Arachidonic Acid Induced Thrombosis in Zebrafish Larvae for Assessing Human Anti-Thrombotic Drug
- 1. Hunter Biotechnology, China
- 2. Early Test Base of Zhejiang Province Key Laboratory of Human Supplier Safety Evaluation, China
- 3. Center of Safety Evaluation, Zhejiang Academy of Medical Sciences, China
- 4. Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences, China
Abstract
Thrombosis is a prominent cause of many cardiovascular diseases, novel anti-thrombosis drugs are still in emergency. However, the development of effective and safe therapeutic agents for thrombotic diseases faces various challenges, especially on animal model. Here, we described the development and validation of a new thrombosis model induced by arachidonic acid (AA), a platelet-coagulation agonist, for drug screening and efficacy assessment, using larval zebrafish which were transparency in their early life period. Zebrafish at 3 days post fertilization (dpf) were pre-treated with tested drug for 3 hours (h), followed by AA treatment at a concentration of 80μM for 1.5 h. Tested drugs were administered into zebrafish either by direct soaking or circulation microinjection. Antithrombotic efficacy was quantitatively evaluated based on an image analysis of the heart red blood cells (RBCs) stained with Odianisidine, and this technology has been patented previously. Thrombosis induced by AA in live zebrafish was visually confirmed under a dissecting stereomicroscope and quantified by the image assay. All the six human antithrombotic drugs (aspirin, clopidogrel, diltiazem hydrochloride injection, xueshuantong injection, salvianolate injection, astragalus injection) showed significant anti-thrombotic effects (p < 0.05, p < 0.01 & p < 0.001) in this zebrafish thrombosis model. In this study, we introduced a new larval zebrafish thrombosis model, which would be an attractive alternative for in vivo thrombosis studies and for rapid screening and efficacy assessment of antithrombotic drugs.
Keywords
Zebrafish; Thrombosis; Arachidonic acid; O-dianisidine; Anti-thrombotic drugs; Traditional Chinese medicine.
Citation
Zhang Y, Guo SY, Zhu XY, Zhou J, Liao WH, et al. (2017) Arachidonic Acid Induced Thrombosis in Zebrafish Larvae for Assessing Human Anti-Thrombotic Drugs. JSM Cell Dev Biol 5(1): 1023.
ABBREVIATIONS
H: Hours; DPF: Days Post Fertilization; DMSO: Dimethyl Sulfoxide; NOAEL: No Observed Adverse Effect Level; RBCs: Red Blood Cells; AA: Arachidonic Acid; TCM: Traditional Chinese Medicine
INTRODUCTION
There are dozens of pharmaceutical agents aiming to attenuate platelet function and prevent the formation of thrombosis, which leads to myocardial infarction, ischemia and stroke[1]. Various anticoagulants and anti-platelet therapies have been proved successful in the treatment of thrombosis over the past several decades; however, the defect of anti-platelet drugs would increase the risk of bleeding after prolonged usage causes serious concern[2,3]. In vitro and ex vivo thrombosis assays have a limited value due to the lack of organ structures and whole organism metabolism, extrapolation of these results to mammal and human being is often challenging. Conventional mammalian thrombosis models are usually time-consuming, labor-intensive, expensive and different from human in the cause of disease and clinical presentation, restricting their applications in thrombosis research and early screening of anti-thrombotic drugs [4]. An animal model that mimics human thrombosis, allows tracking and a detailed analysis of thrombosis would be highly valuable for thrombosis studies and for anti-thrombotic drug screening.
