |
30 (4.8%) |
|
|
Patient Alcohol Use, n (%) |
|
|
|
|
|
0.007 |
|
Recent Alcohol Use Documented |
1,167 (25%) |
690 (26%) |
337 (22%) |
34 (28%) |
106 (30%) |
|
|
(Missing) |
3,059 |
1,685 |
1,031 |
70 |
273 |
|
|
Elixhauser Comorbidity Index, Median (IQR) |
2.00 (1.00 - 3.00) |
2.00 (1.00 - 3.00) |
2.00 (1.00 - 3.00) |
1.00 (0.00 - 3.00) |
1.00 (0.00 - 3.00) |
<0.001 |
|
Arrhythmia, n (%) |
|
|
|
|
|
<0.001 |
|
Diagnosed |
579 (7.5%) |
296 (6.8%) |
241 (9.5%) |
12 (6.3%) |
30 (4.8%) |
|
|
Current/Active Venous Thromboembolism, n (%) |
|
|
|
|
|
0.009 |
|
Diagnosed |
27 (0.4%) |
24 (0.6%) |
3 (0.1%) |
0 (0%) |
0 (0%) |
|
|
Current/Active Deep Vein Thrombosis, n (%) |
|
|
|
|
|
0.007 |
|
Diagnosed |
20 (0.3%) |
19 (0.4%) |
1 (<0.1%) |
0 (0%) |
0 (0%) |
|
|
Current/Active Pulmonary Embolism, n (%) |
|
|
|
|
|
>0.99 |
|
Diagnosed |
7 (<0.1%) |
5 (0.1%) |
2 (<0.1%) |
0 (0%) |
0 (0%) |
|
|
Condition Predisposing Clotting Issues, n (%) |
|
|
|
|
|
<0.001 |
|
Diagnosed |
882 (11%) |
472 (11%) |
351 (14%) |
15 (7.9%) |
44 (7.0%) |
|
|
Pre-Admission VTE Prophylaxis Usage, n (%) |
|
|
|
|
|
<0.001 |
|
Prior Prophylaxis Use Documented |
1,776 (23%) |
777 (18%) |
862 (34%) |
28 (15%) |
109 (17%) |
|
|
Any Pharmacologic Contraception Usage, n (%) |
|
|
|
|
|
0.54 |
|
Pharmacologic Contraception Use Documented |
49 (0.6%) |
31 (0.7%) |
12 (0.5%) |
1 (0.5%) |
5 (0.8%) |
|
|
|
Combination of Inpatient and Discharge VTE Prophylaxis |
|
||||
|
Patient Characteristic |
Overall, N = 7,6851 |
Group 1 Both Inpatient/Discharge Prophylaxis Supplied, N = 4,3321 |
Group 2 Inpatient Prophylaxis Only, N = 2,5351 |
Group 3 Discharge Prophylaxis Only, N = 1911 |
Group 4 Neither Inpatient/Discharge Prophylaxis Supplied, N = 6271 |
p-value2 |
|
First Documented Albumin Level, Mean (SD) |
4.11 (5.23) |
4.34 (6.15) |
3.83 (4.03) |
3.77 (1.58) |
3.72 (1.32) |
0.44 |
|
(Missing) |
2,316 |
1,258 |
748 |
65 |
245 |
|
|
First Documented Platelet Level, Mean (SD) |
258.79 (92.65) |
263.53 (96.13) |
253.97 (89.22) |
249.08 (83.88) |
246.98 (80.00) |
<0.001 |
|
(Missing) |
194 |
58 |
50 |
16 |
70 |
|
|
First Documented Hemoglobin Level, Mean (SD) |
12.63 (2.09) |
12.59 (2.09) |
12.65 (2.10) |
12.85 (1.89) |
12.77 (2.10) |
0.077 |
|
(Missing) |
144 |
37 |
40 |
13 |
54 |
|
|
Tibial Fracture Location, n (%) |
|
|
|
|
|
0.026 |
|
Tibial Shaft Fracture |
3,115 (41%) |
1,725 (40%) |
1,076 (42%) |
76 (40%) |
238 (38%) |
|
|
Lower Tibial Fracture |
549 (7.1%) |
283 (6.5%) |
200 (7.9%) |
16 (8.4%) |
50 (8.0%) |
|
|
Upper Tibial Fracture |
4,021 (52%) |
2,324 (54%) |
1,259 (50%) |
99 (52%) |
339 (54%) |
|
|
Fracture Severity, n (%) |
|
|
|
|
|
<0.001 |
|
Closed Fracture |
6,688 (87%) |
3,761 (87%) |
2,176 (86%) |
177 (93%) |
574 (92%) |
|
|
Open Fracture |
997 (13%) |
571 (13%) |
359 (14%) |
14 (7.3%) |
53 (8.5%) |
|
|
Mechanism-of-Injury Severity, n (%) |
|
|
|
|
|
0.009 |
|
Low Energy |
1,738 (38%) |
964 (36%) |
626 (39%) |
37 (42%) |
111 (41%) |
|
|
Medium Energy |
1,090 (24%) |
640 (24%) |
349 (22%) |
27 (31%) |
74 (28%) |
|
|
High Energy |
1,773 (39%) |
1,055 (40%) |
610 (38%) |
24 (27%) |
84 (31%) |
|
|
(Missing) |
3,084 |
1,673 |
950 |
103 |
358 |
|
|
Type of VTE Prophylaxis Utilized in Inpatient, n (%) |
|
|
|
|
|
|
|
Heparin/Warfarin |
349 (4.5%) |
