Increased Lysophosphatidyleth-anolamine and Diacylglycerol levels in Alzheimer
- 1. Department of Physiology and Pharmacology, Lincoln Memorial University, USA
- 2. Department of Neurology, Oregon Health Science University, USA
Abstract
Background: Previous studies have demonstrated deficits in biosynthesis of plasmalogens and sphingolipids in Alzheimer ’s Disease (AD)
Objective: We undertook an analysis of alternate biomarkers of lipid synthesis, metabolism, and remodeling “Lands” cycle in the plasma of control and cognitively impaired patients based upon their Mini-Mental State Examination (MMSE) scores.
Methods: Plasma levels of 600 lipids were measured utilizing a high-resolution mass spectrometric shotgun analysis platform.
Results: The lyso metabolites of phosphatidylethanolamines and phosphatidylglycerols, along with diacyglycerols were all elevated in the plasma of patients with cognitive impairment.There were no significant differences in the plasma levels of fatty acids metabolized by peroxisomes.
Conclusion: The increased levels of lysolipids and diacylglycerols in theplasma of all patient groups suggest that lipid remodeling is dysfunctional early in the AD disease process. It remains to be determined if this involves increased phospholipase A2 activity, decreased acyltransferase activity, or decreased cellular uptake of lysophospholipids for subsequent acylation.
Keywords
• Lysophosphatidylethanolamine
• Lysophosphatidylglycerol
• Diacylglycerol
• Lipid remodeling
• Alzheimer’s disease
• Mild cognitive impairment
• Plasma biomarker
• High-resolution lipidomics
• Phospholipase A2
• Lysophospholipid acyltransferase
INTRODUCTION
The search for robust blood-based biomarkers for AD has involved both proteomics [1] and lipidomics [2] approaches.Plasma lipidomics findings include decrements in peroxisomaldependent biosynthesis of plasmalogens in AD, altered ceramide levels in Mild Cognitive Impairment (MCI) and AD, and decreases in sphingomyelins in AD [2]. The major goals of current lipidomics research efforts are: i) to identify biomarkers of early-stage disease processes such that preclinical therapeutic interventions can be initialized, ii) to monitor disease progression and the efficacy of therapeutic interventions, and iii) to increase our understanding of upstream disease pathophysiology in efforts to define new therapeutic targets that might halt neurodegeneration.
Since several alterations in the levels of circulating plasmalogens have been reported in AD plasma [3,4], we decided to evaluate glycerophospholipid metabolites to more fully
characterize potential early alterations in lipid dynamics. Thisevaluation involved monitoring lysoglycerophospholipids asbiomarkers of lipid remodeling via deacyltaion/reacylation atsn-2 of the glycerol backbone [2,5-7].
Previous studies have demonstrated decreases in the abilityof peroxisomes to synthesize plasmalogens in AD liver and brain [8,9]. We therefore investigated if peroxisomal-dependent lipid metabolism might also be altered in AD. To this end, we measured the branched chain fatty acid, phytanic acid and the very-longchain fatty acid (VLCFA), hexacosanoic acid (26:0), both of which are dependent upon peroxisomes for their metabolism [10].
CITATION
: Wood PL, Phillipps A, Woltjer RL, Kaye JA, Quinn JF (2014) Increased Lysophosphatidylethanolamine and Diacylglycerol levels in Alzheimer’s Disease Plasma. JSM Alzheimer’s Dis Related Dementia 1(1): 1001.
MATERIALS AND METHODS
Study sample
Plasma samples were obtained from cognitively healthy controlsubjects and from ADpatients (Table 1) followed at the Oregon Health and Science University Aging and Alzheimer’s disease center. Alzheimer’s disease was diagnosed by NINDS ADRDA criteria and patients were stratified by clinical severity measured by the Mini-Mental State Examination (MMSE) score. The clinical studies were approved by the OHSU Institutional Review Board.
