Brugada Phenocopy Following Aluminum Phosphide Poisoning A Case Report and Literature Review
- 1. Department of Cardiology, School of Medicine, Zanjan University of Medical Science, Iran
- 2. Department of Cardiology, School of Medicine, Zanjan University of Medical Science, Iran
- 3. Department of Cardiology, School of Medicine, Zanjan University of Medical Science, Iran
- 4. 2Brigham and Women Hospital, Harvard Medical School, USA
- 5. Department of Cardiology, School of Medicine, Zanjan University of Medical Science, Iran
- 6. Department of Cardiology, School of Medicine, Zanjan University of Medical Science, Iran
- 7. 3Cardiovascular Diseases Research Institute, Tehran University of Medical Sciences, Iran
Abstract
Introduction: Aluminum phosphide (ALP) is a highly toxic pesticide with no specific antidote which is widely used in developing countries. Upon ingestion, it releases phosphine gas, leading to severe metabolic derangements and cardiovascular collapse.
Case Presentation: We report a case of a 28-year-old woman who presented to the emergency department following suicidal ingestion of aluminum phosphide. Shortly after admission, she developed a type 1 Brugada ECG pattern along with metabolic acidosis and cardiogenic shock. Despite intensive supportive care, including vasopressors, mechanical ventilation, and high-dose insulin therapy, the patient died on the fourth day due to refractory shock and acute respiratory distress syndrome (ARDS).
Conclusions: This case illustrates a rare but critical manifestation of ALP poisoning: Brugada phenocopy. Clinicians should be aware of this ECG pattern in the context of toxic ingestion, as it may indicate underlying cardiac instability. Recognition of this ECG pattern in toxicological emergencies is essential for early diagnosis and intervention
Citation
Ghajar M, Tarkiani S, Fard MHS, Ramezani A, Palangi MG, et al. (2025) Brugada Phenocopy Following Aluminum Phosphide Poisoning: A Case Report and Literature Review. Clin J Heart Dis 4(1): 1012.
Keywords
• Aluminum Phosphide
• Alp Poisoning
• Brugada Pattern
• St-Segment Elevation
• Phosphine Gas
• Cardiac Toxicity
• Multiorgan Failure
• Toxicological Emergency
INTRODUCTION
Aluminum phosphide (AIP), commonly known as “rice tablet” in some regions, is a highly toxic fumigant used for grain preservation. Its easily accessibility and low cost have made it a frequent agent in cases of deliberate self-poisoning, particularly in developing countries. Upon contact with moisture, ALP releases phosphine gas, which inhibits mitochondrial oxidative dysfunction. The absence of a specific antidote and the rapid onset of toxicity make ALP poisoning a major medical emergency with a high mortality rate [1-3].
Cardiovascular toxicity is a leading cause of death in ALP poisoning, often presenting as hypotension, arrhythmias, myocardial depression, and in rare cases, sudden cardiac death. Electrocardiographic abnormalities are commonly observed, ranging from nonspecific ST-T changes to lifethreatening arrhythmias.
Brugada pattern, an electrocardiographic finding typically linked to Brugada syndrome, has also been observed as a transient manifestation during acute illnesses—a phenomenon referred to as Brugada phenocopy. This reversible ECG pattern has been documented in association with metabolic disturbances, ischemia, and various toxic exposures. However, its occurrence in the context of aluminum phosphide (ALP) poisoning is exceedingly rare [4].
In this report, we present the case of a young woman who developed a Brugada-like ECG pattern shortly after ingesting ALP, and we discuss the potential pathophysiological mechanisms underlying this presentation in the context of existing literature.
CASE PRESENTATION
A 28-year-old woman was admitted to the emergency department of Valiasr General Hospital, affiliated with Zanjan University of Medical Sciences, approximately three hours after ingesting a tablet of Aluminum Phosphide (ALP). Prior to admission, she received initial treatment at a local clinic, including intravenous fluids, oxygen therapy, gastric decontamination with paraffin oil, and cardiac monitoring. Subsequently, she was transferred via emergency medical services (EMS) to the tertiary care center for further evaluation and management.
At presentation, she was alert but hypotensive (Blood Pressure: 85/65 mmHg) with a pulse rate of 115 bpm, respiratory rate of 19/min, and a Glasgow Coma Scale (GCS) of 13/15. She reported a single episode of vomiting. There was no relevant past medical or drug use history.
