Efficacy and Safety of Partial Splenic Artery Embolization in Patients with Decompensated Cirrhosis Awaiting Liver Transplantation
- 1. Liver Transplantation Program, Loma Linda University Medical Center, USA
- 2. Interventional RadiologyLoma Linda University Medical Center, USA
- 3. Division of Gastroenterology and Hepatology, University of Southern California, USA
ABSTRACT
Partial splenic artery embolization (PSAE) is an invasive procedure that is used to treat certain complications of cirrhosis. It is safe and effective in selective patients with compensated cirrhosis when it is used appropriately. The aim of the study is to determine the safety and efficacy of PSAE in patients with decompensated cirrhosis awaiting liver transplant.
Study: This is a single—center retrospective study. Patients with decompensated cirrhosis and hypersplenism listed for liver transplantation were included. Patients with HIV, compensated cirrhosis, trans jugular portosystemic shunt (TIPS), active sepsis, receiving steroids or lost follow up were excluded.
Results: Thirteen patients met the inclusion criteria. One-year survival rate was 70 %. PSAE significantly increased platelets count compared to baseline, and this effect was sustained for forty-eight weeks. There were no significant changes in Child-Pugh and MELD scores after week 4 of the procedure. Only one patient had splenic abscess. PSAE did not affect post liver transplantation outcome. The major cause of death prior to liver transplantation was systemic sepsis.
Conclusion: Although PSAE is associated with significant morbidity, it is a safe and effective treatment in well-selected patients awaiting liver transplantation when it is performed in a transplant center and by an experienced radiologist. A prospective large multi-center study is needed to determine the independent factors that correlate with survival pre-and-post liver transplantation.
KEYWORDS
Cirrhosis , Ascites , Thrombocytopenia , Splenic embolization
CITATION
Trimzi M, Smith J, Smith D, Hadi R, Kayali Z (2013) Efficacy and Safety of Partial Splenic Artery Embolization in Patients with Decompensated Cirrhosis Awaiting Liver Transplantation. JSM Gastroenterol Hepatol 1(1): 1004.
INTRODUCTION
Hypersplenism is a major complication related to portal hypertension that can lead to thrombocytopenia, leukopenia and extensive collaterals and portal shunting [1-5]. Profound thrombocytopenia in cirrhotic patients is an ominous and a major risk factor for death due to bleeding [6]. If it is uncorrected, it may jeopardize patients’ candidacy for liver transplantation. Medical treatment is of limited efficacy. Thrombopoietin-receptor agonist (TRA) has been approved for patients with idiopathic thrombocytopenia purpura (ITP) [7], but later results have shown an increased risk for hypercoagulation and portal vein thrombosis [8]. Although TRA was effective and safe in compensated hepatitis C cirrhotic patients [9], its safety and efficacy in decompensated cirrhotics who are waiting for liver transplantation has yet to be determined. The risk of portal vein thrombosis may limit the indication of using of TRA in these patients. Partial splenic artery embolization (PSAE) aiming at decreasing sequestration and portal shunting has been reported as a salvage treatment for patients with profound thrombocytopenia who did not respond to medical treatment [10-14]. PSAE has also been used to treat other portal hypertension complications such as refractory hepatic encephalopathy (HE), gastric varices and portal hypertensive gastropathy not responding to conventional treatment. PSAE improves portal hypertension by decreasing the blood flow from the splenic vein into portal vein and hence decreasing the pressure in the portal vein [15-17]. Infection and splenic abscess are common side effects [15-17]. The majority of PSAE data has been reported in compensated cirrhotic patients, and its efficacy and safety in decompensated cirrhotics has been reported in a small number of patients [10-22]. Yet, it is unknown if PSAE will jeopardize patients’ candidacy to receive liver transplantation by increasing their risk for infection or worsening their Model for End Stage Liver Disease (MELD) score. The aim of this study is to determine the safety and efficacy of PSAE in patients with decompensated cirrhosis awaiting liver transplantation.