Laboratory methods
Plasma samples were processed utilizing tert-butyl methylether and methanol for extraction of lipids [11-12]. The extraction solution contained [2 H8 ]arachidonic acid, [2 H3 ] phytanic acid, [2 H4 ]hexacosanoic acid, [13C16]palmitic acid,[2 H7 ] cholesterol sulfate, [2 H5 ]MAG 18:1, [13C3 ]DAG 36:2, [2 H31]PtdEtn 34:1, [2 H54]PtdEtn 28:0, [2 H31]PtdCh 34:1, [2 H54]PtdCh 28:0, [2 H62] PtdCh 32:0, [2 H31]SM 16:0, [2 H31]PtdSer 36:1, and [2 H31]PA 34:1 as internal standards
Extracts were dried by centrifugal vacuum evaporation prior to dissolution in isopropanol: methanol: chloroform (4:2:1) containing 7.5 mM ammonium acetate. Shotgun lipidomics were performed utilizing high-resolution (140,000 at 200 amu) data acquisition, with sub-ppm mass accuracy on an orbitrap mass spectrometer (Thermo Q Exactive) with successive switching between polarity modes [11]. Washes between samples with hexane/ethyl acetate (3:2) were used to minimize ghost effects. In negative ion ESI, the anions of ethanolamine plasmalogens, phosphatidylglycerols, phosphatidic acids, phosphatidylinositols, phosphatidylserines, lysophosphatidylethanolamines, lysophosphatidylinositols, lysophosphatidylglycerols, cerebrosides, sterol sulfates, and fatty acids were quantitated and lipid identities validated by MS/MS. In