161 (3.7%) |
188 (7.4%) |
0 (0%) |
0 (0%) |
|
|
Enoxaparin |
4,329 (56%) |
2,731 (63%) |
1,598 (63%) |
0 (0%) |
0 (0%) |
|
|
Rivaroxaban |
143 (1.9%) |
108 (2.5%) |
35 (1.4%) |
0 (0%) |
0 (0%) |
|
|
Dabigatran |
3 (<0.1%) |
1 (<0.1%) |
2 (<0.1%) |
0 (0%) |
0 (0%) |
|
|
Apixaban |
179 (2.3%) |
98 (2.3%) |
81 (3.2%) |
0 (0%) |
0 (0%) |
|
|
Edoxaban |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
|
|
Fondaparinux |
2 (<0.1%) |
1 (<0.1%) |
1 (<0.1%) |
0 (0%) |
0 (0%) |
|
|
Aspirin |
519 (6.8%) |
333 (7.7%) |
186 (7.3%) |
0 (0%) |
0 (0%) |
|
|
ADP Inhibitors |
10 (0.1%) |
4 (<0.1%) |
6 (0.2%) |
0 (0%) |
0 (0%) |
|
|
Dipyridamole |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
|
|
Cilostazol |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
|
|
Vorapaxar |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
|
|
Multiple Therapeutic Agents |
1,333 (17%) |
895 (21%) |
438 (17%) |
0 (0%) |
0 (0%) |
|
1Mean (SD) or Median (IQR) or Frequency (Column %)
2Pearson's Chi-squared test; Kruskal-Wallis rank sum test; Fisher's exact test
Overall, 171/7,685 patients (2.2%) were diagnosed with a VTE within 1 year of treatment of their tibia fracture. When a diagnosis of VTE was made it was most commonly made within 60 days of the initial admission for tibia fracture. Incidence of VTE based on chemical prophylaxis status was found to be: 98/4,332 (2.3%) in Group 1 (inpatient and outpatient VTE prophylaxis group); 59/2,535 (2.3%) in Group 2 (those who received inpatient VTE prophylaxis without outpatient prophylaxis) were diagnosed with VTE after treatment of their tibia fracture; in Group 3 (no inpatient VTE prophylaxis but prescribed outpatient prophylaxis) 3/191 (1.6%) were diagnosed with VTE; and 11/627 (1.8%) of patients in Group 4 (no inpatient or outpatient chemical VTE prophylaxis) were diagnosed with VTE after isolated tibia fracture (p=0.88). Logistic regression was performed and in both the full and reduced models (Table 2), increased age (65 and over compared to those under 65) was found to be statistically significant in predicting the odds of a patient developing VTE (p=0.004, odds ratio (OR) 1.765, confidence interval (CI) [1.193, 2.647]). Smoking status was predictive of the odds of VTE as well (p=0.026, OR 1.504, CI [1.050, 2.140]). Interestingly, the use of VTE prophylaxis in the period after isolated tibia fracture was not found to be significantly predictive of the odds of developing VTE (p=0.962 for Group 1 vs Group 2; p=0.698 for Group 1 vs Group 3; and p=0.469 for Group 1 vs Group 4). In the full model only, conditions predisposing to clotting were also significant for the development of VTE (p=0.049, OR 1.550, CI [1.002, 2.333]). Pertinent patient variables that were not found to be associated with VTE after chi squared analysis were Elixhauser Comorbidity Index, fracture location in tibia (proximal, shaft, distal), treatment (surgery versus nonsurgical treatment) and immobilization of the fractured limb (boot/splint/or cast). A wide confidence interval was calculated for the effect of pharmacologic contraception due to small numbers of patients receiving these medications, suggesting that the estimate of these effects may be critically biased. Outcome data are further summarized in Table 2.
