Involvement of Gicerin, a Cell Adhesion Molecule, in the Progression of Meningioma
- 1. Department of Animal Hygiene, Graduate School of Biology and Environmental Sciences, Kyoto Prefecture University, Japan
Abstract
Meningioma is tumors with variable evolution and prognosis. Several molecules seem to be involved in their behavior, including cell adhesion molecules. We previously found the heterogeneous expression of gicerin in four sporadic cases of Meningioma. Gicerin is an immunoglobulin super family cell adhesion molecule with both homophilic adhesion activity (gicerin – gicerin) and heterophilic adhesion with neurite outgrowth factor (NOF). This study was performed to clarify the importance of gicerin expression in the meninges. In the present study, the cell adhesiveness and behavior of gicerin-expressing meningioma cells in animals were examined to clarify the biological significance of this molecule in the malignant aspects of meningiomas. Firstly, transfectants with stable gicerin expression were established by introducing gicerin cDNA into the endogeneous gicerinnegative KPU-YT1 cells derived from a spontaneous rat meningioma. The transfectants showed enhanced cell-cell binding activity and also increased adhesion on either gicerin or NOF proteins. Next the transfectants were implanted into a cervical subcutaneous site of nude mice and were examined for their tumorigenicity and invasiveness. Interestingly, the gicerin transfectants formed larger tumors in the injection sites of most mice, which was inhibited by pre-treatment with an anti-gicerin polyclonal antibody. In addition, gicerin transfectants were enhanced their invasiveness into surrounding tissues. These findings suggest that gicerin might promote the malignant aspects of meningioma with respect to cell adhesion.
Keywords
Meningioma; Cell adhesion molecule; Gicerin; Implantation.
Citation
Utsumi K, Shiba H, Adachi K, Tsukamoto Y (2014) Involvement of Gicerin, a Cell Adhesion Molecule, in the Progression of Meningioma. JSM Cell Dev Biol 2(2): 1011.
INTRODUCTION
Meningiomas are usually benign tumors which grow slowly, and they typically demonstrate a good outcome after a complete removal. However, from 0.9-11% of all meningiomas is either atypical or malignant meningiomas that grow very fast and infiltrate the surrounding tissue. There is usually a good correlation between the cytological criteria and the subsequent clinical behavior. Most meningiomas are attached to the dura and press on the brain or spinal cord through the arachnoid, although unusual examples can arise within the brain’s ventricles or in the leptomeninges without dural attachment [1].
There have been many reports concerning the developments in the diagnosis and treatment of meningioma [2]. However, the causes of malignancy in meningioma are currently poorly understood. We previous found a heterogeneous expression of gicerin, a cell adhesion molecule (CAM), in sporadic cases of meningioma, and thought that it might have a role in determining the malignant potential of meningioma, since gicerin promotes the metastatic and invasive activities of various other tumor cells, including Wilm’s tumors, oviductal adenocarcinomas, lymphomas, colorectal carcinomas and mammary gland adenocarcinomas due to its adhesive activities [3-9].
Gicerin is an immunoglobulin super family CAM and has both a homophilic adhesion activity (gicerin-gicerin) and exhibits heterophilic adhesion with neurite outgrowth factor (NOF), an extracellular matrix protein [10,11]. Gicerin is expressed in developing, regenerating tissues and also in tumors: gicerin’s adhesive activities are known to be involved in neurite extension, cell migration, epithelization during development, and in the reorganization of the respiratory and renal epithelia during the regeneration process after viral infections [12-15]. However, the potential role of gicerin in the progression of meningioma in vivo remains unclear.
Transfection of gicerin cDNA into cells and a subsequent analysis of their tumorigenic, invasive and metastatic abilities in animals provide a model for this issue. In the present study, we investigated the actions of gicerin-transfected KPU-YT1 cells, an implantable rat meningioma cell line, by implanting it into mice and herein provide evidence that gicerin promotes tumor growth and increases the invasive properties of tumor cells. This is a first report to show the involvement of gicerin expression in the progression of meningiomas.
MATERIALS AND METHODS
Establishment of gicerin-expressing meningioma cells
The KPU-YT1 cell line is a newly-established implantable meningioma cell line derived from an experimental F344 rat with sporadic meningioma. We have cultured the tumor cells in vitro over 20 passages, and found that the cells were implantable in nude mice, but not in rats. In our preliminary study, the KPU-YT1 cells were found to be negative for gicerin expression.