Zebrafish (Danio rerio) is emerging as a predictive vertebrate animal model for in vivo assessment of drug efficacy, toxicity and safety [5-9]. An important advantage of the zebrafish model is that the morphological and molecular basis of tissues and organs is either identical or similar to mammals, including humans [10]. The sequence and presumed function of many genes that are important for vertebrates are conserved in the zebrafish [11]. As a thrombosis model, zebrafish has several advantages, such as the capacity to follow thrombus formation at high resolution in real time using intravital microscopy without the need of complex surgical techniques, and to rapidly knockdown gene expression using morpholino antisense approaches [12,13]. Platelet has emerged as a pivotal entity in primary hemostasis and is a key mediator of thrombosis [14]. And, zebrafish thrombocytes are morphological and functional equivalent to human platelets, which share most of the central factors of platelet adhesion, activation, aggregation and release reaction with humans, including the von Willebrand factor (VWF) receptor, αIIb integrin receptor, membrane protein αIIb, GPIb, protease-activated receptors (PAR)1-4, P2Y1/P2Y12 receptors, cyclooxygenase-1 and cyclooxygenase-2, Phospholipase A2 and thromboxane A synthase [15-18]. The functional responses, such as aggregation, calcium release, P-selectin exposure, annexin V binding, dense-granule secretion, and thrombus formation, have all been demonstrated in zebrafish [4,18-22]. All of these factors make zebrafish an appropriate organism to study thrombocyte function, thrombosis and hemostasis. It has been demonstrated that zebrafish thrombocyte would aggregate in response to platelet agonists, such as collagen, adenosine diphosphate (ADP) and AA [20,23].
AA is the precursor of thromboxane A2 (TxA2) and hydroxyl fatty acids, which could be converted to TxA2 by cycoloxygenase and thromboxane synthase. TxA2 mobilizes calcium from intracellular storage sites, and then calcium activates phospholipase A2 which liberates AA from phosphatidylcholine and phosphatidylethanolamine. Calcium also activates myosin light-chain kinase. TxA2 and activated myosin light-chain kinase together lead to platelet coagulant activation by stimulating secretion of platelet granules, which promote tenase and prothrombinase formation [24]. AA has been shown to evoke aggregation response of zebrafish thrombocytes in microtiter tilt plate, whole blood aggregometry (WBA) and flow cytometry (FC) assays [15,25]. However, AA induced thrombocytes aggregation in larval zebrafish has not been reported as a live thrombosis model and not validated for drug screening yet.
Aspirin has been proven to inhibit platelet activation through suppressing cyclooxygenase system, TxA2 production and the synthesis of prostacyclin [26]. Clopidogrel (Plavix) is a selective and irreversible inhibitor of adenosine diphosphate (ADP)- induced platelet aggregation [27]. Diltiazem could potentially block thrombosis by blocking calcium channel [28]. In China, Traditional Chinese Medicines (TCMs) have been proved effective in preventing and treating thrombotic diseases in a very long history [29]. Xueshuantong is a drug prepared from Panax notoginseng extract that has been used for the treatment of thrombotic diseases in China for more than three decades, and proved highly effective [29,30]. Salvianolate, an injectable drug extracted and prepared from a Chinese herb Salvia miltiorrhiza, is commonly used to treat cardiovascular disease in eastern countries, particularly in China, and has been proven could inhibit platelet aggregation and adhesion and reduce thrombus formation[31]. Astragalus as a herb has been used for thousands of years [32], numerous studies have shown that astragalus has obvious effects of inhibiting thrombus formation in vivo and resolving blood clot in vitro [33].
In this study, we have developed and validated a novel zebrafish thrombotic model that is convenient and predictive for rapid in vivo screening and efficacy assessment of antithrombotic drugs. Our results from zebrafish thrombosis model in combination with literature support TCM as a treasure resource of developing new therapy of thrombotic disease.
MATERIALS AND METHODS
Zebrafish care and maintenance
Adult Albino strain zebrafish were housed in a light- and temperature-controlled aquaculture facility with a standard light: dark cycle of 14:10 h/day and fed with live artemia larvae twice daily and dry flake as supplement once a day. Zebrafish embryos were generated by natural pair-wise mating, on average, 200-300 embryos were generated. Both adults and embryos were maintained at 28°C in fish water (0.2% Instant Ocean Salt in deionized water, pH 6.9-7.2, conductivity 480-510 mS cm-1 and hardness 53.7-71.6 mg l-1 CaCO3 ). For experiments, the embryos were collected, washed and and staged at 6 and 24 hpf (hours post fertilization) [34]. The zebrafish facility at Hunter Biotechnology, Inc. is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAA LAC) International and the Science and Technology Department of Zhejiang Province, China (SYXK20120171).
