Evaluation of Oral Persian Clover Sticks Effect on Platelet Count in Children with Chronic Immune Thrombocytopenic Purpura: A Novel Approach to Herbal Medicine in Hematology
- 1. Department of Pediatrics, Pediatric Hematology and Oncology, Tehran University of Medical Sciences, Iran
- 2. Assistant professor, PhD in Epidemiology, Lorestan University of medical science, Iran
- 3. Associate Professor nutritional Health Research Center, Lorestan University of Medical Sciences, Iran.
- 4. MD General Pediatrician, Iran
- 5. Associate Professor of Pediatric infectious disease, Dr. Alborzi Research center, Shiraz University of Medical Sciences, Iran
- 6*. Pediatric Infections Research Center (PIRC), Research Institute for Children’s Health (RICH), Shahid Beheshti University of Medical Sciences, Iran
Highlights
1. Palliative care in the field of hematology and pediatric oncology is one of the requirements for correcting the complications of immunosuppressive treatments.
2. Herbal medicine in the form of palliative care and complementary alternative medicine helps patients with blood diseases under immunosuppressive therapies.
3- Clover and its related species are one of the native plants of Iran and have medicinal properties.
4. Clover tea can increase platelet count in patients with chronic thrombocytopenia .
Abstract
Objective: People with a high platelet count are at high risk of severe bleeding. The substances contained in clover can have medicinal properties and are useful for humans. Clover was measured for platelet count in children with thrombocytopenia.
Design: This randomized, double-blind clinical trial was performed in Shahid Madani Hospital in Khorram-abad in 1400. Forty patients were randomly and equally divided into two groups of drugs and placebo and received clover or placebo 5cc every eight hours for 4 months. Assays included platelet count, BT, and CBC before and after the intervention. The statistical methods used were paired t-test and independent t-test.
Results: The difference of the mean platelet count in the two groups was statistically significant (P-value <0.004), so that in the group receiving clover infusion a significant increase in the number of Platelets was observed relative to the placebo group. However, the mean BT in the two groups was not statistically significant (P-value<0.507), although in clover group, the mean BT was more decreasing than the placebo group. There was no significant difference between the study groups in terms of demographic characteristics such as gender, family history, and place of residence in this study (P-value> 0.05).
Conclusion: Based on this study, the platelet count increased, and it seems in this regard improves the platelet level of children with thrombocytopenia.
Keywords: Thrombocytopenia; Clover; Herbal Medicine; Palliative Therapy
Introduction
Thrombocytopenia refers to an abnormal decrease in platelet count to less than 150,000 per microliter, and is caused by one or more of the Following three processes: Decreased production in the bone marrow; Retention (separation), usually enlarged in the spleen; and Increased platelet degradation. According to the World Health Organization (WHO), supportive and palliative care is an approach that improves the quality of life of patients and their families in the face of problems caused by life-threatening diseases. These cares identify and control physical, psychological, social, and spiritual challenges in the patient and family. On the other hand, the WHO identifies key components in cancer control: prevention, early detection, treatment, and supportive and palliative care [1]. From the distant past until now, medicinal plants have been important in providing health and well-being for humans both in terms of treatment and prevention of diseases. From a historical point of view, plants have been of great importance in the development of societies, and extensive research has been conducted to find natural herbal extracts and medicinal substances throughout history. Also, the emphasis of the WHO on the gradual replacement of natural substances instead of chemicals has led different countries to invest, plan planting and mass production of medicinal plants, and use it in the pharmaceutical, health and food industries [2]. At present, most of the palliative medicine of medicinal plants is also used as one of the important factors in this field. Chemotherapy uses powerful anti-cancer drugs that often cause severe side effects. Some natural supplements significantly alleviate these problems and completely eliminate jaundice. Various drugs with natural or synthetic origins are effective in the process of platelet proliferation. Clover is the general name of plants of the genus Trifolium and includes about 300 species of plants of the family Fabaceae which have a high nutritional value as animal fodder. In traditional Iranian medicine, Clover has been mentioned as one of the therapies for increasing platelets and coagulability, but few studies have proved this hypothesis. Based on some studies, the substances in clover can have medicinal