MATERIALS AND METHODS
Study Design and Patients
This is a retrospective study targeting all patients who underwent PSAE at the Loma Linda University Medical Center from January 2005 to October 2010. Candidates are patients with decompensated cirrhosis (Child class B or C) have hypersplenism (>12 cm) and listed for liver transplantation. Spleen size was determined by two imaging methods (CT and/or MRI of abdomen) before PSAE. Decompensated cirrhosis was diagnosed based on laboratory and clinical signs of decompensation with or without the presence of ascites on imaging. Patients were included regardless of the indication for PSAE.
In order to estimate the efficacy of PSAE in improving profound thrombocytopenia, patients with thrombocytopenia related to other etiology such as idiopathic thrombocytopenia (ITP), drugs, or autoimmune disorders were excluded based on appropriate blood and clinical testing. Patients with contraindication to liver transplantation or already received liver transplantation, compensated cirrhosis, normal spleen size, transjugular intrahepatic portosystemic shunt (TIPS), active sepsis and receiving steroids were excluded. Patients were followed until death or liver transplantation. The primary end point was survival and the secondary endpoints were safety of PSAE in pre-and-post liver transplant and its effect on improving hematological parameters. Safety was determined based on MELD and Child-Pugh-Turcotte (CPT) scores, as well as procedural related complications.
Patients’ medical records were reviewed and clinical and laboratory findings were collated. Spleen size was measured pre-and-post PSAE by independent radiologists using clinically indicated abdominal CT and/or MRI. The study was approved by the institutional board review.
Partial splenic artery embolization
Interventional radiologist experienced in splenic arterial interventions performed partial splenic artery embolization. Via a common femoral arterial approach, the celiac or splenic artery was catheterized using four or five French diagnostic catheters, and a diagnostic digital subtraction angiogram was then performed. In coaxial fashion, a microcatheter was advanced either into the distal splenic artery or into subsegmental, intrasplenic branches according to operator preference. Embolization was performed with polyvinyl alcohol particles and/or gelfoam slurry soaked in gentamycin and penicillin antibiotic solution. Intrasplenic branches were routinely “capped” off with deployment of platinum coils soaked in topical thrombin. Embolization continued until approximately 60-70% of the splenic parenchyma was devascularized on completion diagnostic angiogram.
Statistical analysis
Statistical analysis was performed by using SPSS statistics 18 [Version 18.0.0. Release date (July 30, 2009) Chicago, IL]. Data are presented as mean or median (range) as indicated. As our study is a repeated-measured design in which blood parameters of the same patients are taken at different time intervals (baseline, one week after, four weeks after, etc), a simple one-way ANOVA cannot be used. In addition, the data collected was not normally distributed and so a Friedman test was used to compare the medians. Statistically significant results are reported in terms of P value, which was set to < 0.05. Post Hoc analysis was done using multiple Wilcoxon Signed-Rank tests with applied Bonferroni Corrections.
DISCUSSION
Splenic artery embolization is an invasive procedure that can lead to a wide variety of complications. It has been used cautiously in cirrhotic patients to treat the consequences of hypersplenism such as profound thrombocytopenia. The procedure did not initially gain popularity in cirrhotic patients because of its serious consequences such as sepsis and death. A modified approach, including stricter antibiotic prophylaxis and partial splenic devascularization, was introduced by Spigos et al in 1979 [10]. Since then, multiple studies of PSAE have reported relatively effective and safe results in patients with Child class A [10-26]. Although, PSAE is effective in treating thrombocytopenia and other portal hypertension complications, it has shown conflicting results regarding its safety in patients with Child class B and C [10-26]. Because of the relative unpopularity of the procedure, the majority of the data about its safety has been reported in small case series. Furthermore, limited data are available in patients with decompensated cirrhosis who are waiting for liver transplantation.