Table 2: Coefficients and model output with full and reduced models. Variable comparisons with OR of development of VTE.
|
|
Combination of Inpatient and Discharge VTE Prophylaxis |
|
||||
|
Patient Characteristic |
Overall, N = 7,6851 |
Group 1 |
Group 2 |
Group 3 |
Group 4 |
p-value2 |
|
Both Inpatient/Discharge Prophylaxis Supplied, N = 4,3321 |
Inpatient Prophylaxis Only, N = 2,5351 |
Discharge Prophylaxis Only, N = 1911 |
Neither Inpatient/Discharge Prophylaxis Supplied, N = 6271 |
|||
|
Boot/Splint as Treatment, n (%) |
|
0.43 |
||||
|
Treatment Utilized |
150 (2.0%) |
78 (1.8%) |
52 (2.1%) |
6 (3.1%) |
14 (2.2%) |
|
|
Cast as Treatment, n (%) |
|
0.64 |
||||
|
Treatment Utilized |
10 (0.1%) |
5 (0.1%) |
5 (0.2%) |
0 (0%) |
0 (0%) |
|
|
Immobilization as Treatment (Boot/Splint or Cast), n (%) |
|
0.48 |
||||
|
Treatment Utilized |
160 (2.1%) |
83 (1.9%) |
57 (2.2%) |
6 (3.1%) |
14 (2.2%) |
|
|
External Fixation Device as Treatment, n (%) |
|
0.32 |
||||
|
Treatment Utilized |
26 (0.3%) |
14 (0.3%) |
12 (0.5%) |
0 (0%) |
0 (0%) |
|
|
Internal Fixation Device as Treatment, n (%) |
|
<0.001 |
||||
|
Treatment Utilized |
3,053 (40%) |
1,879 (43%) |
923 (36%) |
71 (37%) |
180 (29%) |
|
|
Intramedullary Nailing as Treatment, n (%) |
|
0.71 |
||||
|
Treatment Utilized |
545 (7.1%) |
312 (7.2%) |
183 (7.2%) |
11 (5.8%) |
39 (6.2%) |
|
|
Other Treatment, n (%) |
|
<0.001 |
||||
|
Treatment Utilized |
3,209 (42%) |
1,960 (45%) |
981 (39%) |
74 (39%) |
194 (31%) |
|
|
Tibial Surgery as Treatment, n (%) |
|
<0.001 |
||||
|
Treatment Utilized |
3,775 (49%) |
2,277 (53%) |
1,174 (46%) |
94 (49%) |
230 (37%) |
|
|
SURG_NON_TIB, n (%) |
|
>0.99 |
||||
|
Treatment Utilized |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
|
|
Estimated Blood Loss in Milliliters, Mean (SD) |
98.86 (148.60) |
103.96 (127.45) |
95.45 (184.03) |
106.20 (221.03) |
68.13 (87.79) |
<0.001 |
|
(Missing) |
6,044 |
3,363 |
2,024 |
145 |
512 |
|
|
ASA Score (Categorized), n (%) |
|
|
||||
|
ASA 1, Normal Healthy Patient |
373 (9.9%) |
210 (9.2%) |
120 (10%) |
13 (14%) |
30 (13%) |
|
|
ASA 2, Moderate Systemic Disease |
1,824 (48%) |
1,137 (50%) |
519 (44%) |
49 (52%) |
119 (52%) |
|
|
ASA 3, Severe Systemic Disease |
1,424 (38%) |
850 (37%) |
476 (41%) |
25 (27%) |
73 (32%) |
|
|
ASA 4, Life-Threatening Disease |
132 (3.5%) |
67 (2.9%) |
53 (4.5%) |
5 (5.3%) |
7 (3.0%) |
|
|
(Missing) |
3,910 |
2,055 |
1,361 |
97 |
397 |
|
|
Post-Surgical Length-of-Stay in Days, Median (IQR) |
2.00 (1.00 - 4.00) |
3.00 (2.00 - 5.00) |
2.00 (2.00 - 4.00) |
1.00 (1.00 - 2.00) |
1.50 (1.00 - 3.00) |
<0.001 |
|
(Missing) |
3,913 |
2,057 |
1,362 |
97 |
397 |
|
|
Total Length-of-Stay in Days, Median (IQR) |
4.00 (2.00 - 7.00) |
4.00 (2.00 - 8.00) |
3.00 (2.00 - 6.00) |
2.00 (1.00 - 3.00) |
2.00 (1.00 - 4.00) |
<0.001 |
|
Readmission for Venous Thromboembolism, n (%) |
|
0.85 |
||||
|
Diagnosed |
171 (2.2%) |
98 (2.3%) |
59 (2.3%) |
3 (1.6%) |
11 (1.8%) |
|
|
Readmission for Deep Vein Thrombosis, n (%) |
|
0.88 |
||||
|
Diagnosed |
146 (1.9%) |
85 (2.0%) |
49 (1.9%) |
3 (1.6%) |
9 (1.4%) |
|
|
Readmission for Pulmonary Embolism, n (%) |
|
|
|
|
|
0.81 |
|
Diagnosed |
47 (0.6%) |
26 (0.6%) |
18 (0.7%) |
0 (0%) |
3 (0.5%) |
|
|
Time to Readmission for VTE in Days (Average), Mean (SD) |
1.74 (17.30) |
1.60 (15.97) |
1.89 (18.76) |
2.08 (19.01) |
2.03 (19.38) |
0.91 |
|
Time to Readmission for VTE in Days (Stratified), n (%) |
|
|
||||
|
Readmission with VTE within 30 Days |
54 (0.7%) |
37 (0.9%) |
15 (0.6%) |
0 (0%) |
2 (0.3%) |
|
|
Readmission with VTE within 60 Days |
33 (0.4%) |
17 (0.4%) |
13 (0.5%) |
1 (0.5%) |
2 (0.3%) |
|
|
Readmission with VTE within 90 Days |
18 (0.2%) |
9 (0.2%) |
7 (0.3%) |
0 (0%) |
2 (0.3%) |
|
|
Readmission with VTE within 180 Days |
30 (0.4%) |
18 (0.4%) |
10 (0.4%) |
1 (0.5%) |
1 (0.2%) |
|
|
Readmission with VTE within 1 Year |
23 (0.3%) |
10 (0.2%) |
8 (0.3%) |
1 (0.5%) |
4 (0.6%) |
|
DISCUSSION