positive ion ESI, the cations of choline plasmalogens, phosphatidylcholines, lysophosphatidylcholines, alkenyl-acylglycerols, and sphingomyelins and the ammonium adducts of diacylglycerols were quantitated and lipid identities validated by MS/MS.
Statistical analysis
Data were analyzed by 1-way ANOVA, followed by the Tukey Kramer test to determine differences between groups.
RESULTS
Sample storage effects
Since the majority of the plasma samples used in this study were acquired and stored since 2000-2001, we included 4 samples that had been stored no longer than 4 months to assess potential storage artifacts. While most lipids were stable with storage at -80o C, we found 3- to 5-fold elevations of lysophosphatidylcholines and 6- to 10-fold elevations of lysophosphatidylinositols with long-term sample storage
Fatty acids metabolized by peroxisomes
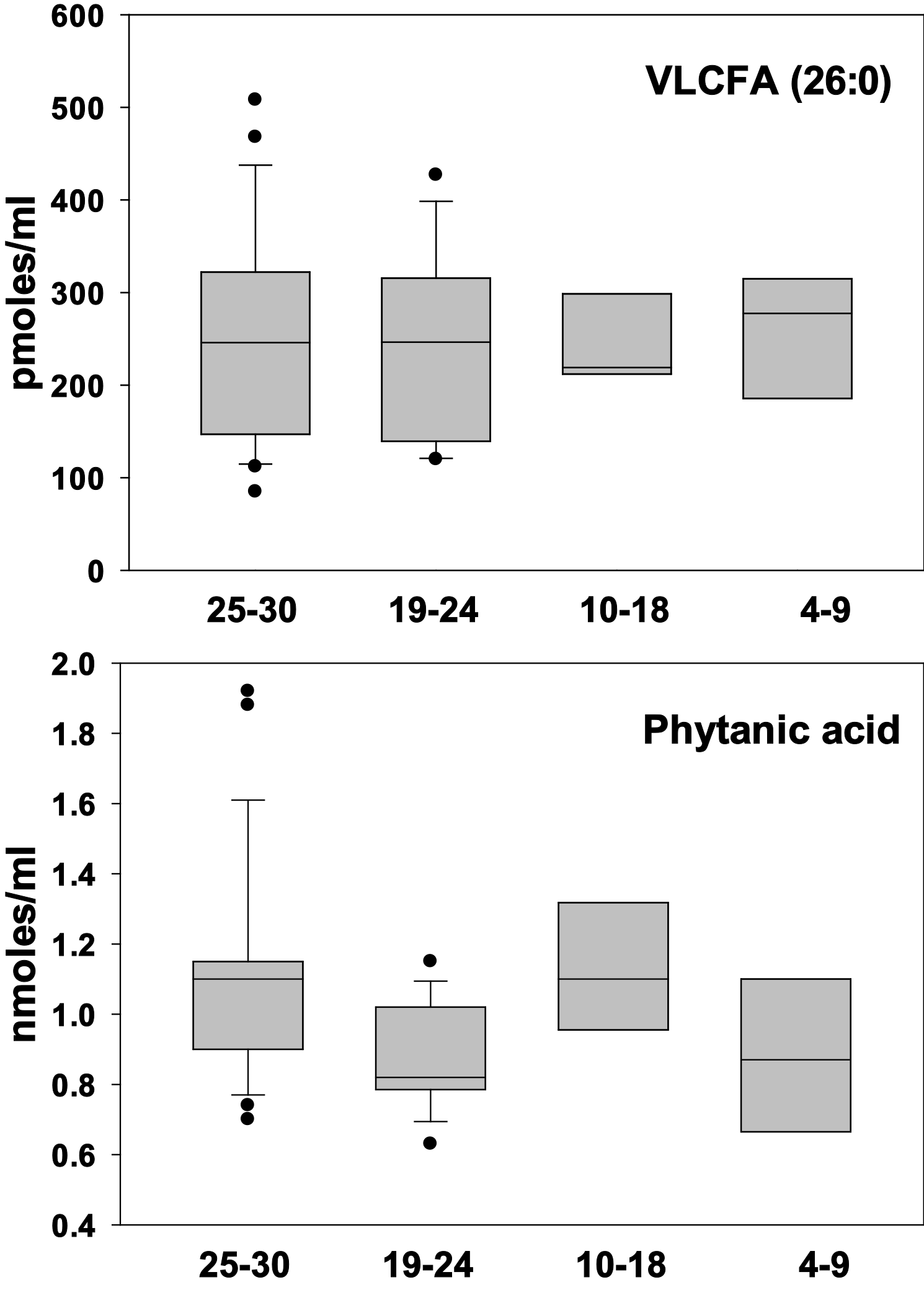
Neither phytanic acid or docosanoic (26:0) acid levels were altered in cognitively impaired patients suggesting that peroxisomal metabolism of branched chain fatty acids and VLCFA is unaltered in AD (Figure 1).
Figure 1 Box plots of plasma levels of the branched chain fatty acid, phytanic acid and the VLCFA, hexacosanoic acid (26:0). There were no statistically significant differences between groups. X Axis = MMSE scores.
