In vitro Selection and Hormonal Regulation in Cell Culture of Artemisia annua L. Plant
- 1. Green Technology Department, Ipca Laboratories Ltd., India
- 2. Department of Botany, Faculty of Science, University of Baroda, India
- 3. Division of Gastroenterology and Liver Disease, Case Western Reserve University, USA
Abstract
An efficient in vitro method for multiple shoot bud induction and regeneration has been developed in Artemisia annua L. plant using leaf and stem explants in various concentrations and combinations of plant growth regulators to evaluate the frequency of regeneration. Callus cultures were induced from leaf and stem explants of A. annua L., at different auxin and cytokinin concentrations. Moderate concentrations of plant growth regulators either in combination or in single in Murashige and Skoog (MS) medium produced friable, light green and non-embryogenic callus from both explants. These totipotent cells gave rise to shoots when transferred to same or different plant growth regulator containing medium as second subculture. Direct shoot induction was raised from leaf and stem explants. High percentages of direct regeneration were obtained from leaf and stem explants on medium supplemented of BAP/NAA (1.5/0.05mg/L). Complete rooting was achieved on full and half strength basal MS medium supplemented with different auxin concentrations. Synergetic effect of plant growth regulator plays an important role in callus induction and cell differentiation. This system exhibited a potential for a rapid propagation of shoots from the leaf and stem explants and makes it possible to develop a clonal selection and propagation of high artemisinin yielding A. annua L. plants.
Keywords
Artemisia annua; Plant growth regulators; Artemisinin; Clonal selection.
Citation
Dangash A, Ram M, Niranjan R, Bharillya A, Misra H, et al. (2015) In vitro Selection and Hormonal Regulation in Cell Culture of Artemisia annua L. Plant. JSM Cell Dev Biol 3(1): 1013.
ABBREVATIONS
2,4-D: 2,4-Dichloro Phenoxy Acetic Acid; NAA: Nephthalene Acetic Acid; IBA: Indol Butyric Acid; IAA: Indol Acetic Acid; BAP: Benzyl Amino Purine; Kin: Kinetin
INTRODUCTION
Artemisia annua, also known as Sweet Wormwood, Sweet Annie, Sweet Sagewort or Annual Wormwood, belonging to the family Asteraceae, is a common type of wormwood that grows throughout the world. It is a crop for the production of antimalarial and possibly antibacterial agents and natural pesticides. It was originally collected by the Chinese as an herbal medicine and is currently processed by pharmaceutical firms for the production of artemisinin for artemisinin-based combination therapies (ACTs) in the treatment of malaria. ACTs have been shown to have rapid resolution to fever and parasitaemia, low toxicity and are well tolerated. Artemisinin, a sesquiterpenelactone isolated from the aerial parts of Artemisia annua L. plants. Artemisinin has proved to be a dramatically effective anti-malarial against multi-drug resistant Plasmodium spp [1,2]. Besides being currently the best therapeutic agent against both drugresistant and cerebral malaria causing strains of Plasmodium sp., [3]. It is also effective against other infectious diseases such as Schistosomiasis, hepatitis B and Lishmmaniasis [4-7]. More recently, it has also been shown to be effective against a variety of cancer cell lines including breast cancer, human leukemia, colon cancer and small cell-lung carcinomas [8,9]. Due to its current use in artemisinin based-combination therapy (ACT), its global demand continuously is increasing. The relatively low yield of artemisinin in A. annua L. plant leaves (0.01-0.8%) however, a serious limitation to the commercialization of the drug [10- 12]. However, cross-pollination in A. annua L. plants is serious limitation to maintain the genetic fidelity and higher artemisinin yield throughout the population.
Micropropagation and organogenesis of different Artemisia species have been previously established by using several parts of plants, in order to obtain large number of plants, such as A. mutellina [13]; A. scorpia [14]; A. vulgaris [15]; A. absinthium [16]. An efficient in vitro method for multiple bud induction and regeneration has been developed in Artemisia annua, using leaf, stem, shoot tip, explants or by using young inflorescence segments [17-21].
In vitro direct organogenesis of different parts of Artemisia annua was investigated, in this research, to obtain a large number of plants true to type. The ultimate goal was the multiplication of the selected clones with high levels of secondary metabolites and the utilization of the protocol in any future genetic transformation of this species.