Chemicals and tested drugs
AA (lot #: F1418031) purchased from Aladdin (Shanghai, China) was used to induce thrombosis in live zebrafish. Six human antithrombotic drugs aspirin, clopidogrel, Diltiazem hydrochloride injection, Xueshuantong injection, Salvianolate injection, Astragalus injection were selected as positive controls. Aspirin (lot #:059K0199) was purchased from Aladdin (Shanghai, China), clopidogrel (lot #:R4411) were purchased from SigmaAldrich (St.Louis, USA).Diltiazem hydrochloride injection (lot #: V025) was from Mitsubishi Tanabe Pharma Corporation (Tianjin, China), Xueshuantong injection (lot #: 14015103) was from Wuzhou Pharmaceutical Corporation (Guangxi, China), Salvianolate injection (lot #: 1405131) was from Shanghai Green Valley Pharmaceutical (Shanghai, China), Astragalus injection (lot #: 1405213) was from Chiatai Qing chunbao (Shanghai, China). Stock solutions were prepared in either 100% dimethyl sulphoxide (DMSO; lot #: BCBN0845V, Sigma) or 0.9% sodium chloride, and serial dilutions were made before each experiment. O-dianisidine (ODA) was purchased from Sigma-Aldrich (lot#: MKBG4648V,St. Louis, MO, USA).
Zebrafish thrombosis model development
A few number of precursors of zebrafish thrombocyte can be detected at 35 hpf around [15-17,35,36] and mature thrombocytes are abundant in the circulation by 72 hpf [2]. Consequently, 3 dpf zebrafish were chosen as an appropriate stage to start AA treatment for the thrombosis model development. Thirty 3 dpf zebrafish were distributed into 6-well plates (Nest Biotech., Shanghai, China) in 3 mL fresh fish water [10, 37]. To optimize the AA treatment concentration and treatment time period, zebrafish were treated with 20, 30, 40, 60, 80 and 100 μM of AA for 1, 1.5 and 2 h, respectively, to induce thrombosis. Zebrafish treated with 1% DMSO served as a vehicle control. Untreated zebrafish were used to confirm vehicle solvent did not have adverse effect on zebrafish. After treatment, zebrafish were stained with ODA, and then 10 zebrafish from each group were randomly selected for visually qualitative assessment of the thrombus formation in the caudal vein under a dissecting stereomicroscope, and also for the quantitative analysis of thrombosis. Based on the qualitative and quantitative result, the optimal AA treatment concentration and treatment time period for inducing thrombosis were selected.
O-dianisidine (ODA) staining and quantification
For quantitative analysis of thrombosis, zebrafish were stained with ODA as described before [38]. Briefly, zebrafish were treated with 0.6 mg/mL ODA in the dark for 15 min and then washed several times. After ODA staining, red blood cells (RBCs) are easily visualized in the heart, caudal vein and artery under a dissecting stereomicroscope. Our patented zebrafish thrombosis assay technology suggested that the heart RBC amount is reversely correlated with the thrombus severity and, thus, thrombosis in zebrafish could be quantified by measuring heart RBCs (China patent number: 201110126427.2). The heart RBCs images were taken following the method as reported before [12]. Quantitative image analysis of RBCs was performed by measuring heart RBC intensity (S) using NIS-Elements D3.10 image analysis software (Nikon) and the data are expressed as mean ± SEM. The effect of a test drug was calculated based on the formula below:
Efficacy (%) = [S(drug) – S(model)]/[S(vehicle) - S(model)] × 100%
A positive percentage means that a tested drug could prevent thrombus and a negative percentage suggests that the tested drug had no effect on thrombosis in the zebrafish model.
Determination of no observed adverse effect level (NOAEL) of drugs
To determine no observed adverse effect level (NOAEL) of a testing drug, 3 dpf zebrafish were treated with the drug for 4.5 h, mortality and toxicity was record at the end of treatment. In the initial tests, five concentrations (0.1,1,10,100 and 500 μg/mL for soaking drugs) or doses (0.1,1,10,100 and 500 ng/zebrafish for injected drugs) were used for each drug. If an NOAEL could not be found from the initial tests, additional concentrations or doses within the range of 0.01-2000 mg/L or 0.01-2000 ng/zebrafish were tested. The NOAEL of a test drug was defined as a maximum concentration or maximum dose that induced neither death nor any observable adverse effect on zebrafish and was determined under a dissecting stereomicroscope. Dead zebrafish was defined as the absence of heartbeat under a dissecting stereomicroscope.