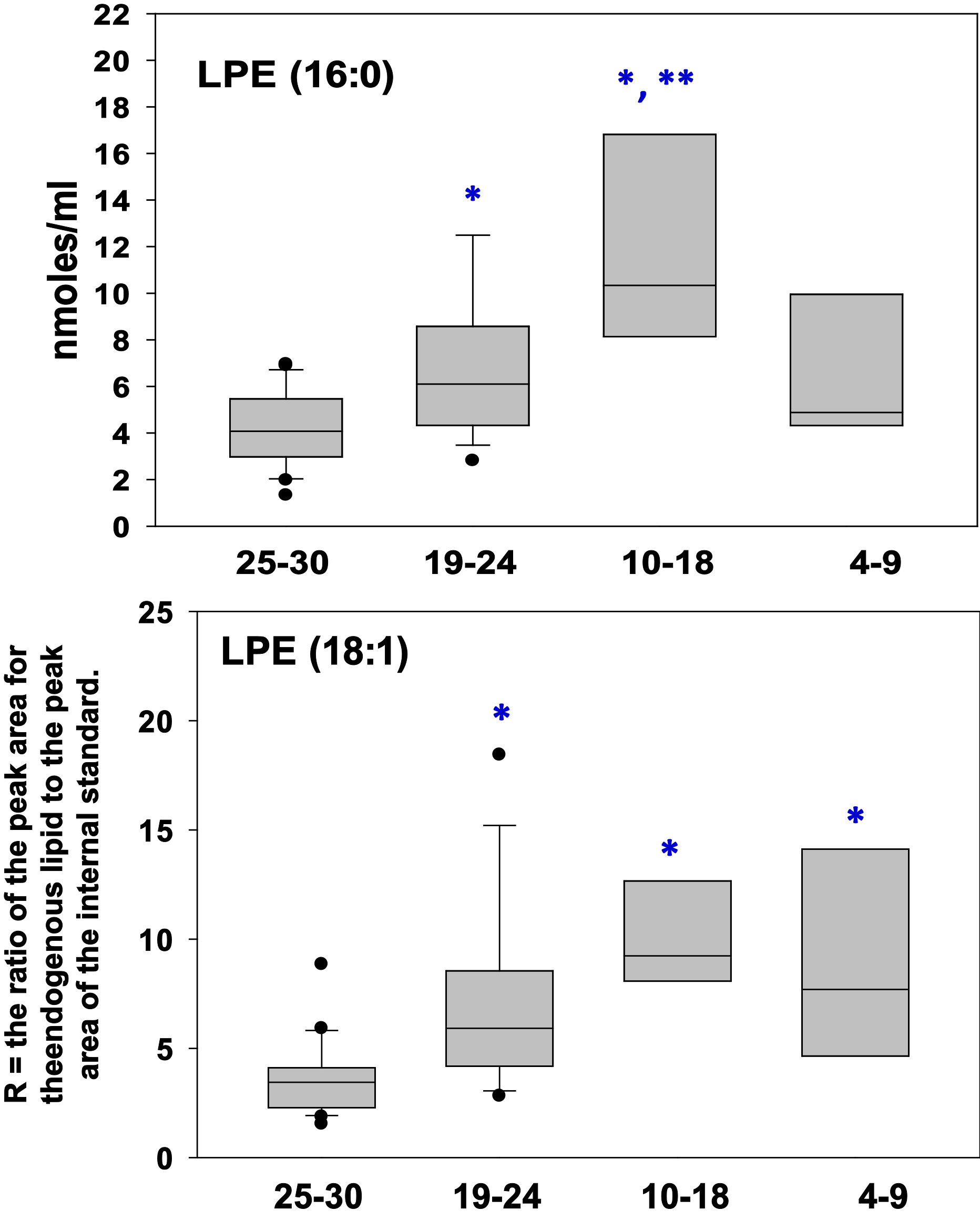
Figure 2 Box plots of plasma levels of the lysophosphatidylethanolamines LPE 16:0 and LPE 18:1. X Axis = MMSE scores. *, p < 0.05 vs. normal cognition (MMSE 25-30); ** p < 0.05 vs. Mild AD (MMSE 19-24).