Although the sample size is small, our study demonstrated a significant improvement in platelets count after PSAE. This effect was sustained for at least twelve months. This is an important finding for patients with profound thrombocytopenia who are awaiting liver transplant. Currently, no effective treatment is available for these patients as the safety of using TRA in decompensated cirrhotics is not clear and the latest reports indicate a high risk of portal vein thrombosis [11,12]. Baseline WBC values also improved significantly post PSAE and remained within normal range after four weeks of treatment. It is unknown if this improvement in peripheral WBC can help decrease the rate of infection in these patients. This could be addressed in a larger prospective trial.
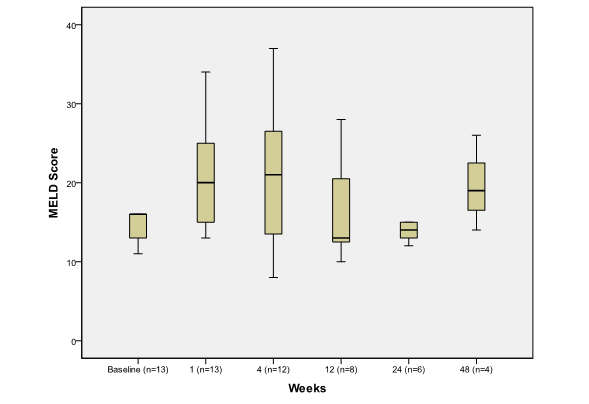
Within four weeks of the procedure, there was a trend towards increasing MELD score, which was reflective of mild increase in INR, total bilirubin and creatinine, as these are the variables that constitute the MELD equation. The effect of PSAE on MELD score was not significant after week 4. This could be attributed to the improvement in azotemia induced by contrast material used during the procedure. This is an important observation for patients waiting for liver transplantation, as the risk of worsening MELD score for a short period of time should be weighed against the benefit of the procedure.
PSAE resulted in multiple side effects and morbidity; however, the study showed a surprisingly good one-year survival rate (70%). We considered patients who received liver transplantation in the survival calculation because our cohort had higher than usual mortality from their liver decomposition regardless of the procedure. A Kaplan Meir curve could not be utilized because of the sample size. Four patients died in this series and surprisingly only two patients died within forty-eight weeks of the procedure. The major cause of death was sepsis. Since it is unethical to have a control group (no treatment) and there is no alternative treatment for PSAE in these patients, it is a matter of speculation to determine if PSAE was the independent factor for death in these patients. Furthermore, sepsis is the leading cause of death in patients with decompensated cirrhosis who do not have PSAE and who are waiting for liver transplantation.
One patient developed a splenic abscess that was treated successfully with antibiotics and percutaneous drainage. This complication did not affect his candidacy to receive liver transplantation. The low incidence of splenic abscess in this study is similar to the findings reported in other studies [10-22]. This could be attributed to adequate use of antibiotic prophylaxis and the diligent sterile technique that is used. Furthermore, the results from this study suggest that PSAE could be a reasonable tool to treat serious bleeding from portal hypertension in patients awaiting liver transplantation who failed to respond to, or who were not candidates to receive, conventional treatment.
The major limitation is that this is a retrospective uncontrolled study with small size sample. Overcoming this bias and limitation is challenging because most of these patients are managed by tertiary care and transplant centers, and the uncommonness of the procedure limits the ability to enroll large number of patients and have a control arm.
In conclusion, PSAE is an effective procedure in patients with Child class B and C cirrhosis who are awaiting liver transplant. It can be used successfully to treat profound thrombocytopenia and other refractory portal hypertension related complications before liver transplantation. PSAE can result in significant morbidity and its impact on the outcome of liver transplantation needs to be studied in a larger study. The selection of appropriate candidates in a multi-disciplinary transplant center, the performance of PSAE by experienced interventional radiologists following strict sterile technique, as well as thorough antibiotic prophylaxis, will minimize risks and improve outcomes. A multi-center prospective study is needed to determine the independent factors that predict survival after PSAE in patients with decompensated cirrhosis.