Assessment of drugs effect on zebrafish thrombosis
To assess drug effect on thrombosis in live zebrafish, six known human antithrombotic drugs (aspirin, clopidogrel, Diltiazem hydrochloride injection, Xueshuantong injection, Salvianolate injection, Astragalus injection) were selected for the validation of zebrafish thrombosis model. Thirty 3 dpf Albino strain zebrafish were distributed into 6-well plates in 3 mL fresh fish water. Zebrafish were first treated with drugs for 3h at 3 concentrations or dosages (1/10 NOAEL, 1/3 NOAEL, NOAEL) as indicated in Table 1, followed by treatment with 80 μM AA (the optimum AA concentration) for 1.5h (the optimum AA treatment time period). Zebrafish only treated with AA served as the thrombosis model.
Aspirin and clopidogrel were directly delivered by soaking. The four injectable drugs (Diltiazem hydrochloride injection, Xueshuantong injection, Salvianolate injection, Astragalus injection) were delivered into zebrafish by circulation microinjection as we reported previously [8], 10 nL of drug solution was delivered to the circulation of each zebrafish. Zebrafish treated with 1% DMSO or injected with 10 nl 0.9% sodium chloride served as vehicle controls, and untreated zebrafish were used to confirm that the vehicle solvent did not have an adverse effect on the zebrafish. After treatment, zebrafish thrombus was quantified using a method as described above.
Statistical analysis
All experiments results repeated at least three times and all data were presented as mean ± SEM. One-way ANOVA followed by the Dunnett’s test was used to compare differences among groups, especially drug-treated and vehicle-treated zebrafish groups. All statistical analyses were performed using the SPSS 16.0 software (SPSS, USA), and p<0.05 was considered statistically significant.
RESULTS AND DISCUSSION
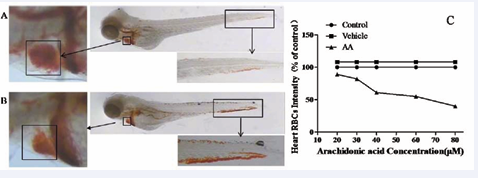
To develop a zebrafish thrombosis model for drug screening and efficacy assessment, the optimal concentration and treatment time period of the thrombosis inducer AA were first determined. Zebrafish at 3 dpf were treated with 6 various concentrations of AA (20 μM, 30 μM, 40 μM, 60 μM, 80 μM and 100 μM) for 1,1.5 and 2 h, the thrombosis was first visually confirmed under a dissecting stereomicroscope, and then quantified by image analysis of the heart RBCs, stained by ODA, as measured by hemoglobin levels.
We found that treatment with 20, 30, 40 and 60 μM AA for 1 h did not induce thrombosis in zebrafish at all, 80 μM AA induced moderate thrombosis, 100μM caused severe thrombosis but also various degrees of cardiovascular toxicity, including pericardial edema, arrhythmia (bradycardia and atrial fibrillation), or no blood circulation. When the treatment prolonged to 1.5 h, 40, 60 and 80 μM AA all induced thrombosis and remarkable decrease of RBCs intensity, the heart RBCs intensity is reversely correlated with the thrombus severity in the caudal vein (Figure 1) as we indicated in another phenylhydrazine induced zebrafish thrombosis model[12]. Meanwhile, 100μM AA caused death.
Figure 1: Zebrafish heart red blood cells (RBCs) amount is reversely correlated with the thrombus length/severity. The RBCs, stained with O-dianisidine, was apparently decreased of 3dpf zebrafish had thrombosis in caudal vein induced by 80 μM AA for 1.5 h (Figure 1B), as compared with control (Figure 1A). The RBCs intensity of 3 dpf zebrafish was reduced as treated with 5 various concentrations of AA for 1.5 h (Figure 1C).
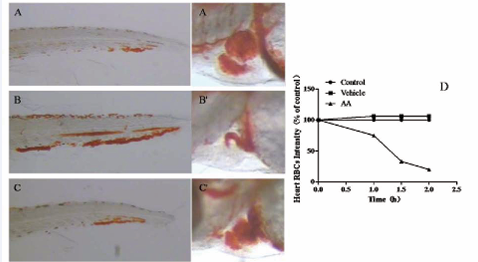
Time-dependent thrombus formation was demonstrated in zebrafish treated with 80 μM AA, of which treatment for 1.5 h induced thrombosis in the caudal vein in 100% zebrafish without observable toxicity, and the thrombus formation was markedly reduced when the zebrafish were co-treated with a well-known antithrombotic drug aspirin (Figure 2A, B, C).