The KPU-YT1 cells were cultured in dishes with Dulbecco’s modified Eagle’s medium (DMEM) (Nacarai) containing 10% fetal calf serum (FCS) (Gifco) at 37°C. The cells were transfected with a pcDNA 3.1 plasmid vector (Invitrogen) containing gicerin cDNA [8]. As a mock transfection, cells were transfected with an empty vector plasmid without gicerin cDNA. These transfectants were selected in DMEM containing 10 mg of G418 (Gibco). Gicerin transfectants were established that expressed the gicerin protein, as confirmed by immunocytochemistry using a polyclonal antibody against gicerin (described below). Mock-transfectants were also established and examined for their gicerin expression by immune cyto chemistry.
Expression of gicerin in the KPU-YT1 meningioma cell line
KPU-YT1 cells on culture dishes (Falcon) were fixed in Zamboni’s solution [0.21% picric acid, 2% paraformaldehyde, and 130mM phosphate buffer (pH 7.49)and incubated with a rabbit polyclonal antibody against the gicerin (1:500) [8] protein at 4°C overnight after being washed in PBS. The cells on dishes were then reacted with afluorescein isothiocyanate (FITC) -conjugated swine antibody against rabbit Igs(Dako) at 37°C for 1 hr following sufficient washing with PBS. Finally, the cells were mounted with glycerol, and then the specific signals for the gicerin protein were examined under a phase-contrast equipped fluorescent microscope with a B-filter (Nikon).
Cell aggregation assay
Monolayer cultures of gicerin transfectants and mocktransfected cells were digested with 0.05% trypsin and 0.53 mM EDTA (Gifco) and collected by centrifugation at 800 x g then were re-suspended in DMEM at 6x106 cells/ml. In some experiments, an anti-gicerin polyclonal antibody or a preimmune IgG (as a control) were added to the cultures at a final concentration of 0.2 mg/ml, and the cells were incubated for 1 hr before starting the aggregation assay [8]. To quantify the aggregation, a 1-ml aliquot of the cell suspension was transferred to a 50-ml polypropylene tube (Falcon) coated with FCS. The assay was carried out by incubating the cell suspension at 37°C without agitation. A small aliquot (10 µl) was withdrawn on a slide glass at 1hr, and then the cell aggregations were observed under a microscope. The examination was performed in five times.
In vitro cell adhesion of meningioma cells onto the ligands of gicerin
Bovine serum albumin (BSA) (Nacarai), gicerin proteins and NOF proteins were independently coated onto culture dishes at 2 µg/cm2 and were incubated for 2 hr at room temperature. The KPU-YT1 cells were seeded onto these culture dishes, and then were incubated in DMEM for 1 h at37°C. After a gentle and brief wash with PBS, the adherent cells on the dishes were observed under a microscope, and the cell numbers were counted per area. This examination was performed in five areas for each protein coating in five culture dishes, the score was averaged and the standard deviations (SD) were calculated.
Injection of meningioma cells into a subcutaneous site of nude mice
KPU-YT1 cells were pre-incubated with an anti-gicerin rabbit polyclonal antibodies or a preimmune IgG (as a control) for 1 hr at 37°C, and then were suspended at 1.8× 108 cells/ mL in DMEM. These cells (0.3 mL) were implanted into the cervical subcutaneous site of nude mice (five animals for each cell type with/without anti-gicerin antibodies). At 15-days postinjection, the mice were sacrificed under deep anesthesia with pentobarbital solution, and the injection sites were removed and fixed with buffered formalin for a histopathological evaluation. The tumor volume was determined using the formula: (A×B2 /2), where A is the largest diameter and B is the smallest diameter. All of the animal experiments were performed in accordance with the guidelines for studies with laboratory animals of the Kyoto Prefectural University Experimental Animal Committee (No. KPU220912)
Histopathology
The samples were embedded in paraffin and cut into 3-μm sections with a microtome. Then, the sections were stained with hematoxylin and eosin (H&E) (Sigma) using routine procedures and were observed under a light microscope.
