Type V Collagen as a Low Thrombogenic Surface Coating for a Biological Vascular Prosthesis
- 1. CSIRO Manufacturing, Australia
- 2. Veterinary Hospital, The University of Melbourne, Australia
- 3. School of Veterinary and Animal Sciences, Charles Sturt University, Australia
- 4. Department of Anatomy and Neuroscience, University of Melbourne, Australia
Abstract
There is a significant unmet need for a biomaterial that is suitable for use as a small diameter vascular prosthesis for coronary by-pass surgery. The Omniflow Vascular Prosthesis? has proved clinically successful at larger diameters, for example for femoral artery replacement. The present study has examined whether type V collagen can provide an improved surface with poor thrombogenic properties that may assist in development of a coronary by-pass device. Biotinylated type V collagen was bound to biotinylated Omniflow Vascular Prosthesis? through use of a streptavidin bridge. The treated prosthesis showed a major reduction in the extent of platelet attachment, indicating that type V collagen coating should assist in the development of small diameter prosthesis.
Keywords
Collagen; Platelet; Adhesion; Vascular prosthesis.
Citation
Werkmeister JA, Casagranda F, Edwards GA, White JF, Glattauer V, et al. (2017) Type V Collagen as a Low Thrombogenic Surface Coating for a Biological Vascular Prosthesis. JSM Cell Dev Biol 5(1): 1022.
ABBREVIATIONS
OVP: Omniflow Vascular Prosthesis?; GA: Glutaraldehyde; BSA: Bovine Serum Albumin; TCP: Tissue Culture Plate; PRP: Platelet-Rich Plasma
INTRODUCTION
Currently there is no ideal small diameter (3-4mm) vascular prosthesis for coronary by-pass surgery, and this remains a significant unmet clinical need. The critical problem associated with failure in these small diameter prostheses is thrombosis. The initial contact between blood and vascular prosthesis is a critical determinant for the success of any vascular implant. One approach has been to seed and culture endothelial cells on the inner surface of the prosthesis, so that a natural nonthrombogenic surface is present from the time of implant [1,2]. Alternatively, after allowing growth, the cells can be removed, leaving an intact basement membrane on the ‘conditioned’ surface of the prosthesis that promotes in situ endothelialisation [3]. More recently, tissue engineering approaches have been suggested [4], however, durability over long time frames is not yet clear. For any construct type, if the endothelial lining is incomplete or if some of the lining sloughs off, the potential for thrombosis is present. An alternative approach is to covalently coat the inner surface of the prosthesis with a biological molecule that prevents thrombosis. If this coating is then seeded and/or subsequently replaced during in situ endothelialisation, there is, nevertheless, continuity in the protection against thrombosis.
In the present study, we have examined a biosynthetic vascular prosthesis [5], the Omniflow Vascular Prosthesis? (OVP) (Bionova International, North Melbourne; see also LeMaitre Vascular). This bioprosthesis is a collagen-polyester composite [5,6], that has proved durable and successful in clinical use [7- 10]. At a diameter of 5-6mm the OVP has been shown to perform with high patency, display long-term structural stability and have good compliance [11]. It has also been promising in popliteal artery replacement applications [11], and as an arterio-venous access device [8]. The inner surface of the composite material consists of loosely organised collagen [6], including the fibril forming type I and type III collagens [12]. These collagens in fibrils are generally seen as highly thrombogenic [13], although in the OVP this is substantially passivated by the glutaraldehyde (GA) crosslinking that is used during the manufacture of the device [5]. However, this passivation has not been sufficient to allow consistent success in small diameter applications such as for cardiac applications. However, the proven clinical performance at larger diameters provides a good starting point for the development of effective smaller diameter prosthesis.
In the present study, we have examined type V collagen, in comparison to type I and III collagens and fibronectin, as a potential biological blood contact surface on the OVP biosynthetic vascular prosthesis, and make it more haemocompatible for use as small diameter prosthesis. In particular, we have examined if a surface can be obtained that will prevent platelet adhesion and activation, as these are the critical initial steps in thrombus formation.