Shortly after admission, her clinical condition deteriorated, marked by worsening hypotension, metabolic acidosis, and decreased level of consciousness, necessitating endotracheal intubation and transfer to the intensive care unit (ICU) Table 1.
Table 1: Laboratory Data (Selected).
|
Parameter |
Day1 |
Day3 |
Reference range |
|
pH (VBG) |
7.31 |
7.25 |
7.35-7.45 |
|
pCO2 (mmHg) |
31.4 |
58.7 |
35-45 |
|
HCO3 (mEq/L) |
15.4 |
25.5 |
22-26 |
|
Serum calcium (mg/dL) |
6.5 |
-- |
8.5-10.5 |
|
Serum magnesium (mg/Dl) |
1.4 |
-- |
1.7-2.2 |
|
AST (U/L) |
1930 |
-- |
<40 |
|
ALT (U/L) |
1090 |
-- |
<41 |
|
LDH (U/L) |
6670 |
-- |
<280 |
|
Total bilirubin (mg/dL) |
1.0 |
-- |
0.3-1.2 |
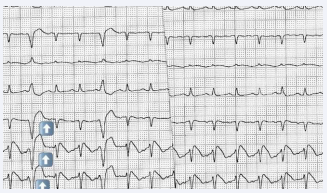
Echocardiography revealed global hypokinesia and a reduced ejection fraction of 20-25%. A Brugada type 1 ECG pattern was observed in leads V1-V3, along with STsegment elevations and T-wave inversions, accompanied by incomplete right bundle branch block (RBBB) (Figure 1). These changes were transient and possibly associated with electrolyte disturbances and hepatic dysfunction.
Figure 1: Electrocardiogram (ECG) presentation ECG showing pulse rate (PR) 132/min, coved ST-segment elevation of more than 5 mm in lead V1 to V3, with incomplete right bundle branch block (RBBB) morphology, and T-wave inversion in V2 and V3, suggestive of type 1 Brugada pattern.
Despite supportive interventions-including high-dose insulin therapy, electrolyte correction, and advanced cardiac monitoring-the patient developed progressive multi-organ dysfunction. Toxicology screening was positive for nicotine, amphetamines, methamphetamine, and lidocaine; however, no other pesticides or toxins were detected in the serum.
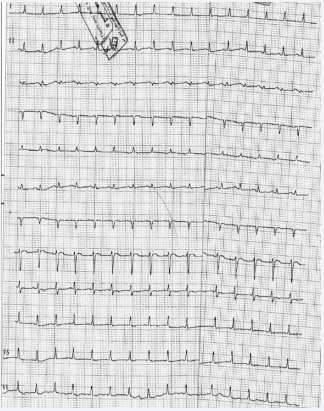
During her three-day ICU stay, Table 2 serial ECGs taken every three hours showed low voltage QRS complexes (Figure 2 illustrates the follow-up ECG showing resolution of the Brugada pattern). On the third day, she developed acute pulmonary edema along with multiple arrhythmias, including supraventricular tachycardia (SVT), atrial fibrillation (AF), and atrial tachycardia (AT). Chest radiography showed bilateral basal infiltrates consistent with acute respiratory distress syndrome (ARDS). Broadspectrum antibiotics were initiated following a rise in in fever and worsening respiratory acidosis. Later that evening, the patient suffered a cardiac arrest. Despite aggressive resuscitative efforts, she unfortunately passed away.
Figure 2: Electrocardiogram (ECG) after 3 days of treatment. ECG showing pulse rate (PR) 140/min, low voltage QRS complexes, ST-segment elevation became isoelectric in lead V1 and decreased to 1 mm in lead V2-V3.
Table 2: Serial ECG Findings.
|
Timepoint |
Findings |
|
ICU admission |
Brugada pattern (type 1) in V1-V3, ST elevation, T-wave inversion |
|
Day 2 |
Low-voltage QRS complexes |
|
Day 3 |
Resolution of Brugada pattern, new-onset SVT, atrial fibrillation, AT |
DISCUSSION
Aluminum Phosphide (ALP) is a widely used pesticide in developing countries, primarily because of its low cost and minimal impact on seed viability. However, it is highly toxic when ingested and poses serious risks upon inhalation, including cardiac failure, arrhythmias, acute respiratory distress syndrome (ARDS), hepatic and renal toxicity, seizures, and coma [5,6]. Although rare, dermal absorption through prolonged skin contact [7,8]. has also been reported to cause dermatitis.