RESULTS AND DISCUSSION
Expression of gicerin in transfectants
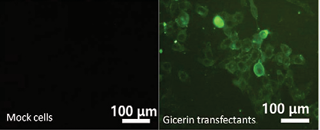
The gicerin expression in Mock- and gicerin-transfected KPU-YT1 cells was examined by an immunofluorescent assay. Gicerin was not found in the mock transfectants. In contrast, membranous expression of gicerin was clearly observed in the gicerin transfectants (Figure 1).
Figure 1: The results of the immunocytochemical analysis of the gicerin expression in KPU-YT1 cells. The expression of gicerin in the gicerin-transfected KPU-YT1 cells (gicerin transfectants) and mock-transfected KPU-YT1 cells (mock cells) was examined by immunocytochemistry. Gicerin was not observed in mock cells. In contrast, gicerin transfectants showed gicerin expression on their cell surface.
Thus, stable gicerin transfectants were established by the introduction of gicerin cDNA into the KPU-YT1 meningioma cell line, and these cells were used for further experiments.
In vitro aggregation of gicerin transfectants
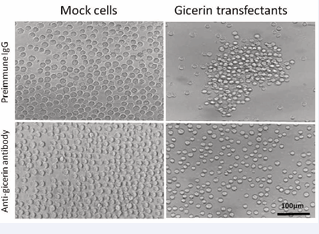
The cell aggregation activities of the mock- and gicerintransfected cells were analyzed by a convenient in vitro assay. After 1-hr incubation, the gicerin transfectants bound each other and formed large cell clusters, whereas the mock transfectants showed only slight aggregation (Figure 2).
Figure 2: The aggregation patterns of KPU-YT1 cells after a 1-hr incubation with preimmune IgG or an anti-gicerin antibody. The aggregation of mock cells was weak in the presence of either the preimmune IgG and anti-gicerin antibody. In contrast, gicerin transfectants showed strong cell aggregation activity: cells begun to aggregate and formed large cell clusters. The anti-gicerin antibody inhibited the aggregation of the gicerin transfectants. The examination was performed in five times and the same results were found.
The examination was performed in five times and the same results were found. Accordingly, the KPU-YT1 cells obtained a hemophilic cell-cell aggregation activity following gicerin expression.
In vitro adhesion of gicerin transfectants on gicerin ligands
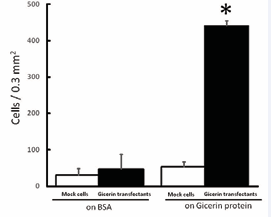
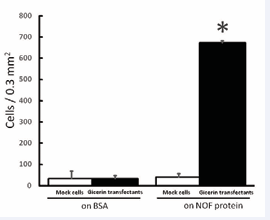
Gicerin- and mock-transfected cells were seeded onto culture dishes coated with gicerin’s ligands, gicerin or NOF. Theadhesion activities of the gicerin transfectants were enhanced on the dishes coated with gicerin or the NOF; numerous gicerin transfectants were adhered onto the dishes compared with the mock cells (Figures 3 and 4).
Figure 3: The cell adhesion activity of the KPU-YT1 cells on gicerin protein-coated cell culture dishes. Mock cells and gicerin transfectants were seeded onto BSA- or gicerin-coated dishes. The number of adhesive cells on the dishes was counted in an area of 0.3 mm2 using light microscopy. Note that the adhesive activity of gicerin transfectants was enhanced on the dishes coated with the gicerin protein. Each bar represents the mean ± SD of five areas in each cell culture dish. *P<0.01 compared with the data of mock cells (Student’s t-test).
Figure 4: The cell adhesion activity of KPU-YT1 cells on the NOF protein. Mock cells and gicerin transfectants were seeded onto BSA- or NOF-coated dishes. The number of adhesive cells on the dishes was counted in an area of 0.3 mm2 using light microscopy. Note that the adhesive activity of gicerin transfectants was enhanced on the dishes coated with the NOF protein. Each bar represents the mean ± SD of five areas in each cell culture dish. *P<0.01 compared with the data of mock cells (Student’s t-test).
Thus, the KPU-YT1 cells acquired adhesive properties with respect to both homophilic and heterophilic binding activities.