MATERIALS AND METHODS
Vascular prosthesis
Samples of partly processed, trimmed OVP were a gift from Bionova International, North Melbourne. The OVP samples had an internal diameter of 4 mm and were prepared using a polyester mesh on a silicone mandrel implanted in sheep as previously described [5]. At explant, after 12 weeks, the tubes were trimmed following normal production procedures (Bionova International, North Melbourne). However, the stabilisation by glutaraldehyde (GA) and subsequent steps were not performed.
Biological materials
Collagens were prepared from minced bovine corium digested at 4°C for 24 - 48 h with pepsin (1 mg/ml) in 100 mM acetic acid adjusted to pH 2.3 with HCl. After centrifugation, a fraction containing solubilised type I and type III collagens and another containing type V collagen were collected using NaCl fractionation at pH 2.3 [14]. Types I and III collagens were further purified by NaCl precipitation at pH 7.2 [15], while type V collagen was purified by precipitation with 4 M NaCl at pH 7.2 followed by precipitation from 50mM sodium acetate at pH 4.8 [16]. Collagen purity was examined by SDS-polyacrylamide gel electrophoresis, using delayed reduction to identify type III collagen [17].
Biological coatings
Five test proteins, namely bovine serum albumin (BSA), fibronectin (both from Sigma) collagen type I, collagen type III and collagen type V, were used for coatings. For preliminary examination of these coatings, the surface of 24-well tissue culture plates (TCP) were passively coated. Coating of TCP was by 20µg protein per well from 0.5 mg/ml solutions in phosphate buffered saline (PBS) [18], for 16 h at 4°C.
For coating the OVP samples, more efficient and higher binding efficiency was sought. The 5 test protein molecules in 50 mM sodium bicarbonate, pH 9.6 were biotinylated using a calculated amount of biotinyl-N-hydroxy-succinimide ester in 50mM sodium bicarbonate, pH 9.6 for 2.5 h at room temperature. The amount of reagent was calculated to react with 20 % of the available lysine residues of each protein. After reaction, the solutions were dialysed extensively at 4°C against PBS.
Commercial OVP is stabilised with 2% GA [5], leaving no available lysine for surface modification. In the current procedure, partly processed non-crosslinked OVP samples were coated using a biotin/streptavidin-based attachment method. The OVP samples, after washing extensively with 50 mM sodium bicarbonate, pH 9.6, were held vertically, closed at the bottom with a clip and were biotinylated by filling with 3 mg/ml biotinylN-hydroxy-succinimide ester in 50 mM sodium bicarbonate, pH 9.6. The outside of the samples were kept moist by irrigation with the same buffer and the reaction allowed to proceed for 4 h at room temperature. After removal of clips, the samples were washed once with 50 mM sodium bicarbonate, pH 9.6 and the multiple times with PBS. After trimming of unreacted ends, the samples were replaced on a silicone mandrel and were fixed in 2% w/v GA in PBS at room temperature for 16 h. After fixation, samples were rinsed in PBS and were then stored in 50% v/v aqueous EtOH at 4°C until analysis. Control samples with 2% w/v GA fixation only were also prepared. Prior to analysis samples were again rinsed multiple times in PBS.
Biotin availability after the GA stabilisation and EtOH storage was verified using 3 mm x 4 mm ID samples cut open to provide a flat surface. H2 02 in MeOH, 0.15 % w/v, was added for 15 min at room temperature to remove any endogeneous peroxidase activity. The samples were then rinsed in PBS and added to 10% BSA in PBS for 1 h. After rinsing in PBS, avidin-horseradish peroxidase (Bio-Yeda), 12.5 µg/ml in PBS, was added for 30 min [19]. After rinsing again, samples were covered with 5 mg/ml solution of 2,2’-azinobis [3-ethylbenzothiazoline-6-sulfonate] in 50 mM potassium phosphate at pH 5.0, followed by 0.3% (w/v) H2 O2 solution.
Biotinylated test proteins were attached to the biotinmodified OVP via a streptavidin bridge. Biotin-modified OVP samples were washed in PBS and closed at one end as above. They were then reacted with 0.8 mg/ml streptavidin (Boehringer Mannheim) in PBS for 2.5 h at 4°C, followed by washing in PBS. Solutions of the biotinylated test protein molecules were added, at 0.5 mg/ ml, and left to react with the streptavidin coupled OVP for 1 h at 4°C, and then washed with PBS.