Figure 2: Determination of AA treatment concentrations and treatment time periods for inducing zebrafish thrombosis. 3 dpf Zebrafish treated with 80 μM AA for 1.5 h visually showed severe thrombosis in the caudal vein (Figure 2B) and heart RBCs dramatically reduced (Figure 2B’) as compared with untreated (Figure 2A, 2A’). Thrombus formation was markedly reduced in the caudal vein (Figure 2C) and heart RBCs was obviously increased (Figure 2C’) when the zebrafish were co-treated with a well-known antithrombotic drug aspirin. Time-dependant reduction of heart RBCs intensity was also found in zebrafish treated with 80 μM AA (Figure 2D).
In quantitative analysis of thrombosis, time-dependent heart RBC intensity reduction (Figure 2D) was observed in zebrafish treated with 80 μM AA, further supporting our patented technology that indicates the heart RBCs intensity is reversely correlated with the thrombus severity and measuring heart RBCs could quantify thrombosis in zebrafish (China patent number: 201110126427.2). Based on these results, zebrafish treated with 80 μM AA for 1.5 h was selected as the optimum treatment concentration and treatment period for subsequent experiments.
To determine whether the zebrafish thrombosis model response to antithrombotic drugs was similar to the response of mammalian model systems, zebrafish was pre-treated with six known drugs (aspirin, clopidogrel, diltiazem hydrochloride injection, xueshuantong injection, salvianolate injection, astragalus injection) currently used in clinical practice individually for 3 h at first, and then co-treated with 80 μM AA for 1.5h. Three concentrations or dosages at 1/10 NOAEL, 1/3 NOAEL and NOAEL were assessed for each drug (Table 1).
|
Table 1: Drug concentrations or dosages used for assessing anti-thrombosis in zebrafish. |
|||
|
Drugs |
Drug concentrations through soaking (μg mL-1) |
||
|
1/9 NOAEL |
1/3 NOAEL |
NOAEL |
|
|
aspirin |
3.3 |
10 |
30 |
|
clopidogrel |
0.8 |
2.5 |
7.5 |
|
- |
Drug dosages through microinjection (ng/zebrafish) |
||
|
1/9 NOAEL |
1/3 NOAEL |
NOAEL |
|
|
diltiazem hydrochloride injection |
2.5 |
7.4 |
22.2 |
|
xueshuantong injection |
16.7 |
50 |
150 |
|
salvianolate injection |
9.3 |
28 |
83 |
|
astragalus injection |
0.4 |
1.1 |
3.3 |
|
NOAEL: no observed adverse effect level |
|||
NOAEL was 30 μg mL-1 for aspirin, 7.5 μg mL-1 for clopidogrel, 22.2 ng for diltiazem hydrochloride injection, 150 ng for xueshuantong injection, 83 ng for salvianolate injection and 3.3 ng for astragalus injection, respectively.
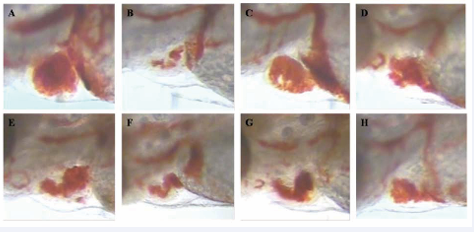
As expected, after a 3h pre-treatment, human anti-thrombotic drugs aspirin, clopidogrel, diltiazem hydrochloride injection, xueshuantong injection, salvianolate injection, astragalus injection significantly increased the heart RBCs/hemoglobin staining intensity (Figure 3),
Figure 3: Increased heart RBCs in thrombotic zebrafish pre-treated with antithrombotic drugs for 3 h. A: Vehicle-treated zebrafish; B: zebrafish treated with AA alone; C-H: thrombotic zebrafish pre-treated with human antithrombotic drugs aspirin, clopidogrel, diltiazem hydrochloride injection, xueshuantong injection, salvianolate injection and astragalus injection significantly increased heart RBCs , indicating the reduced thrombosis after drug treatment.