Upon exposure to atmospheric moisture or gastric hydrochloric acid, ALP releases phosphine gas-a highly toxic compound that is rapidly absorbed through the gastrointestinal tract. Once absorbed, Phosphine is then oxidized to oxyacid, resulting in widespread multi-organ damage [9].
While the exact mechanism of phosphine toxicity remains unclear, it is believed to interfere with mitochondrial oxidative phosphorylation [10,11]. More specifically, phosphine likely inhibits cytochrome c oxidase and catalase, thereby increasing reactive oxygen species (ROS) production and depleting cellular energy reserves [10-12]. This oxidative stress is exacerbated by a reduction in intracellular glutathione [9], and further intensified through phosphine’s interaction with hydrogen peroxide and iron, leading to even greater ROS generation [13], and further intensified through phosphine’s interaction with hydrogen peroxide and iron, leading to even greater ROS generation [14-16].
A dose as small as 0.5 g of ALP is considered potentially lethal. Survivors of ALP poisoning often ingested only a small quantity, an expired tablet, or a tablet exposed to air that had lost its phosphine content [17].
It is estimated that 95% of ALP-related deaths occur within the first 24 hours post-ingestion due to refractory shock and arrhythmias. Deaths occurring beyond this time frame are often associated with ARDS, metabolic acidosis, and cardiac dysrhythmias [18].
ALP poisoning has multi-organ effects, with initial symptoms including nausea, vomiting, retrosternal and epigastric pain, dyspnea, anxiety, agitation, garlic odor on the breath [13]. Without prompt and appropriate intervention, patients may deteriorate due to myocarditis, circulatory collapse, and multi-organ failure [17].
Among affected systems, the cardiovascular system is particularly vulnerable, with cardiac dysfunction being a major contributor to mortality [19, 20].
ECG abnormalities are frequently observed in ALP poisoning and may include bradycardia, QRS widening, PR prolongation, ST-segment elevation, and QTc prolongation [21]. In rare cases, ST- segment elevation in the right precordial leads is noted, in the absence of identifiable structural abnormalities-a pattern referred to as Brugada phenocopy [22,23]. Brugada phenocopy must be distinguished from Brugada syndrome, a congenital channelopathy associated with sudden cardiac death, as both share similar ECG findings-especially, coved STsegment elevation in leads V1-V3 [24]. Brugada phenocopy is typically reversible upon resolution of the underlying cause, such as metabolic or electrolyte disturbances, whereases Brugada syndrome may be unmasked by sodium channel blockers or other pharmacologic triggers [25]. Differentiating Brugada phenocopy from Brugada syndrome is critical, as it directly influences management strategies. Patients with Brugada syndrome may require implantable cardioverter-defibrillators (ICDs) to prevent fetal arrhythmias, whereases treatment of Brugada phenocopy is directed toward correction of the precipitating conditions [25,26]. A scoring system known as PGI (ph., Glasgow Coma Scale, and systolic blood pressure impairment) has been proposed to predict mortality and cardiotoxicity in ALP poisoning. A higher PGI score correlates with increased mortality, need for mechanical ventilation, greater ALP dose ingestion, more significant ECG changes, higher troponin levels, and greater vasopressor requirements [26]. Management of ALP poisoning remains highly challenging due to the lack of a specific antidote. Early diagnosis, aggressive supportive care, and close monitoring are currently the mainstays of treatment [27]. Several adjunct therapies, have been proposed with varying success, including gastric decontamination with non-absorbable oils like castor oil [28], intravenous lipid emulsion, (ILE) to mitigate metabolic acidosis [28], potassium permanganate lavage, antiarrhythmic agents, early fluid resuscitation and vasopressors, IV sodium bicarbonate, digoxin, hydroxyethyl starch, Veno-arterial extracorporeal membrane oxygenation (VA-ECMO), magnesium sulfate, and glucose,-insulin-potassium (GIK) infusion [29].
Recent evidence supports the use of ILE as an adjunct treatment. One study demonstrated that ILE significantly reduced the need for mechanical ventilation and intubation in ALP-poisoned patients, suggesting its potential role in improving outcomes [5-35].