Platelet adhesion evaluation
Platelets were prepared as previously described [20]. Briefly, whole canine blood was added to 0.1 vol. of 3.8% sodium citrate and platelet-rich plasma (PRP) prepared by centrifuging at 160 g for 20 min. Canine blood was selected as trials of OVP in dogs had shown compatibility [5,12]. The upper PRP fraction was collected and the lower fraction was further centrifuged at 3000 g for 10 min for preparation of platelet-poor plasma (supernatant). Platelet counts were in the order of 3.0 x 108 platelets/ml.
Platelet adhesion was performed in two ways. For the preliminary assessment on the various coatings on TCPS, an indirect method was used where platelet counts were assessed using a Coulter Counter. The % Adhesion of PRP on the various coated TCP surfaces was the percent difference in PRP before and after incubation. For platelet adhesion to the modified OVP surfaces, absorption of 51Cr-labelled platelets was examined. Platelets were labelled with 100µCi 51Cr as previously described [21,22]. Lengths of the prosthesis (40 mm) were mounted vertically and clamped as above and then filled with 51Cr-labelled platelets. After 4 min the OVP sample was unclamped, rinsed in PBS and measured for bound radioactivity of adhered platelets. The % Adhesion of labelled platelets was expressed by comparing the amount of bound labelled platelets to those on an unmodified GA processed OVP.
SEM evaluation
Prosthesis modified samples were also treated with unlabelled platelets as well as whole blood and processed for SEM by dehydrating in ethanol, critical point drying from Peldri II, and gold coating. Samples were examined using a JEOLJSMT20 instrument
RESULTS AND DISCUSSION
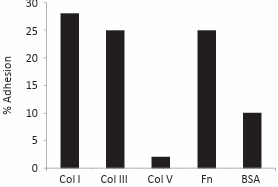
The three collagen types that were prepared from the bovine hide, collagen types I, III and V, were examined by electrophoresis and shown to be pure. The use of delayed reduction SDS-PAGE [17], enabled type III collagen to be identified separate from type I collagen. Most importantly, the type V collagen was confirmed to be free of any contamination by type I or III collagens (data not shown). Previous studies [18], had indicated that type V collagen showed poor platelet adhesion. On the other hand, type I and type III collagens have been shown to have strong platelet attachment [13]. Hence any contamination would adversely change the low attachment properties of type V collagen. In the preliminary assessment on modified TCP, the collagens were passively absorbed on TCP, as previously reported [18], and type V collagen was shown to have very low platelet attachment, contrasting to the high attachment shown by types I and III collagens (Figure 1).
Figure 1: Binding of platelets in PRP onto TCP coated with various test proteins. (Col I = collagen type I, etc, Fn = fibronectin).
Thrombogenicity is a major hurdle in the use of biological and synthetic vascular grafts, particularly for small diameter coronary grafts. In the normal vasculature, damage to the endothelial cell lining exposes collagen types I, III. IV and V [23]. It is the exposure to these components that can lead to platelet adhesion, activation and thrombus formation. In the case of the OVP some components, particularly collagens type I and III, are already present and pose as potential candidates for thrombus formation. The current OVP is, however, passivated to some degree by the potential presence of endogenous collagen type V as well as by the GA treatment that can mask the potential binding sites for the α2β1 integrin and glycoprotein VI complex receptors [24]. In order to examine the effects of these collagens on the surface characteristics of the OVP prosthesis, biotin-streptavidin biochemistry was utilised to bind 5 test proteins onto the surface of the material, including fibronectin and BSA as controls. Fibronectin is well established as providing a strongly adherent surface for platelets [25], while BSA has been considered to provide a low adherent surface [26].
Prior to testing for platelet adhesion, the biotin coupling onto the OVP was verified, to ensure that the biotin was still available and had not been lost during the GA treatment or during storage. The avidin-horseradish peroxidase conjugate bound readily to the modified OVP and the test reaction led to a strong, positive green colour, indicating binding. The control OVP, which had not been biotinylated but had received the 2% GA treatment gave little or no green colour reaction. Together these indicate that the biotin was still present and accessible for reaction with the range of biotinylated components.
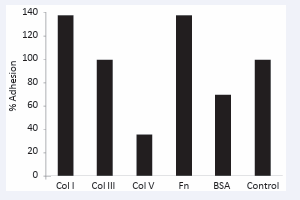
Platelet adhesion tests, using 51Cr labelled platelets, showed a range of different extents of adhesion depending on the particular protein (Figure 2).