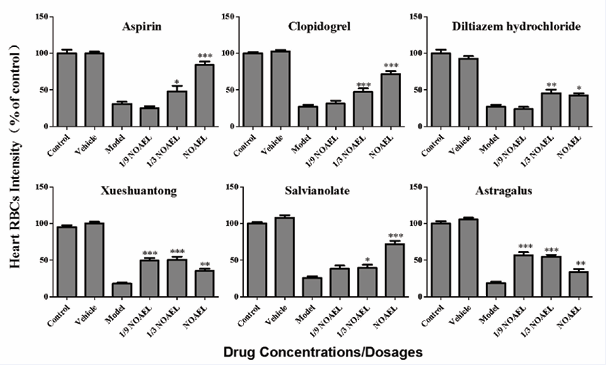
implying the reduced thrombosis in zebrafish. The efficacy of thrombosis was -8%-71% for aspirin, 5%-59% for clopidogrel, -5%-23% for diltiazem hydrochloride injection, 38%-21% for xueshuantong injection, 15%-56% for salvianolate injection, and 44%-17% for astragalus injection, respectively. Statistically significant effects on the zebrafish thrombosis were observed at 1/3NOAEL (p<0.05)and NOAEL (p<0.001) for aspirin; 1/3NOAEL (p<0.001) and NOAEL (p<0.001) for clopidogrel; 1/3 NOAEL (p<0.01) and NOAEL (p<0.05) for Diltiazem hydrochloride injection; 1/9 NOAEL(p<0.001), 1/3 NOAEL (p<0.001) and NOAEL (p<0.01) for Xueshuantong injection; 1/3 NOAEL (p < 0.05) and NOAEL (p < 0.001) for Salvianolate injection; 1/9 NOAEL(p < 0.001), 1/3 NOAEL (p < 0.001) and NOAEL (p < 0.01) for Astragalus injection (Table 2, Figure 4).
|
Table 2: Efficacy of anti-thrombotic drugs on zebrafish thrombosis model. |
|||
|
Efficacy (%) # |
|||
|
1/9 NOAEL |
1/3 NOAEL |
NOAEL |
|
|
-8 |
47** |
71*** |
|
|
5 |
27** |
59*** |
|
|
diltiazem hydrochloride injection |
-5 |
28*** |
23*** |
|
xueshuantong injection |
38*** |
40*** |
21** |
|
salvianolate injection |
15 |
17* |
56*** |
|
44*** |
41*** |
17** |
|
|
# compared with vehicle control: *p < 0.05, **p < 0.01, ***p < 0.001. |
|||
Figure 4: Quantitative analysis of antithrombosis efficacy in thrombotic zebrafish after drug pre-treatment for 3 h. Drug effect was measured based on quantitative image analysis of the heart RBCs intensity in zebrafish. Compared with vehicle control: *p < 0.05, **p < 0.01, ***p < 0.001.
The reduced thrombus formations in the caudal vein of the zebrafish pre-treated with anti-thrombotic drugs were also confirmed by visual observations (data not shown).
Platelets play a major role in the blood coagulation system. The activity of platelets dependents on the degree of their activation, both in physiological processes and pathological conditions, such as hemostasis and thrombosis. And there have been many anti-platelet drugs in the market and proven to be effective [14]. AA is a crucial actor in the activation of platelet, it would be metabolized into pro-inflammatory prostaglandins and pro-thrombotic TXA 2 by cyclooxygenase (COX), a pathway that will be triggered by oxidative stress [39]. And, it has been demonstrated that the addition of AA addition can radically cause TXA 2 generation and platelet aggregation [40]. Therefore, an AA induced in vivo thrombosis model would be very useful for anti-thrombosis drugs screening. However, it’s difficult to develop an AA induced mammalian thrombosis, since AA induced inflammatory and thrombotic response is so acute that it’s hard to keep the balance between thrombosis and fatality.