Lysoglycerophospholipids
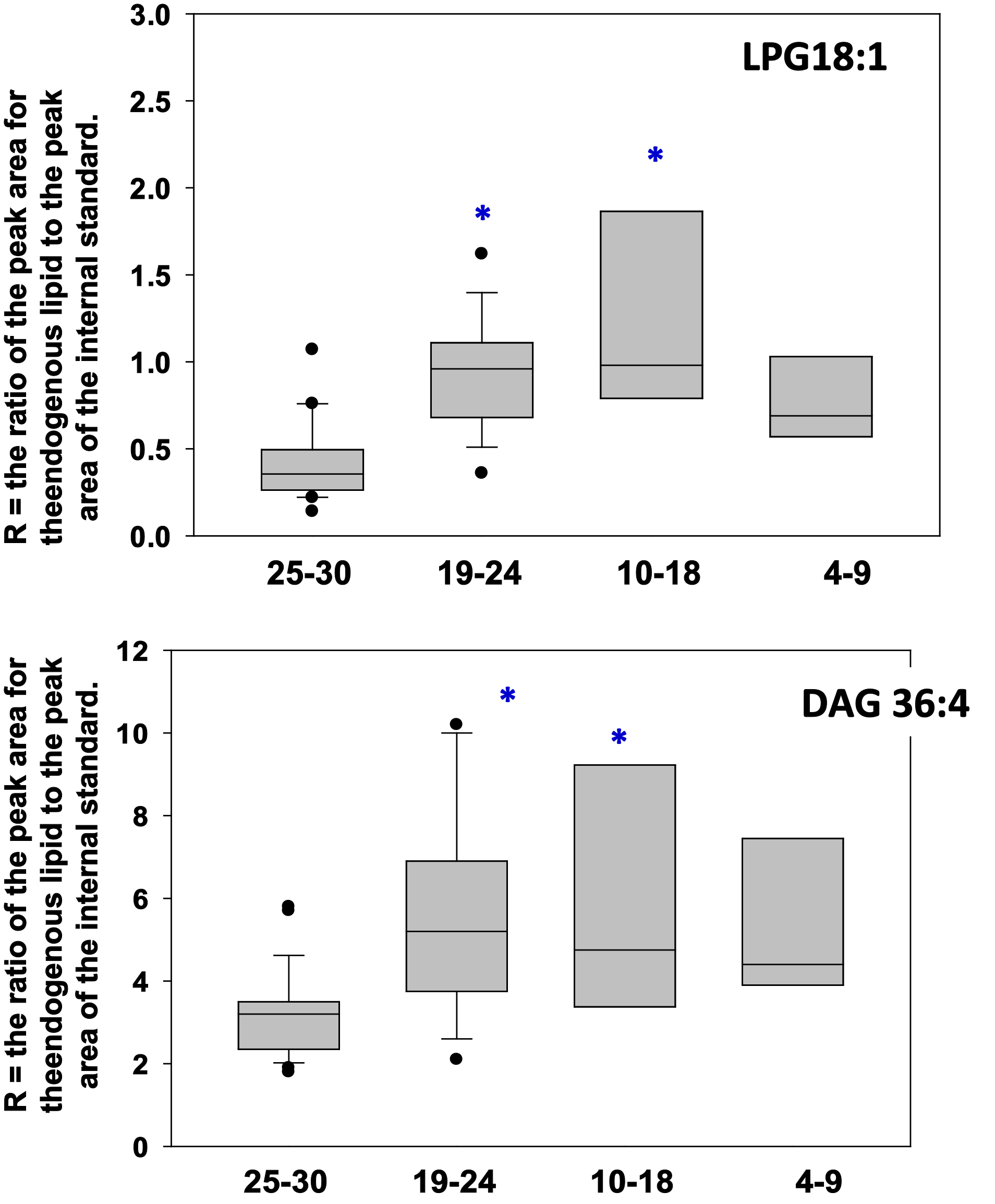
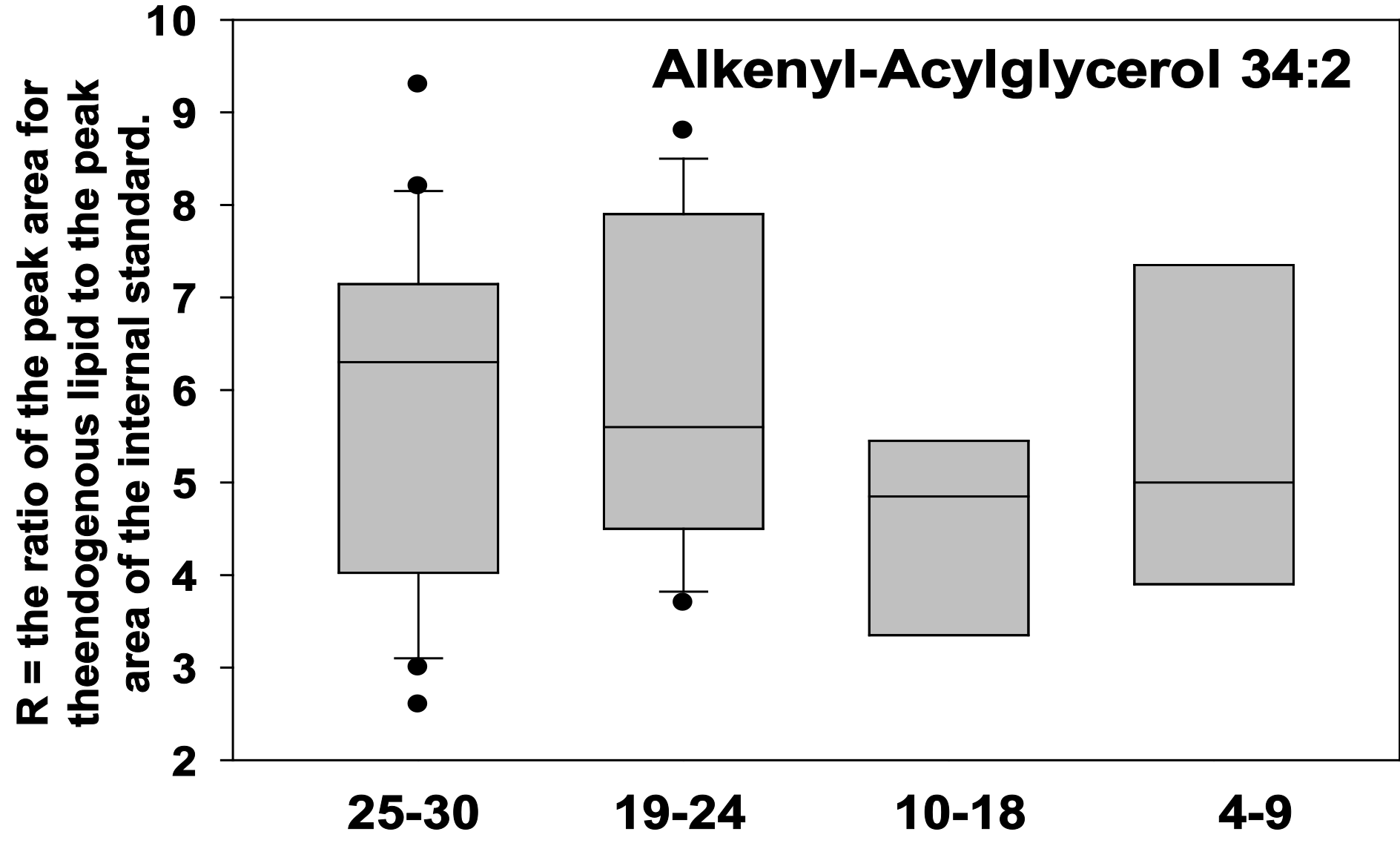
LPE 16:0 and 18:1 were both increased in the plasma of cognitively impaired patients (Figure 2). Similarly, diacylglycerols like DAG 36:4 and lysophosphatidylglycerols like LPG 18:1 were elevated in cognitively impaired patients (Figure 3). In contrast, to these phospholipase A2 (PLA2) metabolic products, phospholipase D products like alkenyl-acylglycerol 34:2 were not altered in the plasma of cognitively impaired patients (Figure 4).
Figure 3 Box plots of LPG 18:1 and diacylglycerol (DAG 36:4) in plasma. Both LPG 18:1 and DAG 36:4 levels were elevated in plasma from mild and moderate AD patients. *, p < 0.05 vs. normal cognition (MMSE 25-30).X Axis = MMSE scores.
Figure 4 Box plots of alkenyl-acyl glycerol 34:2 in plasma. There were no statistically significant differences between groups. X Axis = MMSE scores.