properties and be useful for humans [3]. The clover contains vitamins A, K and E, and Fresh clover is also a rich source of vitamin C. Enzymes in clover include amylase, emulsifying anorectase. Clover leaves have about 20% protein, which contains the amino acids lysine, arginine, adenine, histidine, phenylalanine, asparagine and, cysteine [4]. In Iranian clover, the number of basic genomes of the genus Trifolium is eight and it is genetically a self-priming diploid. However, in one of the sources, there is strong evidence about the transformation of the plant. This type of clover has many ecotypes; each ecotype is named after its planting area. Among these names, we can mention Haft Chin of Markazi Province (Shandz), Haft Chin of Anaj, Chin and Dochin of Kermanshah, Ghorchi Bashi, Aleshtar of Lorestan, Herati Boroujerd, Surian Abadeh, Eghlid Fars, Lordegan of Chaharmahal and Bakhtiari, etc. Persian clover is an annual and autumn plant, whose main growth in winter is vegetative and lying on the ground and it grows upright, in spring. It has a height of 45 to 90 cm. it grows betterin alkaline (pH>6), wet and heavy soil. It can be consumed wet, dry, silos, pastures and pastures, it is cultivated alone or mixed with grasses such as chicken. The weight of seed is about 0.7 grams. It is adapted to most cold and semi-cold regions. The average yield of dry forage is 2 to 5 tons per hectare, but in experiments it has been reported up to 10 tons per hectare [5]. In this study, we investigated the effectiveness of clover on platelet proliferation in children with thrombocytopenia.
Materials and Methods
Study Design and Subjects
This study was designed as an interventional study (clinical trial). The study population was all children aged 1 to 18 years with thrombocytopenia treated in the pediatric hematology clinic of Shahid Madani Hospital in Khorram-Abad in 1400.This study was performed randomly, bilaterally, placebo-controlled, in two stages of interventional or clinical trial and single-center. The Haphazard sampling method was used for sampling. The inclusion criteria were including; Age between 1 year and 18 years; No spontaneous bleeding; No other blood diseases; not have systemic diseases such as lupus erythematous; Conscious consent of parents of children involved in the project and Platelet count above 50,000. The children with Active and spontaneous bleeding during the study, Infectious diseases affecting platelet count, Severe clover allergy, Lymphatic or Chronic intermittent neutropenia disease were excluded from study. All participants received main treatment in addition to intervention drug. The parents of participants, also signed the clinical trial consent, in addition to the consent to participate in the study.
In order to equalize the distribution of two important confounders of age and sex, a class was created based on these two variables. Then, "Age group 1 to 10 years, age group 11 to 18 years" and "Boys and girls" were randomly divided into two groups of treatment (Clever group) and control (Placebo group) in the same way. The size of each block was 4 items, so that 6 different combinations of 4 blocks were created and were selected randomly by placing the blocks. Using this method, the sample size in the two study arms was equal (balance) and the difference between the two groups in terms of sample size was a maximum of half a block (two people). Using this method of random allocation, the maximum power can be expected in the study results.
In each study arm, considering type one error (alpha) equal to 0.05, type two error (beta) /20. Based on the mean changes in platelet count of the two intervention and control groups in a similar study equal to 13479 and the minimum clinically acceptable difference equal to 4000 and the polarized standard deviation equal to 10438, the sample size was estimated to be equal to 38 people. Therefore, the sample size for the whole study was 76 people. Because the study was longitudinal and there was a possibility of falls in the subjects, 2 people (considering a 15% drop) were added to each study arm. As a result, the total sample size was 76 people.
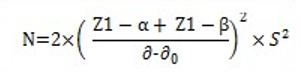
(In the placebo group, apple essential oil was added to distilled water, but in the intervention group, only apple essential oil was added to distilled water).
Cloverstick Drug and Placebo Formulation and Implement
The clover in this study was obtained froma genus of the genus Trifolium dark in the family Fabaceae and an agronomic species that grows in Lorestan province. The brew of clover stick was prepared as described below. The clover was cleaned and boiled in water higher than 90? in the particle size. Then the clover container was put in the steam bath for 30 minutes and was stirred during this time. After that, it was strained hot. Pour it on the filtered plant residue of boiling water to reach the previously determined weight after a short pressing. This solution contains 650 mg of clover extract per 5cc. The Clevergroup was given 5cc of this main drug every 8 hours or three times a day for 4 months.