Conflict of Interest
None of the authors has conflict of interest that is related to the study or to the content of the manuscript.
RESULTS
Demographics and Laboratory
Nineteen patients underwent PSAE during this period of time were identified. Thirteen patients (8 females and 5 male) met the inclusion criteria and were included in the analysis. Six patients excluded due to 3 patients already had liver transplant prior to the procedure, 2 patients were not transplant candidates, and one patient was not cirrhotic. There was no patient lost to follow up. Mean follow up was 8 months. The mean age was 50.8± 7.9 years and BMI was 32.5 ± 1.68. Characteristics of patients are summarized in Table 1. Baseline platelets count was 33.4 ± 29.4 (103 /mm3 ). MELD and CPT score were 16.6 ± 6 and 9.5 ± 1.3 respectively. Spleen size was 19.9 4.5 cm. There were six patients with platelets count <20,000. Seven patients had Child class C cirrhosis and six had Child class B. All patients had detectable ascites on physical exam. The major reasons for PSAE were profound thrombocytopenia (6 patients), uncontrolled bleeding from portal hypertension gastropathy (4 patients), gastric variceal bleed (2 patients) and refractory hepatic encephalopathy due to splenorenal shunt (1 patient).
Table 1: Patients’ Demographics Data (n = 13 patients). Data are presented as mean ± SD. HCV: Hepatitis C Virus, ALD: Alcohol Liver Disease, NASH: Non-alcoholic Steatohepatitis, HBV: Hepatitis B Virus, MELD: Model For End Stage of Liver Disease, CPT: Child-Pugh-Turcotte. WBC: White Blood Cell, INR: International Normalized Ratio.
| Age (yr) | 50.8±7.9 |
| Male (n) | 5 |
| Female (n) | 8 |
| Race (n) Hispanic White Asian |
10 2 1 |
| Etiology of cirrhosis HCV HCV+ALD NASH HBV Budd-Chiari |
4 3 4 1 1 |
| MELD score | 16.6 ± 6 |
| CPT score | 9.5 ± 1.3 |
| WBC (103 /mm3 ) | 3.6 ± 3 |
| Hemoglobin (mg/dl) | 9 ± 1.8 |
| Platelets (103 /mm3 ) | 33.4 ± 29.4 |
| Sodium Na (mg/dl) | 137 ± 3.4 |
| Creatinine (mg/dl) | 1.4 ± 1.1 |
| Total Bilirubin (mg/dl) | 2.7 ± 3.1 |
| INR | 1.5 ± 0.3 |
Survival
One-year survival rate was 70 % (9 patients). Four patients received liver transplantation at 2, 4, 12 and 16 weeks after PSAE. Four patients died at 6, 28, 74, and 104 weeks after the procedure, and only one of them received a viable organ offer during these periods of time. All patients remained on the list for transplant and only one of them died from variceal hemorrhage shortly (6 weeks) after the procedure. The other three patients died from systemic infection.
Thrombocytopenia and Laboratory
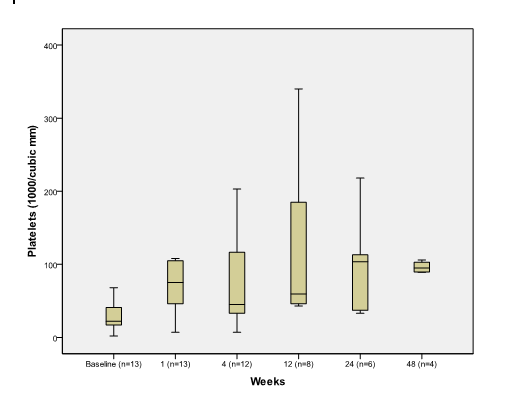
Median platelet count increased significantly one week after PSAE compared to baseline 75 (7, 290) vs. 22 (2, 110) (p=0.002) (Figure 1A).