In this large patient population consisting of 7,685 patients with tibia fractures, no benefit in the form of decreased VTE events was found with the use of chemical VTE prophylaxis compared with no chemical VTE prophylaxis. This was consistent with prior literature on fractures below the knee and chemical VTE prophylaxis [4,5]. This is believed to be the largest study of its kind that focused specifically on isolated tibia shaft fractures in a single hospital system database. Goel et al., published a double blinded randomized controlled trial using low molecular weight heparin (LMWH) versus saline placebo in patients 18-75 with isolated fractures below the knee requiring operative fixation [4]. Age and type of fracture were found to be associated with the rate of DVT, but there was no difference between those who were treated with LMWH versus placebo [4]. Furthermore, and consistent with the current study, Goel found no difference in the incidence of DVT depending on the type of post-operative immobilization used. Several large studies have found very low rates of DVT and PE after fractures below the knee both with and without the use of chemical VTE prophylaxis. Griffiths et al studied a consecutive series of 2,654 patients undergoing foot and ankle surgery, of which 1,078 received aspirin VTE prophylaxis while 1,576 patients received no chemical prophylaxis [6]. There was no difference in DVT and PE between the two groups, with an overall incidence of 0.27% for DVT and 0.15% for PE. The authors of that study recommended against the routine use of chemical VTE prophylaxis in foot and ankle surgery. Jameson et al performed a review of a large national database looking at 45,949 patients undergoing foot and ankle surgery and found rates of DVT (0.12%) and PE (0.17%) to be extremely low after ankle fracture surgery, and even lower after elective foot and ankle surgeries [7]. The authors in that study similarly stated that “…prophylaxis was not required in most of these patients”. Previous randomized placebo controlled studies have found no difference between VTE rates following surgical treatment of isolated below the knee fractures treated with or without outpatient chemical VTE prophylaxis [4,8,5,9]. These studies have primarily focused on foot and ankle fractures, however. A registry study from Wahlsten et al in 2015 looked at isolated tibia, ankle, and patella fractures in their cohort of surgically treated patients followed for 180 days treated without chemical VTE prophylaxis post-hospital discharge. That study found VTE in 594/57,619 (1.0%) patients [10]. In a review of the National Trauma Databank, Auer et al looked at the incidence of DVT and PE in patients with tibia fractures without other fractures, with or without additional non-orthopaedic trauma [9]. This study looked at 86,076 patients in the database and found an overall incidence of DVT of 0.53% and PE of 0.35%. Age greater than 50 and administration of chemical VTE prophylaxis were found to be risk factors for VTE [9]. Additionally, male gender, increased ISS, higher BMI, impaired sensorium, pneumonia, and compartment syndrome were all found to be risk factors for VTE. Conclusions reached from the review of the NTDB were that “chemical thromboprophylaxis was not warranted in every patient’s case” in treatment of tibia fractures [9]. In the current study, upon logistic regression analysis current smokers and patients aged 65 years and older were also more likely to develop VTE (OR 1.504 and 1.765, respectively). Age and smoking are inconsistently listed in the literature as potential risk factors for VTE. Age has been discussed in the studies referenced above, and at least one large meta analysis study previously performed found smoking to be associated with a slightly increased risk of VTE [10]. Some of the risk factors that were hypothesized to be relevant that did not show any increased risk of VTE in the current study with or without chemical prophylaxis were BMI, ECI, oral contraceptive use, fracture location, high-energy injury, surgical treatment, and the use of post-operative immobilization. There are several important limitations to consider in the current study. This study shares limitations present and inherent in any database study, namely