CONCLUSION
This case highlights the severe toxicity of aluminum phosphide ingestion and its rare manifestation as a Brugada phenocopy, identified by characteristic ECG changes. Although uncommon, this presentation underscores the potential arrhythmogenic effects of aluminum phosphide and the importance of considering such findings in the clinical setting. Early recognition, continuous cardiac monitoring, and aggressive supportive management are crucial to minimize complications and improve outcomes. Further research is needed to better understand the underlying mechanism linking aluminum phosphide toxicity to cardiac and respiratory dysfunction.
DECLARATION
Consent for Publication
Written informed consent for publication was obtained from the patient’s next of kin.
Availability of Data and Materials
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
REFERENCES
- Bumbrah GS, Krishan K, Kanchan T, Sharma M, Sodhi GS. Phosphide poisoning: a review of literature. Forensic Sci Int. 2012; 214: 1-6.
- Hosseini SF, Forouzesh M, Maleknia M, Valiyari S, Maniati M, SamimiA. The Molecular Mechanism of Aluminum Phosphide poisoning in Cardiovascular Disease: Pathophysiology and Diagnostic Approach. Cardiovasc Toxicol. 2020; 20: 454-461.
- Yilmaz R, Yilmaz E, Ozdemir V, Can M, Pakis I, Piskin IE, et al. An evaluation of childhood deaths in Turkey due to yellow phosphorus in firecrackers. J Forensic Sci. 2015; 60: 648-652.
- Guru S, Kumar R, Behera A, Patra S, Kumar P Jr. Aluminium Phosphide-Induced Expression of Covertly Present Brugada Pattern in Electrocardiogram: A Rare Case Report. Cureus. 2020; 12: e10552.
- Gurjar M, Baronia AK, Azim A, Sharma K. Managing aluminum phosphide poisonings. J Emerg Trauma Shock. 2011; 4: 378-384.
- Sudakin DL. Occupational exposure to aluminium phosphide and phosphine gas? A suspected case report and review of the literature. Hum Exp Toxicol. 2005; 24: 27-33.
- Aliasghar Manouchehri, Shiva Ghareghani, Shabnam Shamaei, Maede Nilechi, Fatemeh Bossaghzadeh. A review on Aluminum phosphide (Rice Tablets) Poisoning; From Exposure to the Applicable and New Strategies of Clinical Management. 2021; 8: 326-332.
- Kapil Kumar Garg. Review of Aluminium Phosphide Poisoning. Int J Med Sci Public Health. 2020; 9: 392-400.
- Gurjar M, Baronia AK, Azim A, Sharma K. Managing aluminum phosphide poisonings. J Emerg Trauma Shock. 2011; 4: 378-384.
- Anand R, Sharma DR, Verma D, Bhalla A, Gill KD, Singh S. Mitochondrial electron transport chain complexes, catalase and markers of oxidative stress in platelets of patients with severe aluminum phosphide poisoning. Hum Exp Toxicol. 2013; 32: 807-816.
- Fahimeh Nourbakhsh, Samira Barangi, Bita Dadpour, shahrad Tajoddini. Aluminum Phosphide Poisoning, Mechanism of Action and Treatment: a Literature Review. J Kerman University Med Sci. 2019; 26: 406-419.
- Singh S, Bhalla A, Verma SK, Kaur A, Gill K. Cytochrome-c oxidase inhibition in 26 aluminum phosphide poisoned patients. Clin Toxicol (Phila). 2006; 44: 155-158.
- Nath NS, Bhattacharya I, Tuck AG, Schlipalius DI, Ebert PR. Mechanisms of phosphine toxicity. J Toxicol. 2011; 2011: 494168.
- Yadav D, Bhattacharyya R, Banerjee D. Acute aluminum phosphide poisoning: The menace of phosphine exposure. Clin Chim Acta. 2021; 520: 34-42.
- Gary B Quistad, Susan E Sparks, John E Casida. Chemical model for phosphine-induced lipid peroxidation. Pest Management Science. 2000; 56: 779-783.
- Banerjee D, Madhusoodanan UK, Nayak S, Jacob J. Urinary hydrogen peroxide: a probable marker of oxidative stress in malignancy. Clin Chim Acta. 2003; 334: 205-209.