Behavior of gicerin transfectants in nude mice after subcutaneous implantation
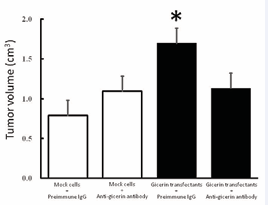
The tumorigenicity of the gicerin transfectants was examined by an in vivo implantation assay in nude mice. On day 15 postimplantation, the tumor volume was measured and scored as the mean ± SD of five mice (Figure 5).
Figure 5: The growth of KPU-YT1 tumors after subcutaneous implantation in nude mice. The tumor volume was monitored for 15 days after a subcutaneous injection of mock cells or gicerin transfectants pretreated with preimmune IgG or an anti-gicerin antibody. The data are expressed as the average tumor volume of five mice. The results are reported as the means ± SD. *P<0.01 compared with data from tumors generated by mock cells incubated with preimmune IgG.
The gicerin transfectants formed larger masses in the injection sites of nude mice compared with those of mock transfectants. However, pretreatment of transfectants with an anti-gicerin polyclonal antibody inhibited the growth of the gicerin transfectant tumors. The doubling times of mock- and gicerin-transfectants in DMEM containing 10% FCS were 22.3±3.7 and 20.7±2.5hrs, respectively. This indicated no difference of in vitro cell growth among these cells. Since no obvious difference in doubling time was recorded between mock and gicerin transfectants in a culture system, changes in cell-ECM or cell-cell interactions by gicerin expression might affect the growth of meningioma cells in mice.
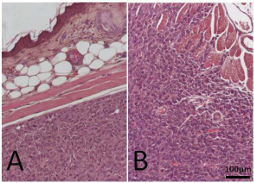
Histopathologically, the tumor tissues had typical meningioma appearances: round to spindle atypical cells grew in sheets or epithelial structures with less connective stroma. Interestingly, the gicerin transfectants had enhanced malignancy with respect to invasiveness; the tumor cells frequently invaded into the surrounding skeletal muscular tissues (Figure 6).
Figure 6: The histopathological findings of the subcutaneous tumors from nude mice collected 15 days post-implantation. Paraffin sections of tumors were cut and stained with H&E. The tumors showed appearances typical of primary sporadic meningioma in experimental rats : atypical tumor cells were forming poorly differentiated epithelia-like structures by round to spindle shaped cells, and mitotic figures were also seen. In the tumors formed by mock cells, the cells were not invading into the adjacent tissues, including the dermis and skeletal muscle (A). In contrast, gicerin transfectants showed more infiltrative growth patterns: cells were invading into the dermis of the skin and destroyed adjacent skeletal muscle (B).There were no histopathological variations among five mice in each group.
In contrast, no tumor invasive legions were observed in the mock transfectant tumors or in the mice injected with gicerin transfectants pretreated with an anti-gicerin antibody. These findings indicated that gicerin expression promoted the progression of meningioma. There were no metastatic legions in any other organs in the mice injected with the gicerin- and mock-transfected cells.
CONCLUSION
The cell-to-cell and cell-to-ECM adhesion are essential properties involved in the development and maintenance of tissues [16]. These cell adhesion reactions are also involved in tumor progression, including the growth, invasion and metastasis of tumors [17,18]. In the present study, we have demonstrated that gicerin expression promoted the tumor growth and invasiveness of a rat meningioma cell line toward more malignant behavior. The gicerin transfection in endogeneous gicerin-negative KPUYT1 cells enhanced the cell-cell and cell-ECM interactions, leading to tumor growth and enhanced invasiveness in experimental animals.
It has been reported that various CAMs are being developed as potential biomarkers for the diagnosis of meningioma, and are also involved in the tumor progression [19-21]. We believe that gicerin is a potential enhancer of the progression of meningioma with respect to cell-cell and cell-ECM interactions, since gicerin’s ligands (gicerin and NOF) are enriched in blood vessels, skeletal muscle cells and the basement membrane. CD146, a gicerin analogue in the human species is a potential biomarker of malignant melanoma [22]. Recently, CD146 is attempted to be developed as a new cell surface marker for cartilage progenitor cell population in the late-stage osteoarthritis [23].We are now attempting to elucidate the expression patterns of gicerin in various sporadic cases of meningiomas for use as a potential diagnostic marker for the malignancy of meningioma.