incorrect and incomplete data entry. Improper coding can be a particularly problematic issue in database studies, causing cohorts to be incorrectly skewed in one direction or another. Database studies may not provide details that may be of interest in orthopaedic surgical series such as type of surgery, relative implant size and positioning, fracture reduction, etc. As mentioned above, a database study confined to one hospital system also will not capture patients who present to another hospital with the outcome in question, thereby missing those patients in final analysis (however, this limitation is applied to all groups). We included patients who were prescribed outpatient VTE prophylaxis in groups 1 and 3, but there is no guarantee these patients took their VTE prophylaxis medications as prescribed, if at all. Dosages and length of chemical prophylaxis administration were not standardized, and practices vary widely in clinical practice. Furthermore, time to surgery is not included in this analysis, which could influence VTE rates. Notwithstanding these limitations, however, database studies provide the ability to evaluate large groups of patients which allows for evaluation and treatment comparisons of rare diseases and complications, such as VTE. This provides the ability to study large patient populations and find statistical and clinical significance that would not be possible with other study designs [11-13]. This study was, therefore, unable to evaluate and account for every possible risk factor for VTE. Although there are several risk factors that are commonly thought to play a role in VTE, both prospective and large retrospective studies have failed to consistently reproduce the same independent predictors of VTE. The data currently presented has utilized the ECI to account for multiple comorbidities and an overall measure of patient baseline health, and has attempted to include as many confounding factors as could be provided in a large database. Because the patients reviewed for this study were treated at different locations and not standardized to specific treatment protocols, patient selection for prophylaxis and the diagnosis of VTE may have differed throughout the study population. Location of DVT when present (proximal versus distal) is also unknown. This is another limitation inherent in a database study such as this, namely that diagnosis of VTE for the purposes of the study is made solely based on correct CPT coding and does not take into account individual clinicians and their diagnostic methods. However, this may be preferrable in some respects when compared to studies where routine ultrasound is performed on all patients. In comparison to most prospective studies on this subject, DVT frequency in the current study represents clinically relevant DVT whereas in studies where routine ultrasound is performed DVT rates are vastly over representative of what is clinically relevant. Future research on this topic should focus on the utility of chemical VTE prophylaxis in cases of isolated tibia fractures. Identification of which patients may be at higher risk for VTE and development of prophylaxis guidelines for those patients will help to avoid the exposure of the risk and costs of these medications in patients who do not benefit from their administration. Furthermore, medication, dosage, and duration of treatment guidelines are lacking when such treatment is determined to be beneficial. Further study regarding Thromboelastography (TEG) guided treatment may also be of benefit in the future in these patients. Consistent with the American College of Chest Physicians evidence-based clinical practice guidelines, this review of 7,685 patients with tibia fractures found that the standard of care would dictate routine use of chemical VTE prophylaxis is not necessary. On the contrary, there is no recommendation being made, however, to completely and indiscriminately dispense with the use of thrombo-prophylaxis. Physicians should individualize treatment for their patients and prescribe chemical VTE prophylaxis when clinically indicated.