Tissue culture uses standard protocols with shoot tips of mature field grown plants [22]. Shoot-tips and lateral buds of A. annua L. produced numerous shoots on MS medium and formed 100% roots on half strength Murashige and Skoog minimal organic medium. The medicinal use of A. annua in the tropics should be emphasized. There is therefore an urgent need for the conservation and rapid propagation of the seedlings using tissue culture techniques. This experiment was carried out to study growth response of A. annua L. plant shoot tip and stem explants in vitro, under different combinations of plant growth regulators, to develop a protocol for production of clean healthy plantlets for subsequent multiplication. In vitro selection has been proved very effective method for the selection of high artemisinin yielding genotypes of A. annua L. plants. Thus we have been able to maintain genetic fidelity and production of quality seed of A. annua L. plants
MATERIALS AND METHODS
Plant Materials and Chemicals
This experiment was carried out at Green Technology Department, Ipca Laboratory, Ratlam. Seeds of A. annua L. (CIM AROGYA) were purchased from CIMAP, Lucknow. These seeds were used for the preparation of nursery and 45 days old seedlings of A. annua L. plants were transplanted in the experimental field of Ipca Laboratories Ltd. The leaf and stem explants were selected from high artemisinin yielding A. annua L. plant grown in experimental field of Ipca Laboratory Ltd., Ratlam. Explants were washed under running tap water for 30 min and then surface sterilized with 5% teepol for 5-7 min. Further sterilization of explants was done in Laminar Air Flow; 1.0% bavistin (w/v) and 0.25% streptomycin (w/v) was used for 5 min. 5% solution of sodium hypochlorite (v/v) used for 4 min followed by disinfection with 70% ethyl alcohol for 30 seconds. Explants were then rinsed five times in sterile distilled water before inoculation on media. All chemicals and hormones were procured from Himedia Laboratories (India) and SigmaAldritch (USA). Sucrose and agar were procured from Himedia Laboratories, India. All the buffers and solutions were prepared by using autoclaved MilliQ water.
Media and Culture Conditions
Basal medium used was full strength [23]. The medium containing 3% (w/v) sucrose, B5 vitamins, 0.1 g Inositol, was augmented with different cytokinins and an auxin. For callogenic study, four auxins; 2, 4-D, IAA, NAA and IBA with different concentrations (0.25-1.5mg/L) in combination with two cytokinins; Kinetin and BAP (0.25-1.5mg/L) were used in basal MS. Different concentrations of BAP and Kin (0.25-2.0mg/L) in combination with amino acids (Glutamine-100mg/L; Cystine. HCl-5mg/L; Arginine-50mg/L; Asparagine-40mg/L) and two constant concentrations of NAA (0.05mg/L and 0.1mg/L) were tested for the shoot induction from callus and leaf. While different concentrations of IAA IBA and NAA (0.1-2.0mg/L) were used for root induction. Hormone free MS medium served as control. The pH of the medium was adjusted to 5.8 prior to the addition of 0.8%w/v agar. 20 ml aliquots were dispensed into jam bottles and autoclaved at 121°C at 15 lb pressure for 15 min. Surface sterilized explants were aseptically inoculated in jam bottles and the cultures were maintained at 25 ± 2°C using 16/8 light/dark period, under a light intensity of 3000 lux provided by cool-white fluorescent lamps and 50 to 55% relative humidity.
Statistical analysis
The experiments were entirely randomized with six replicates for each growth regulator(s) concentration(s). Statistical analyses were carried out by the ANOVA and Dunkens Multiple Test, at a 0.5% probability level.
RESULT AND DISCUSSION
Micro propagation is an advanced technique for producing a large number of genetically uniform and pathogen free plants in limited time and space [24]. In vitro clonal propagation of species through tissue culture has been frequently based on the successful adjustment of the type and combinations of plant growth hormones [25,26] .
Callogenic response
The callogenic response form leaf and stem explants was observed at different plant growth regulators concentration either singly or in combination (Table 1).