DISCUSSION
Lipidomics studies have revealed a number of alterations in both peroxisome-dependent synthesis of plasmalogens and in glycerophospholipid and sphingolipid metabolism [2]. Our evaluation of the branched chain fatty acid, phytanic acid and the VLCFA docosanoic acid suggest that the peroxisomal deficit in plasmalogen synthesis is specific and that not all peroxisomal functions are altered in AD.
Our evaluation of glycerophospholipid metabolites demonstrated increases in lysophosphatidylethanolamines (LPE), lysophosphatidylglycerols (LPG), and diacylglycerols (DAG). Increased DAG levels [13] and increased MAG and DAG lipases [14] have been reported in AD brain, suggesting altered lipid metabolism and signaling are present in the AD brain. Our data are the first to report altered DAG levels in the plasma of cognitively impaired patients
The augmented levels of LPE and LPG that we monitored in the plasma of cognitively impaired patients are likely products of augmented PLA2 activity which is involved in the dynamic regulation of fatty acid substitutions at sn-2 of the glycerol backbone [2,15]. However, PLA2 biochemistry is extremely complex with over 26 PLA2 genes and multiple enzyme isoforms [16] and increased levels of different isoforms in AD cortex and CSF [2]. However, plasma lipoprotein-associated PLA2 is unaltered in both MCI and AD patients [17], suggesting that alternate isoforms of circulating PLA2 or tissue PLA2 are involved in augmenting circulating levels of these lipid metabolites. Other factors that might contribute to elevated lysoglycerophospholipids would be an uncoupling of the highly regulated deacylation-reacylation enzymes [2,15,18] and/or dysregulation of cellular uptake of lysoglycerophospholipids and subsequent intracellular acylation [19].
The implications for these findings of altered glycerophospholipid metabolism are twofold. First, alterations in the levels of these complex lipid families will have structural effects on cellular membrane and organelle function [2]. Second, lysoglycerophospholipids are involved in multiple cell signaling functions [20], thereby potentially contributing to altered neurotransmission in AD.
Limitations and future research
This is a robust pilot study except for the small N in the more severely cognitively impaired groups. Further validation of our findings will require augmenting these cohorts. Further investigation of the potential role of changes in DAG and LPE metabolism on cardiovascular health, a risk factor for AD, is warranted.
CONCLUSION
In summary, our data demonstrate significant increases in lysoglycerophospholipids, lysoglycerolipids, and DAG in the plasma of cognitively impaired patients indicating that these lipid changes occur early in the disease process. The next steps will be to define if these changes in the lipidome involve dysfunctional lipid remodeling and/or altered cellular uptake of lysoglycerolipids for acylation. These data will help to define AD lipid pathophysiology in more detail and increase our understanding of the biochemical processes that ultimately result in the cognitive decline associated with AD.
ACKNOWLEDGEMENT
We wish to thank the patients, caregivers, and healthcare staff that all contributed to this study
Support
This study was supported by operating funds from Lincoln Memorial University-Debusk College of Osteopathic Medicine for the Metabolomics Unit and by NIA-AG08017.
REFERENCES
- Snyder HM, Carrillo MC, Grodstein F, Henriksen K, Jeromin A, Lovestone S, et al. Developing novel blood-based biomarkers for Alzheimer's disease. Alzheimers Dement. 2014; 10: 109-114.
- Wood PL. Lipidomics of Alzheimer's disease: current status. Alzheimers Res Ther. 2012; 4: 5.
- Goodenowe DB, Cook LL, Liu J, Lu Y, Jayasinghe DA, Ahiahonu PW, et al. Peripheral ethanolamine plasmalogen deficiency: a logical causative factor in Alzheimer's disease and dementia. J Lipid Res. 2007; 48: 2485-2498.