For placebo formulation, Low dose of the main drug that clinically ineffective was added to boiled water. 1cc of clover extract solution to 250 cc is equivalent to one glass of boiled water. This method makes more the similarity of smell, color and taste, between placebo and main drug and helps maintain blindness in clinical trials. The Placebo group received 5 cc placebos every 8 hours for 4 months.
Sampling and Laboratory Tests
The BT and CBC are measured before and after intervention [6]. Characteristics of children and sensitivity to latex or disinfectants were recorded. Blood sampling test was done in the >5 years old bed in children <5 yrs., and in a chair in children ≥ 5 yrs. In CBC test, arm vein or veins of the back of the hand or ankle were used. In this way, 2cc of blood were taken and pour in CBC vial and mixed with the anticoagulation -Ethylenediaminetriacetic Acid (EDTA) - by moving the number 8.It is best for the participants to observe the fasting conditions before performing this test, although there is no requirement for it. The test method was automated cell counting technique.
The IVY method used for BT measure. The inner part of the forearm was cleaned with alcohol; the armband of the blood pressure monitor was closed above the child's forearm and adjusted to a pressure of 40 mm Hg, and kept constant during the test. Then the bleeding is cleaned once every 30 seconds, when the bleeding stopped, the time was recorded again. The time interval between the beginning and the end of the bleeding was calculated. This time is called BT. then the blood pressure armband was opened and an adhesive bandage was applied to the patient's hand. If the bleeding continues for more than 10 minutes, the test is stopped [7].
Elimination of the Effect of the Main Drug Difference in Participants
To equalize patients for treatment regimens, because the majority of patients were treated with immunosuppression for at least 4 months, the degree of humoral and cellular immunodeficiency was evaluated. The number 1100 was used as the cut-off point. Lymphopenic patients were excluded from the study. Also, the criterion of neutropenia was the Absolute neutrophilic count. Alternately, they were excluded from the study [6].
Ethical Considerations
The study protocol was approved by the Research Ethics Committee of Lorestan University of Medical Sciences (LUNS) with ethical code: IR.lums.REC.1400.185.
Statistical Analysis
In order to determine the descriptive results, the central index and dispersion were calculated depending on the distribution of variables. Then, repeated measures (ANOVA) were used to compare the mean platelet count and BT between the intervention and control groups. As a result, three indicators of time effect, intervention effect and also the interaction of time effect and intervention were examined. Stata software version 16 was used for data analysis.
Table 1
Table 1: Demographic profile.
Table 2
Table 2: Frequency, mean and standard deviation of platelet count in the studied groups according to the time of intervention.
Table 3
Table 3: Frequency, mean and standard deviation of BT in the studied groups patient & control group.
| Group | Number | Mean ± SD | |
| clover | Pre-intervention | 40 | 4.51 ±1.48 |
| control | 38 | 4.56 ± 1.51 | |
| total | 78 | 4.53 ± 1.34 | |
| clover | 1month after intervention | 40 | 4.23 ± 1.34 |
| control | 38 | 4.35 ± 1.41 | |
| total | 78 | 4.29 ± 1.36 | |
| clover | 2month after intervention | 40 | 4.08 ± 1.28 |
| control | 38 | 4.31 ± 1.33 | |
| total | 78 | 4.18 ± 1.29 | |
| clover | 3month after intervention | 40 | 3.72 ± 1.2 |
| control | 38 | 4.08 ± 1.36 | |
| Total | 78 | 3.88 ±1.24 | |
| clover | 4month after intervention | 40 | 2.87 ± 0.7 |
| control | 38 | 3.67 ± 0.7 | |
| total | 78 | 3.19 ± 0.84 | |
Results
In the present study, all 78 children completed the treatment with stick cloverstick drug. The mean and standard deviation of age of the studied children was 3.87±7.2 years in the intervention group (clover) and 4.68±8.8 years in the control group (placebo) and no statistically significant difference was observed (P-value < 0.930) (Table 1). Also, in the Clover group, 70% of the subjects were boys and 30% were girls, and in the Placebo group, 70% of the subjects were boys and 30% were girls.