Table 1: Callogenic response from leaf and stem explant at different growth regulatorsZ.
|
Growth Regulator |
Concentration (mg/l) |
Leaves Explant |
Stem Explant |
||
|
Callus formation (%)X |
Response |
Callus formation (%)X |
Response |
||
|
2, 4-D |
0.25 0.5 0.75 1.0 1.25 1.5 |
62.5 76.0 100.0 100.0 58.0 52.0 |
++ +++ ++++ +++ ++ + |
59.5 76.0 100.0 86.0 42.5 32.5 |
++ +++ ++++ +++ ++ + |
|
IAA |
0.25 0.5 0.75 1.0 1.25 1.5 |
65.0 85.0 100.0 100.0 53.0 44.0 |
++ +++ ++++ ++++ ++ + |
82.5 92.0 100.0 100.0 57.0 41.0 |
++ +++ ++++ +++ ++ + |
|
NAA |
0.25 0.5 0.75 1.0 1.25 1.5 |
76.0 100.0 100.0 100.0 100.0 69.0 |
++ +++ ++++ ++++ +++ + |
71.0 76.5 100.0 100.0 54.0 38.5 |
++ +++ ++++ ++++ +++ + |
|
IBA |
0.25 0.5 0.75 1.0 1.25 1.5 |
77.0 88.0 100.0 100.0 68.0 45.0 |
++ +++ ++++ +++ ++ + |
68.0 79.0 100.0 100.0 53.5 51.0 |
++ +++ ++++ +++ ++ + |
|
BAP |
0.25 0.5 0.75 1.0 1.25 1.5 |
62.0 78.0 85.5 53.5 - - |
+ +++ ++++ +++ + - |
55.0 72.0 80.0 36.5 - - |
+ ++ +++ +++ + - |
|
Kin |
0.25 0.5 0.75 1.0 1.25 1.5 |
51.0 64.0 74.0 22.0 - - |
++ +++ ++++ ++ + - |
47.0 61.0 72.5 14.0 - - |
++ +++ ++++ ++ - - |
|
BAP/IBA |
0.5/0.5 0.5/1.0 0.5/1.5 |
81.5 100.0 100.0 |
++ ++++ +++ |
77.0 100.0 100.0 |
++ ++++ +++ |
|
BAP/NAA |
0.5/0.5 0.5/1.0 0.5/1.5 |
100.0 100.0 100.0 |
++++ ++++ ++++ |
100.0 100.0 100.0 |
++++ ++++ ++++ |
|
Kin/NAA |
0.5/0.5 0.5/1.0 0.5/1.5 |
100.0 100.0 100.0 |
+++ ++++ ++++ |
100.0 100.0 100.0 |
+++ ++++ ++++ |
|
BAP/2, 4-D |
0.5/0.5 0.5/1.0 0.5/1.5 |
87.0 100.0 100.0 |
+++ ++++ +++ |
82.0 100.0 100.0 |
+++ ++++ +++ |
|
Kin/IBA |
0.5/0.5 0.5/1.0 0.5/1.5 |
84.0 100.0 100.0 |
+++ ++++ +++ |
78.0 100.0 100.0 |
+++ ++++ +++ |
|
BAP/IBA/Kin |
0.5/0.5/1.0 0.5/1.0/1.0 0.5/1.5/1.0 |
100 100 100 |
++++ ++++ +++ |
100 100 100 |
++++ ++++ +++ |
|
BAP/NAA/Kin |
0.5/0.5/1.0 0.5/1.0/1.0 0.5/1.5/1.0 |
100 100 100 |
+++ ++++ ++++ |
100 100 100 |
+++ ++++ +++ |
|
BAP/2, 4-D/Kin |
0.5/0.5/1.0 0.5/1.0/1.0 0.5/1.5/1.0 |
100 100 100 |
+++ +++ ++++ |
100 100 100 |
+++ ++++ +++ |
|
Control |
|
64.0 |
++ |
72.0 |
+++ |
X %age response of 6 replicates.
Z Rated after 30 days of culture: + = Low, ++ = good, +++ = very good, ++++ = excellent, - = nil.