- Mehrpour O, Jafarzadeh M, Abdollahi M. A systematic review of aluminium phosphide poisoning. Arh Hig Rada Toksikol. 2012; 63: 61-73.
- Moghadamnia AA. An update on toxicology of aluminum phosphide. Daru. 2012; 20: 25.
- Sahoo D, Kujur ST, Das DS, Dey A, Devi S. Aluminium Phosphide Poisoning: Early Suspicion of Cardiotoxicity Is Necessary for Improved Outcomes. Cureus. 2020; 12: e10237.
- Umair Aziz, Aamir Husain. Frequency of Cardiac Arrhythmias in Patients with Aluminum Phosphide Poisoning. Asia Pacific J Med Toxicol. 2015; 4: 147-150.
- Rahimi Kakavandi N, Asadi T, Hooshangi Shayesteh MR, Baeeri M, Rahimifard M, Baghaei A, et al. The electrocardiographic, hemodynamic, echocardiographic, and biochemical evaluation of treatment with edaravone on acute cardiac toxicity of aluminum phosphide. Front Pharmacol. 2022; 13: 1032941.
- Allam P, Shakya S, Yadav V, Kc S, Sedai H, Poddar E, et al. Induction of Brugada electrocardiogram pattern with aluminum phosphide poisoning: a case report. Ann Med Surg (Lond). 2023; 85: 5105-5109.
- Wilde AA, Antzelevitch C, Borggrefe M, Brugada J, Brugada R, Brugada P, et al. Proposed diagnostic criteria for the Brugada syndrome: consensus report. Circulation. 2002; 106: 2514-2519.
- Nalawade DD, Jadhav A, Wadhokar PS, Khan A, Gupta A, Malani SK. Toxic Myocarditis with Brugada Phenocopy in a Case of Aluminum Phosphide Poisoning. J Tehran Heart Cent. 2024; 19: 147-150.
- Anselm DD, Evans JM, Baranchuk A. Brugada phenocopy: A new electrocardiogram phenomenon. World J Cardiol. 2014; 6: 81-86.
- Marsman EMJ, Postema PG, Remme CA. Brugada syndrome: updateand future perspectives. Heart. 2022; 108: 668-675.
- Yoshino Minoura, Youichi Kobayashi, Charles Antzelevitch. Drug-induced Brugada syndrome. J Arrhythmia. 2013; 29: 88-95.
- Baranchuk A, Nguyen T, Ryu MH, Femenía F, Zareba W, Wilde AA, et al. Brugada phenocopy: new terminology and proposed classification. Ann Noninvasive Electrocardiol. 2012; 17: 299-314.
- Adytia GJ, Sutanto H. Brugada phenocopy vs. Brugada syndrome: Delineating the differences for optimal diagnosis and management. Curr Probl Cardiol. 2024; 49: 102566.
- Samar Sakrorcid, Mona Atef, Nashwa Mohamad Shalaby. PGI Score as a Predictor of Cardiotoxicity and Mortality in Patients with Acute Aluminum Phosphide Poisoning. Zagazig J Forensic Med Toxicol. 2023; 21: 32-48.
- Agrawal VK, Bansal A, Singh RK, Kumawat BL, Mahajan P. Aluminum phosphide poisoning: Possible role of supportive measures in the absence of specific antidote. Indian J Crit Care Med. 2015; 19: 109- 112.
- Sanaei-Zadeh H, Marashi SM. Gastric decontamination in aluminium phosphide poisoning: a case against the use of water-based solutions. Arh Hig Rada Toksikol. 2016; 67: 364-365.
- Gheat HS, Fayed MM, Elgazzar FM, Draz EI, El-Kelany RS. The possible therapeutic role of intravenous lipid emulsion in acute aluminium phosphide poisoning: a randomized controlled clinical trial. Toxicol Res (Camb). 2024; 13: tfae090.
- Gebray HM, Chekol AL. Survival after aluminum phosphide poisoning with cardiotoxicity: a case report. J Med Case Rep. 2024; 18: 614.
- Samar ELabdeen, Khaled Saad, Mervat Oreby, Fatma Elgazzar. Assessment of Intravenous Lipid Emulsion as an Adjuvant Therapy in Acute Aluminum Phosphide Poisoning: A randomized Controlled Trial. Ain Shams J Forensic Med Clin Toxicol. 2020; 34: 51-68.