There was no significant difference in terms of gender distribution between the two groups (P-value=0.517) (Table 1). 65% of the subjects in clover group and 70% in Placebo group lived in the city. There was no significant difference in terms of location between the two groups (P-value=0.488) (Table 1). In Clover and Placebo groups, the family history of thrombocytopenia was 15% and 25% respectively. No significant difference was observed in the study (P-value=0.524) (Table 1-3). Most patients had chronic peripheral thrombocytopenia and most had similar therapies, such as corticosteroids, rituximab, and cyclosporine.
The platelet count and BT in before intervention (P1, bt1) and 4 times after intervention for 4 months in two groups are present in (Table 3). The measures in after intervention were done in one month (P2), two months (P2, bt2), three months (P3, bt3), four months (P4, bt4) and five months (P5, bt5) after the intervention.
Determining the Effect of Time
The mean count of platelets in the Clover group showed an increasing trend in the measurement times, and these values had significant differences statically in the 4 measurement times (P-value<0.003). In the same way, the mean BT had a decreasing trend in 4 measurements in 4 months, but these values had no significant difference statically (P-value<0.137). In Placebo group, the mean of platelets and BT were changed, but not significant difference in 4 measurements (P-value>0.05). The control group can be changed. No attention was paid to the mean platelet count.
Determining the Effect of the Intervention
A comparison of the results between the two clover groups and the control group at each measurement is has shown in (Figure 1,2). As shown in (Figure 1), the change of mean platelet in Clover and Placebo groups were statistically significant (P-value <0.004), but, the mean changes of BT in Clover and Placebo groups were not statistically significant (P-value <0.507) (Figure 2). Comparison between mean BT in clover and control groups showed a significant difference.
Determining the Effect on Intervention Interaction and Time
The Comparison of changes in the mean platelet count and BT in two groups over time is present in (Figure 1). The changes of mean platelet count in overtime were statistically significant between two groups (P-value<0.0001), while, the changes of mean BT in overtime were not statistically significant between two groups (P-value<0.713).
Mean changes of BT in clover and placebo groups over time at different times were statistically significant (P-value <0.713) (Figure 2). As seen in (Figure 1), the mean platelet count in the Clover group had statistically significant changes in P1, P2, P3, P4 and P5, compared to the Placebo group, and, the trend of these changes was incremental. This upward trend is also changeable overtime and there is a significant difference between the two groups (P-value<0.05).
The minimum difference of mean platelet count is seen in P1 and the maximum difference is in P5 between the two groups. As shown in (Figure 2), the mean BT had adecreasing trend overtime, and despite of the difference in bt1, bt2, bt3, bt4, and bt5, between the clover and placebo groups, this difference was not statistically significant(P-value>0.05).However, this decreasing trend also changed over time, so that, the differences between the two groups was significant, significantly. The minimum difference between the mean BT in the Clover and Placebo groups was in bt1, and the maximum one was in bt5.
Figure 1
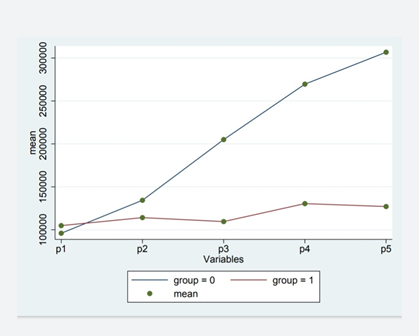
Figure 1: Diagram of determining the effects of time, intervention and on the interaction of time and intervention platelet count (P1: before intervention, P2: 1 month after intervention, P3: 2 months after intervention, P4, 3 months after intervention, P5: 4 months after intervention.
Group 0: intervention group (clover), group 1: control group (placebo), mean: mean platelet count.)
Figure 2
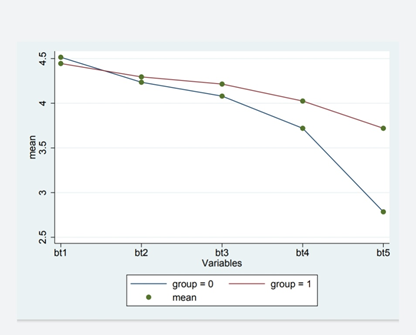
Figure 2: Diagram of determining the effects of time, intervention and on the interaction of time and BT intervention. (bt1: before intervention, bt2: 1 month after intervention, bt3: 2 months after intervention, bt4, 3 months after intervention, P5: 4 months after intervention.