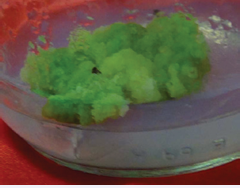
Callogenic response from leaf and stem explants started at the margins or from injuries. Plant growth regulator (PGR)-free basal MS medium also induced callogenic response from both explants where leave explants showed 90% callogenic response while 73% stem explants produced calli. Nin et al. [27] reported no callogenic response from leaf explant on PGR-free medium and explants died after few days. 2, 4-D as callus inducing hormone produced light green, soft, friable and compact callus from leaf and stem explants. But at all concentration of 2, 4-D organogenic response was not observed within observation time. Nin et al. [27] stated that low concentration of 2, 4-D stimulated adventitious root development from 86% of all explants of A. annua L. At all concentrations of BAP and Kin, the callogenic response was poor. Very low callus was developed which was green, soft and compact. Small and few numbers of leaves also emerged, when the callus remained on the same medium for six weeks or the callus turned to hard and embryogenic. Callus produced at different concentrations of IAA and IBA was yellowish, soft and friable and callogenic response was 100% at lower concentration of both hormones. Nin et al. [27] reported that callogenesis occurred in 100% of explants, independent of the cytokinins/auxin ratio. But at different concentrations of NAA, light green, soft and friable callus was observed. At low concentrations of NAA, small shoots emerged while at higher concentrations callus turned hard and compact. The result shows that media supplemented with BAP either with Kin, NAA, 2, 4-D or IBA produced 100% callogenic response from both explants. 0.5 mg/l BAP and 0.5-1.5 mg/l NAA in combination produced green, soft and friable callus from both explants (Figure 1 & 2).
Figure 1: Callus induction from leaf explant of Artemisia annua L. plant on MS medium (BAP 0.5mg/l + NAA 1.5mg/l).
Figure 2: Callus induction from stem explant of Artemisia annua L. plant on MS medium (BAP 0.5mg/l + NAA 1.5mg/l).
Ganesan and Paulsamy [19] observed 98.66% callogenic response from leaf discs with NAA at 0.9mg/l in A. annua L., while Nin et al. [27] reported best callogenic response with BAP and NAA in the medium for A. absinthium whereas Benjamin et al. [28] observed callus induction from shoot buds using BAP plus IAA for Artemisia pallens. 2, 4-D at varying concentration (0.05 - 0.25 mg/l) in combination with BAP (0.5 mg/l) also produced light green and soft callus when supplemented in MS medium. When IBA and NAA were combined with Kin, the callogenic response was also low and callus was not good in texture. Xu and Jia [29] observed best callus result in the presence of 2, 4-D with Kin for Artemisia sphaerocephala.
Shoot Induction and Regeneration
Shoots were induced from callus and leaf explants by supplementing various combinations of BAP, Kin and NAA. All combinations of these plant growth regulators in callus and leaf explant culture could not induce shoot induction (Table 2 & 3). The period of shoot induction was 4 weeks and it varies in different species of Artemisia [13-16,30].
Shoot induction from callus was observed at different concentration of BAP and Kin, alone and in combination with NAA and amino acids (Glutamine-100mg/L; Cystine.HCl-5mg/L; Arginine-50mg/L; Asparagine-40mg/L) (Table 2).
Table 2: Effect of growth regulators on in vitro shoot induction of Artemisia annua from callus on MS mediumZ.
|
Growth Regulator |
Conc. (mg/l) |
Response (%)Y |
Average No. of of shootsXT |
General description |
|
BAP |
0.25 0.5 0.75 1.0 1.5 2.0 |
00.0 28.0 46.0 69.2 79.0 15.0 |
- - 0.83 ± 0.234d 1.66 ± 0.412cd 2.16 ± 0.614ab - |
No response No response 1-2 shoots with small green leaves 1-3 shoots with small green leaves 2-3 shoots with small green leaves No response but embryogenic callus |
|
Kin |
0.25 0.5 0.75 1.0 1.5 2.0 |
00.0 21.0 36.0 56.2 41.0 00.0 |
- - 0.83 ± 0.352d 1.5 ± 0.335cd - - |
No response but embryogenic callus No response but embryogenic callus 1-2 shoots with small green leaves 1-3 shoots with small green leaves No response but embryogenic callus No response but embryogenic callus |
|
NAA |
0.1 0.5 |
00.0 00.0 |
- - |
No response but embryogenic callus No response but embryogenic callus |
|
BAP/NAA |
1.5/0.05 1.5/0.1 |
83.6 26.0 |
2.83 ± 0.234a 2.16 ± 0.271bbc |
3-4 shoots with green leaves 1-2 shoots with green leaves |
|
Kin/NAA |
1.5/0.05 1.5/0.1 |
65.0 16.5 |
2.33 ± 0.724cd 1.16 ± 0.121d |
2-3 shoots with green leaves 1-2 shoots with green leaves |
|
LSD |
|
|
1.366 |
|
XMean ± standard error
Interval of confidence 95%.