Competing Interests/Disclosures
Dr. Riehl is a consultant and receives Royalties from Arthrex Inc.
FUNDING
This research was supported (in whole or in part) by HCA Healthcare and/or an HCA Healthcare affiliated entity. The views expressed in this publication represent those of the author(s) and do not necessarily represent the official views of HCA Healthcare or any of its affiliated entities.
AUTHOR CONTRIBUTIONS
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by John Riehl. The first draft of the manuscript was written by John Riehl.
ACKNOWLEDGEMENTS
The author would like to express special thanks to Jeffery Durbin, M.S., Senior Research Analyst (Biostatistician) for Graduate Medical Education and HCA Healthcare Physician Services Group for his time and effort provided in the statistical analysis that contributed to the creation of this manuscript.
ETHICS APPROVAL
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of HCA Healthcare.
REFERENCES
- Etscheidt J, Shahien A, Gainey M, Kronenfeld D, Niu R, Freccero D, et al. Review of Therapeutic Options for the Prevention of VTE in Total Joint Arthroplasty. Geriatrics (Basel). 2020; 5: 18.
- Shibuya N, Frost CH, Campbell JD, Davis ML, Jupiter DC. Incidence of acute deep vein thrombosis and pulmonary embolism in foot and ankle trauma: analysis of the National Trauma Data Bank. J Foot Ankle Surg. 2012; 51: 63-68.
- Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008; 133: 381S-453S.
- Goel DP, Buckley R, deVries G, Abelseth G, Ni A, Gray R. Prophylaxis of deep-vein thrombosis in fractures below the knee: a prospective randomised controlled trial. J Bone Joint Surg Br. 2009; 91: 388-394.
- Selby R, Geerts WH, Kreder HJ, Crowther MA, Kaus L, Sealey F. A double-blind, randomized controlled trial of the prevention of clinically important venous thromboembolism after isolated lower leg fractures. J Orthop Trauma. 2015; 29: 224-230.
- Griffiths JT, Matthews L, Pearce CJ, Calder JD. Incidence of venous thromboembolism in elective foot and ankle surgery with and without aspirin prophylaxis. J Bone Joint Surg Br. 2012; 94: 210-214.
- Jameson SS, Augustine A, James P, Serrano-Pedraza I, Oliver K, Townshend D, et al. Venous thromboembolic events following foot and ankle surgery in the English National Health Service. J Bone Joint Surg Br. 2011; 93: 490-497.
- Lapidus LJ, Ponzer S, Elvin A, Levander C, Lärfars G, Rosfors S, et al. Prolonged thromboprophylaxis with Dalteparin during immobilization after ankle fracture surgery: a randomized placebo- controlled, double-blind study. Acta Orthop. 2007; 78: 528-535.
- Zheng X, Li DY, Wangyang Y, Zhang XC, Guo KJ, Zhao FC, Pang Y, Chen YX. Effect of Chemical Thromboprophylaxis on the Rate of Venous Thromboembolism After Treatment of Foot and Ankle Fractures. Foot Ankle Int. 2016; 37: 1218-1224.
- Wahlsten LR, Eckardt H, Lyngbæk S, Jensen PF, Fosbøl EL, Torp-Pedersen C, et al. Symptomatic venous thromboembolism following fractures distal to the knee: a nationwide Danish cohort study. J Bone Joint Surg Am. 2015; 97: 470-477.
- Auer R, Riehl J. The incidence of deep vein thrombosis and pulmonary embolism after fracture of the tibia: An analysis of the National Trauma Databank. J Clin Orthop Trauma. 2017; 8: 38-44.
- Cheng YJ, Liu ZH, Yao FJ, Zeng WT, Zheng DD, Dong YG, et al. Currentand former smoking and risk for venous thromboembolism: asystematic review and meta-analysis. PLoS Med. 2013; 10: e1001515.
- Grauer JN, Leopold SS. Editorial: large database studies--what they can do, what they cannot do, and which ones we will publish. Clin Orthop Relat Res. 2015; 473: 1537-1539.