Group 0: intervention group (clover), group 1: control group (placebo), mean: mean BT.)
Discussion
In recent decades, in most countries, the use of alternative therapies, especially herbal medicine and dietary supplements to improve a variety of diseases has increased. One of the major problems facing physicians as well as consumers of medicinal plants is the lack of sufficient information about the health of the drug and, its effect on the disease [8]. Fortunately, over the past 30 years, extensive research has been conducted on the effectiveness of medicinal plants used in traditional medicine, proving their effectiveness or ineffectiveness [9].
The present study evaluated the effect of clover infusion on several parameters related to thrombocytopenia in children with thrombocytopenia. The main findings of this study showed a significant increase in the mean platelet count in the intervention group compared to the control group. In the in-group comparison of separate blood analyses before and after the intervention, a significant difference was observed only in the mean platelet count in the intervention group and no significant difference was observed for BT. A search of scientific databases to compare the results has been not finding a similar study to a clinical trial of patients with thrombocytopenia.
An increasing number of reports indicate that some species of clover (Trifolium) may be of significant medical importance. However, the effects of these plants on blood platelets and homeostasis are not well understood. To this end, Kolodziejczyk et al. conducted one study in 2013 to investigate the effects of extracts of two clover species on human platelet function in vitro. The experiments showed that the extracts may affect platelet function in vitro. Inhibitory and concentration-dependent effects of all tested extracts were found on the adhesion of thrombin-stimulated platelets to collagen. Clover extract reduced thrombin-induced platelet adhesion to fibrinogen. The results showed that these plants may be a promising source of natural compounds that prevent the increase of platelet activity [10].
The results of this study justify the conclusion of our study that clover did not have a significant effect on BT reduction, which is due to the effect of clover, which reduces platelet aggregation and agglutination. Numerous studies have shown that Trifolium-derived plant extracts may exhibit significant antioxidant and free radical scavenging properties [8,11]. In an in vitro study conducted by Kolodziejczyk et alin 2015. Cloamide-rich extract of clover reduced the damage caused by oxidative stress to blood platelets and plasma. The results of this study showed that clover extract is most likely a source of compounds with antioxidant properties that are useful in preventing diseases related to oxidative stress. Nitric Oxide (NO) is a potent plutropic mediator of physiological processes such as smooth muscle relaxation, neural signaling, and inhibition of platelet aggregation. Clover also inhibits nitric oxide in a dose-dependent manner [12].
On the other hand, clover extract contains large amounts of polyphenol antioxidant compounds. In another study, Kolodziejczyk et alexamined the antiplatelet properties of nine clover species in 2016. The results of their studies showed that phenolic fractions are the most effective inhibitors of platelet activation. The obtained results can be the basis for further studies on clover as a source of extracts with anti-platelet properties [11]. The mechanism of action of polyphenols in inhibiting platelet function is not fully understood. It leads to the accumulation of platelets. In addition to the antioxidant effect, other experiments have suggested the possibility of inhibiting some clover on platelet activation [8].
In another laboratory study, the effects of clover on platelet aggregation and adhesion were investigated. The results of this study showed that adhesion of blood platelets to fibrinogen occurs through the αIIbβ3 integrin receptor. In resting platelets, αIIbβ3 receptors are kept in a low-affinity state, but during platelet activation, these receptors rapidly become a high-affinity compound. Platelet response to ADP is mediated by its interaction with G protein-associated P2 receptors (P2Y1 and P2Y12). Interaction of ADP with Gq-associated P2Y1 receptor activates phospholipase C (PLC), Ca 2+ influx, and intracellular Ca2+ mobilization. P2Y12, which binds to Gi, inhibits adenyl cyclase and reduces cAMP levels.
The P2Y12 receptor is a target for several antagonists that are used as antithrombotic/antiplatelet agents such as clopidogrel, prasugrel, ticagrelor, kanglerol, and elinogrel. Thrombin activates platelets due to protease-activated receptors (PARs): PAR-1 and PAR-4. PARs are paired with the Gq protein and transmit cellular signals primarily by PLCβ stimulation [13-17]. According to the above studies and the mechanisms described, it can be concluded that clover infusion reduces platelet aggregation and adhesion, on the one hand,and, it causes increasing bone marrow hematopoiesis platelets in patients, on the other hand. As a result, platelet proliferation also increases BT by activating the coagulation system.