- Wood PL, Mankidy R, Ritchie S, Heath D, Wood JA, Flax J, et al. Circulating plasmalogen levels and Alzheimer Disease Assessment Scale-Cognitive scores in Alzheimer patients. J Psychiatry Neurosci. 2010; 35: 59-62.
- Kainu V, Hermansson M, Somerharju P. Electrospray ionization mass spectrometry and exogenous heavy isotope-labeled lipid species provide detailed information on aminophospholipid acyl chain remodeling. J Biol Chem. 2008; 283: 3676-3687.
- Zhang L, Díaz-Díaz N, Zarringhalam K, Hermansson M, Somerharju P, Chuang J. Dynamics of the ethanolamine glycerophospholipid remodeling network. PLoS One. 2012; 7: e50858.
- Zarringhalam K, Zhang L, Kiebish MA, Yang K, Han X, Gross RW, et al. Statistical analysis of the processes controlling choline and ethanolamine glycerophospholipid molecular species composition. PLoS One. 2012; 7: e37293.
- Astarita G, Jung KM, Berchtold NC, Nguyen VQ, Gillen DL, Head E, et al. Deficient liver biosynthesis of docosahexaenoic acid correlates with cognitive impairment in Alzheimer's disease. PLoS One. 2010; 5: e12538.
- Kou J, Kovacs GG, Höftberger R, Kulik W, Brodde A, Forss-Petter S, et al. Peroxisomal alterations in Alzheimer's disease. Acta Neuropathol. 2011; 122: 271-283.
- Wanders RJ. Metabolic functions of peroxisomes in health and disease. Biochimie. 2014; 98: 36-44.
- Schuhmann K, Almeida R, Baumert M, Herzog R, Bornstein SR, Shevchenko A. Shotgun lipidomics on a LTQ Orbitrap mass spectrometer by successive switching between acquisition polarity modes. J Mass Spectrom. 2012; 47: 96-104.
- Wood PL, Khan MA, Smith T, Ehrmantraut G, Jin W, Cui W, Braverman NE. In vitro and in vivo plasmalogen replacement evaluations in rhizomelicchrondrodysplasiapunctata and Pelizaeus-Merzbacher disease using PPI-101, an ether lipid plasmalogen precursor. Lipids Health Dis. 2011; 10: 182.
- Chan RB, Oliveira TG, Cortes EP, Honig LS, Duff KE, Small SA, et al. Comparative lipidomic analysis of mouse and human brain with Alzheimer disease. J Biol Chem. 2012; 287: 2678-2688.
- Farooqui AA, Liss L, Horrocks LA. Stimulation of lipolytic enzymes in Alzheimer's disease. Ann Neurol. 1988; 23: 306-308.
- Lands WE. Stories about acyl chains. Biochim Biophys Acta. 2000; 1483: 1-14.
- Kitsiouli E, Nakos G, Lekka ME. Phospholipase A2 subclasses in acute respiratory distress syndrome. Biochim Biophys Acta. 2009; 1792: 941-953.
- Davidson JE, Lockhart A, Amos L, Stirnadel-Farrant HA, Mooser V, Sollberger M, et al. Plasma lipoprotein-associated phospholipase A2 activity in Alzheimer's disease, amnestic mild cognitive impairment, and cognitively healthy elderly subjects: a cross-sectional study. Alzheimers Res Ther. 2012; 4: 51.
- Shindou H, Shimizu T. Acyl-CoA:lysophospholipid acyltransferases. J Biol Chem. 2009; 284: 1-5.
- Riekhof WR, Wu J, Jones JL, Voelker DR. Identification and characterization of the major lysophosphatidylethanolamine acyltransferase in Saccharomyces cerevisiae. J Biol Chem. 2007; 282: 28344-52.
- Frasch SC, Zemski-Berry K, Murphy RC, Borregaard N, Henson PM, Bratton DL. Lysophospholipids of different classes mobilize neutrophil secretory vesicles and induce redundant signaling through G2A. J Immunol. 2007; 178: 6540-6548.