Figure 2: Binding of 51Cr-labelled platelets onto protein-modified and GA-treated OVP samples relative to un-modified GA treated OVP (Control). (Col I = collagen type I, etc, Fn = fibronectin).
These data showed, consistent with our tissue culture plastic data and that of others [18], that compared to the GA control, with no other coating, that both type I and type III collagens were strongly adherent for platelets, as was fibronectin (Figure 2). The BSA was seen to passivate the surface to some extent, while much greater passivation was achieved by the type V collagen coating (Figure 2). In our study, platelet adhesion was compared to the unmodified but GA processed OVP that is known to contain collagen type I and III on its inner surface [6,12]. Our results support the concept that OVP can bind platelets and could be prone to thrombogenesis in small diameter applications. Our data also showed that addition of further coating by collagen type I on the surface caused an increase in platelet adhesion compared to OVP. On the other hand, collagen type V binding and coating over any residual un-crosslinked collagen types I and III substantially negates these thrombogenic collagens.
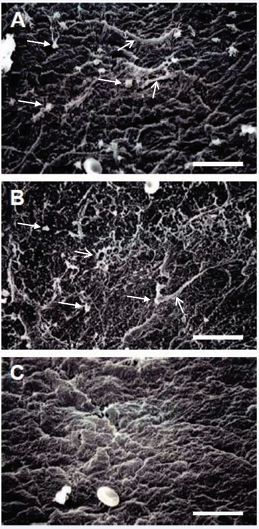
These results were confirmed further by SEM examination of the platelet exposed surfaces, where both the fibronectin (Figure 3a), and type I collagen (Figure 3b), treated surfaces showed a substantial amount of platelet binding as well as fibrin deposition. On the other hand, the type V collagen treated surface (Figure 3c) showed little evidence of any platelet binding or fibrin accumulation.
Figure 3: SEM examination of the surfaces of modified prosthesis samples after absorption of platelets from dog blood. (A) Fibronectin coated vascular prosthesis. (B) Collagen type I coated vascular prosthesis. (C) Collagen type V coated vascular prosthesis. Closed arrows indicate examples of attached platelets. Open arrows indicate examples of fibrin deposition. Bar = 15 µm.
These SEM data (Figure 3) are consistent with the adhesion assays (Figures 1 and 2).
Although all three collagens examined are considered as interstitial, fibril forming collagens, a key difference between types I and III collagen and type V collagen is that the purified type I and III collagens readily associate into large fibrillar structures [27]. Furthermore, it has been shown that collagen types I and III are much more potent in platelet adhesion assays when they are in a fibrillar form [13], and seemingly must have the fibrillar, quaternary structure for the subsequent platelet aggregation and release steps [28]. On the other hand, purified type V shows a much lower tendency to form fibrils and when fibrils are formed they remain small and do not bind the major platelet receptors [24]. This is in agreement with the disposition of these collagens in natural tissues, where type V collagen is readily observed at very early stages of fine fibril formation, but soon becomes fully covered by types I and III collagens as larger collagen fibrils form [29]. Consistent with these observations, we have previously shown that type V collagen is associated with early stages of neointima formation on the inner surface of the 6mm diameter OVP in a dog model [30].
While type V collagen could prove useful in passivating the surface of a vascular prosthesis, a potential problem is purification of this collagen in commercially adequate quantities, although coatings do not require large amounts of material. Type V collagen has a low natural abundance; for example, less than 5% of the major type I and III collagens in bovine hides. An alternative source could be recombinant production in Pichia [31,32] which can provide human, fully hydroxylated protein. Alternatively, other collagen preparations may be suitable. For example, there are other collagens that are not fibril forming [33], including some that are readily produced in recombinant systems [34].
CONCLUSIONS
We conclude that type V collagen could be a useful material for passivating the blood contact surface in the production of small diameter vascular prostheses. Moreover, if type V collagen’s limited fibril formation is a key aspect of its utility, then alternative collagens may also be useful.
ACKNOWLEDGEMENTS
We wish to thank Bionova International for the gift of partly processed Omniflow Vascular Prosthesis material.