Conclusion
Clover infusion, as traditional herbal medicine and adjuvant treatment, increases the number of platelets during the studied periods and in this regard improves the platelet level of children with peripheral immune thrombocytopenia. Based on the results of this study, the effect of cerebrospinal fluid on thrombocytopenia due to decreased megakaryocyte and platelet production in the bone marrow can be studied in controlled and safe conditions for the patient. But the use of traditional Iranian medicine as a complementary medicine alongside classical medicine requires verification with clinical trial studies.
Conflict of Interest
The authors whose names are listed immediately below certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Acknowledgment
I am grateful to all of those with whom I have had the pleasure to work during this and other related projects. Each of the members of my Dissertation Committee has provided me with extensive personal and professional guidance and taught me a great deal about both scientific research and life in general.
References
- IAHPC. Global Directory of Institutions and Organizations.
- Petrovska BB. Historical review of medicinal plants' usage. Pharmacogn Rev. 2012; 6: 1-5.
- Abberton MT, AH. M. Progress in breeding perennial clovers for temperate agriculture. J Agricultural Sci. 2005; 143: 117-135.
- Baghdar HN, Koushyar MM, Hamedi SS, Feyzabadi Z, Ghorbanzade H, Jafarinejad M. Comparison of the Effect of Alfalfa Syrup and Placebo on Platelet Count in Patients with Idiopathic Thrombocytopenic Purpura. Med History J. 2020; 12: 55-62.
- Department of primary industries. Persian clover: NSW Government.
- McCusker C, Upton J, Warrington R. Primary immunodeficiency. Allergy Asthma Clin Immunol. 2018; 14: 61.
- Sang Medicine. The Bleeding Time [BT]: Sang Medicine.
- Kolodziejczyk-Czepas J, Olas B, Malinowska J, Wachowicz B, Szajwaj B, Kowalska I, et al. Extracts from Trifolium pallidum and Trifolium scabrum aerial parts as modulators of blood platelet adhesion and aggregation. Platelets. 2013; 24: 136-144.
- Kolodziejczyk-Czepas J, Nowak P, Kowalska I, Stochmal A. Biological activity of clovers - free radical scavenging ability and antioxidant action of six Trifolium species. Pharm Biol. 2014; 52: 1308-1314.
- Kolodziejczyk-Czepas J, Nowak P, Moniuszko-Szajwaj B, Kowalska I, A. S. Free radical scavenging actions of three Trifolium species in the protection of blood plasma antioxidant capacity in vitro. Pharm Biol. 2014; 52: 1308-1314.
- Kolodziejczyk-Czepas J, Sieradzka M, Wachowicz B, Nowak P, Oleszek W, AS. The anti-adhesive and anti-aggregatory effects of phenolics from Trifolium species in vitro. Mol Cell Biochem. 2016; 412: 155-164.
- Engelmann NJ, Reppert A, Yousef G, Rogers RB, Lila MA. In Vitro Production of Radiolabeled Red Clover (Trifolium pratense) Isoflavones. Plant Cell Tissue Organ Cult. 2009; 98: 147-156.
- Stegner D, Nieswandt B. Platelet receptor signaling in thrombus formation. J Mol Med (Berl). 2011; 89: 109-121.
- Wijeyeratne YD, Heptinstall S. Anti-platelet therapy: ADP receptor antagonists. Br J Clin Pharmacol. 2011; 72: 647-657.
- Ruggeri ZM, Mendolicchio GL. Adhesion mechanisms in platelet function. Circ Res. 2007; 100: 1673-1685.
- Farndale RW. Collagen-induced platelet activation. Blood Cells Mol Dis. 2006; 36: 162-165.
- Heuberger DM, Schuepbach RA. Protease-activated receptors (PARs): mechanisms of action and potential therapeutic modulators in PAR-driven inflammatory diseases. Thromb J. 2019; 17: 4.








































































































