In the present study, we aimed to develop an in vivo thrombosis model induced by AA, and evaluate whether anti-thrombotic drugs treatment has a protective effect on this model. Zebrafish is an excellent model for hemostasis and thrombosis research [4], since it possesses coagulation factors, thrombocyte receptors similar to mammalian, has a thrombocyte which morphological and functional is similar to human platelets, and responds to anti-coagulant and anti-platelet drugs commonly used in clinical treatment [12]. Besides, zebrafish is transparent in its early development stage, this characteristics makes it’s realizable to provide real-time information on thrombus formation with high optical and temporal resolution, hence without needing the surgical expertise and licensing requirements of the murine model [13]. AA used as a platelet agonists in platelet function testing at clinical to evaluate of patients with bleeding problems and in vitro thrombocyte aggregation [23]. ODA has been employed for identification of the red blood cells in developing zebrafish [38]. For quantitative analysis of thrombosis, zebrafish were stained with ODA to quantify the heart red blood cells (RBCs) (hemoglobin level) of zebrafish. Our previously patented zebrafish thrombosis assay technology suggested that the heart RBCs amount is reversely correlated with the thrombus length/severity and thus measuring heart RBCs could quantify thrombosis in zebrafish (Li & Zhu, China patent number: 201110126427.2). In this study, we have optimized AA treatment concentration and treatment time period to develop a live zebrafish thrombosis model. Zebrafish at 3 dpf were treated with AA at a concentration of 80 μM for a time period of 1.5 h were determined as the optimum conditions for the zebrafish thrombosis model development. The thrombosis process in the live zebrafish was visually tracked and confirmed under a dissecting stereomicroscope. The thrombus size or severity in the caudal vein of zebrafish was consistent with the quantitative image assay of the heart RBCs. This zebrafish thrombosis model is stable and highly reproducible with CV values of intra-experiments, inter-experiments and daily-to-daily variations ≤ 25% (data not shown).
In order to validate zebrafish thrombosis model for pharmaceutical testing, 6 marked human antithrombotic drugs aspirin (thromboxane A2 inhibitor) [26], clopidogrel (adenosine diphosphate [ADP] inhibitor) [27], diltiazem hydrochloride injection (calcium channel blocker) [28], xueshuantong injection (inhibiting platelet aggregation, blood coagulation, fibrinolysis activation, and reducing plasma viscosity) [30], salvianolate injection (antiplatelet aggregation, anticoagulant, and antithrombosis) [32], and astragalus injection (restraining thrombosis and platelet aggregation) [33] were chose and tested in this model. All 6 human anti-thrombotic drugs significantly reduced zebrafish thrombosis after 1.5 h treatment, indicating that the zebrafish thrombosis model developed in this report is suitable for in vivo screening and assessment of oral and injectable anti-thrombotic drugs no matter single compound medicines or herb extracts. Many traditional Chinese Medicines (TCMs) have been proven effective in preventing and treating thrombotic diseases in a very long historical period. Our results further support that drugs derived from TCMs are valuable in the prevention and treatment of thrombotic diseases.
The zebrafish as a model organism offer opportunities for rapid in vivo drug discovery and development. Larval zebrafish thrombosis model developed in this study was a live and physiology-associated whole animal assay. This model is a useful tool for whole animal thrombosis studies and for screening preventive and therapeutic agents of thrombus. The use of zebrafish as an alternative animal model for anti-thrombotic drug screening and assessment could save time, decrease costs, and reduce drug failure at later stages of drug development. Extended studies are needed to further validate this model using more human drugs, and to develop this model into a higher throughput screening or even an automatic assay system. We believe that the power of the zebrafish models and assays could be better utilized to advance the basic and translational research and drug discovery and development including in the field of hemostasis and thrombosis.
CONCLUSION
This study developed and validated a zebrafish thrombosis model that could be used for in vivo screening and efficacy assessment of anti-thrombotic drugs. This conventional zebrafish thrombosis model is predictive, easily available, less expensive with a short testing time and could speed up antithrombotic drug research and development.
ACKNOWLEDGEMENTS
Chun-Qi Li and Ye Zuguang designed the research; Yong Zhang, Sheng-Ya Guo, Juan Zhou and Wen-Han Liao performed the research; Yong Zhang and Sheng-Ya Guoanalyzed the data; Yong Zhang and Sheng-Ya Guowrote the paper; Xiao-Yu Zhu assisted the figure and Gao-Li Zheng helped in data analysis. This work was sponsored in part by the National Science & Technology Major Projects of China (No. 2017ZX09301-059) and the Sciences Technology Department of Zhejiang Province (No. 2017F0001).