YData are mean of 6 replicates.
TMean separation by LSD.
ZRated after 30 days of culture.
Values with the different letters on the same column are significantly different.
At 1.5 mg/l BAP, 2.16 ± 0.614 shoots emerged while at 0.5 mg/l no shoot induction was observed. Nin et al. and Zia et al. [16,27] also did not observe any shoot induction at low concentrations of BAP in A. absinthium. However Le (2001) reported that new axillary shoots development was promoted in Artemisia annua by addition of BAP in MS medium. The best shoot induction 83.6% (2.83 ± 0.234) was observed on BAP (1.5 mg/l) in combination with NAA (0.05 mg/l) (Figure 3).
Figure 3: Shoot induction from callus of Artemisia annua L. plant on MS medium (BAP 1.5mg/l + NAA 0.05mg/l + Glutamine-100mg/L; Cystine.HCl-5mg/L; Arginine-50mg/L; Asparagine-40mg/L).
At different concentrations of Kin alone or in combination with NAA response of shoot induction was observed low. At 1.0 mg/l Kin, shoot induction was 56.2% (1.5 ± 0.335) and at higher concentrations response was absent while the callus turned hard, compact and embryogenic. In the present study, direct shoot induction from leaf explant was also carried out. The best shoot induction 85.7% (3.16 ± 0.434) was observed on BAP (1.5mg/l) in combination with NAA (0.05mg/l) (Figure 4).
Figure 4: Direct shoot induction from leaf explant of Artemisia annua L. plant on MS medium (BAP 1.5mg/l + NAA 0.05mg/l).
Geng et al. [31] observed shoot cluster in A. annua L. on MS medium supplemented with BAP and NAA. Mackay and Kitto [32] and Nam-cheol et al. [33] also reported shoot induction on MS medium supplemented with BAP and NAA in different Artemisia species. Shoot induction was very low or absent at different concentrations of Kn alone or in combination with NAA. At 1.5mg/l Kn along with 0.05mg/l NAA, shoot induction was 65% (2.0 ± 0.724) and at higher concentrations, response was absent (Table 3).
Table 3: Effect of growth regulators on in vitro direct shoot induction of Artemisia annua from leaf explants on MS mediumZ.
|
Growth Regulator |
Conc. (mg/l) |
Response (%)Y |
Average No. of of shootsXT |
General description |
|
BAP |
0.25 0.5 0.75 1.0 1.5 2.0 |
00.0 28.0 46.0 69.2 79.0 15.0 |
- - 0.833 ± 0.324d 1.5 ± 0.622cd 2.83 ± 0.615ab - |
No response No response 1-2 shoots with green leaves 1-3 shoots with green leaves 2-3 shoots with green leaves No response but embryogenic callus |
|
Kin |
0.25 0.5 0.75 1.0 1.5 2.0 |
00.0 21.0 36.0 56.2 41.0 00.0 |
- - 0.833 ± 0.352d 2.16 ± 0.335cd - - |
No response but embryogenic callus No response but embryogenic callus 1-2 shoots with green leaves 1-3 shoots with green leaves No response but embryogenic callus No response but embryogenic callus |
|
NAA |
0.1 0.5 |
00.0 00.0 |
- - |
No response but embryogenic callus No response but embryogenic callus |
|
BAP/NAA |
1.5/0.05 1.5/0.1 |
85.7 26.0 |
3.16 ± 0.434a 1.83 ± 0.271bbc |
4-5 shoots with green leaves 2-3 shoots with green leaves |
|
Kin/NAA |
1.5/0.05 1.5/0.1 |
65.0 16.5 |
2.0 ± 0.724cd 1.66 ± 0.121d |
2-3 shoots with green leaves 1-2 shoots with green leaves |
|
LSD |
|
|
1.233 |
|
XMean ± standard error
Interval of confidence 95%
YData are mean of 6 replicates
TMean separation by LSD
ZRated after 30 days of culture.
Values with the different letters on the same column are significantly different.
Vergauwe et al. [34] reported the shoot regeneration from leaf explants of A. annua L. on MS medium with 0.05mg/l NAA and 0.05mg/l BAP after 5 weeks of culture. In the present study, a result of shoot induction rate is in agreement with the report of Banyai et al. [35] who considered 1mg/L BAP with 0.1mg/L NAA as the best supplemented medium for leafexplants-derived shoot regeneration. Almaarri and Yu Xie [20] reported 100 and 66.6% shoot induction in different genotypes of A. annua on MS fortified with TDZ (1 mg/l) and BAP (1 mg/l), respectively. Similar results have also been reported by Sujata and Kumari, Sharma et al. Gonzalez et al. Tahir et al. and Hailu et al. [18,21,36-38].
However, we established an improved protocol for direct shoot regeneration of A. annua L. using leaf explants on MS medium supplemented with BAP and NAA resulting in a rapid and high number of shoots per explant in this study. Therefore, this regeneration system might be a useful method for high regeneration efficiency and has commercial advantage due to the shoot regeneration period over a combination of several plant growth regulators. The regeneration system developed in this study will be useful for plant improvement through micropropagation and genetic engineering of A. annua L. Moreover, this system can be available for the clonal propagation in order to obtain the strain containing a constant concentration of artemisinin in A. annua L.
Root Induction
Regenerated shoots were sub-cultured on same medium and multiplied on BAP (1.5mg/l) in combination with NAA (0.05mg/l) (Figure 5).
Figure 5: Multiplication of regenerated shoots of Artemisia annua L. plant on MS medium (BAP 1.5mg/l + NNA 0.05mg/l).
Individual shoots were isolated and transferred to elongation medium (half strength MS) for 30 days. Elongated shoots were transferred on rooting medium. Rooting was found very well in the present study (Table 4).
Table 4: Effect of growth regulators on in vitro rooting of Artemisia annuaZ.
|
Growth Regulator |
Concentration (mg/l) |
Full MS |
½ MS |
||
|
Response (%)Y |
Average No. of rootsX |
Response (%)Y |
Average No. of rootsX |
||
|
IAA |
0.1 0.25 0.5 1.0 1.5 2.0 |
- 36.0 55.8 23.5 - - |
- 1.16 ± 0.5 2.33 ± 0.6 1.33 ± 0.4 - - |
- - 45.8 - - - |
- - 0.83 ± 0.25 - - - |
|
IBA |
0.1 0.25 0.5 1.0 1.5 2.0 |
- 23.5 59.6 - - - |
- 0.83 ± 0.3 2.0 ± 0.9 - - - |
- 21.9 55.4 - - - |
- 0.66 ± 0.2 1.66 ± 0.7 - - - |
|
NAA |
0.1 0.25 0.5 1.0 1.5 2.0 |
- 50.6 85.8 43.5 18.7 - |
- 1.83 ± 0.6 2.5 ± 0.9 1.33 ± 0.5 1.16 ± 0.3 - |
- 20.2 72.3 20.5 - - |
- 0.83 ± 0.3 1.83 ± 0.9 0.66 ± 0.2 - - |
XMean ± standard error
YData are mean of 6 replicates
ZRated after 30 days of culture
Six concentrations of auxins (IAA, IBA, and NAA) were tested in full and half strength MS medium. Highest response 85.8% with 0.5 mg/l of NAA in full MS followed by IBA (59.6%) and IAA (55.8%) was observed from shoots. At 0.5 mg/l NAA at full MS and ½ MS, 2-3 roots were observed. When these plants were transferred on the same medium, they produced further roots. Similar findings were observed by Zia et al. and Mohammad et al. [16,25]. Plants that produced roots were transferred to pots filled with soil and peat moss (3:1) under high humid condition till maturation of leaves, and then transferred to green house.
ACKNOWLEDGEMENT
Alka Dangash thankful to Ipca Laboratories Ltd., Ratlam for providing lab facility to carry out the present study