Figure 1A. The effect of PSAE on Platelet
Boxplot depicting median and range of platelet counts (1000/mm3) to compare baseline to post procedure findings in week time interval (n = number of patients at each time interval).
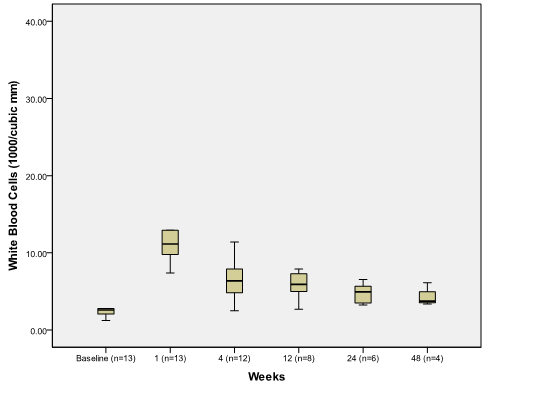
Platelets remained significantly elevated at 12 weeks 59.5 (43,340) compared to baseline (p=0.012). Median white blood cells (WBC) increased significantly from baseline 2.6 (1.2,10.4) to 11.1(2.74, 34.63) at week 1 (p=0.001) and returned to normal range 5 (2.5, 11.4) at week 4 (Figure 1B).
Figure 1B.
The effect of PSAE on white blood cell
Boxplot depicting median and range of white blood cell counts (1000/mm3) to compare baseline to post procedure findings in week time interval (n = number of patients at each time interval).
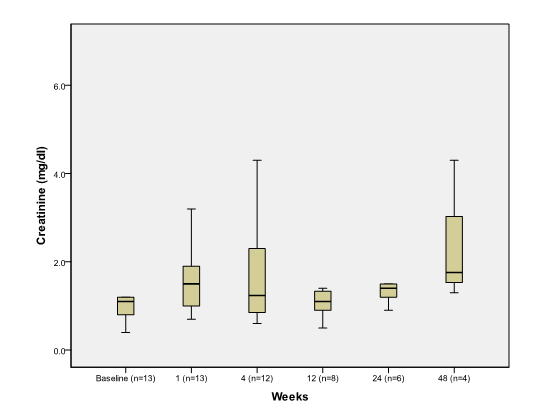
There was significant increase in serum creatinine during the first week after the procedure but became not statistically significant thereafter (Figure 1C).
Figure 1C.
The effect of PSAE on creatinine
Boxplot depicting median and range of creatinine values (mg/dl) to compare baseline to post procedure findings in week time interval (n = number of patients at each time interval).
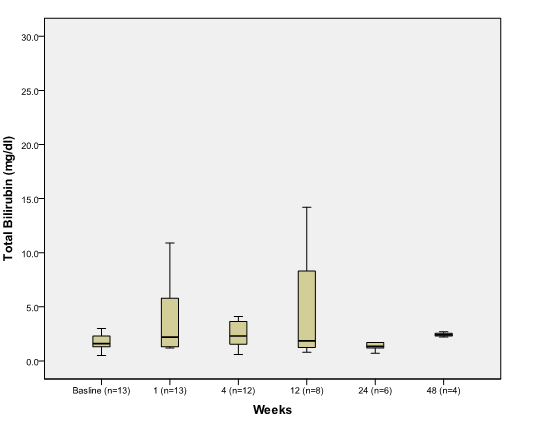
There was no significant difference between baseline and post PSAE in total bilirubin (Figure 1D),
Figure 1D.
The effect of PSAE on total bilirubin
Boxplot depicting median and range of total bilirubin values (mg/dl) to compare baseline to post procedure findings in week time interval (n = number of patients at each time interval).
hemoglobin, albumin, International Normalized Ratio (INR), and serum sodium.
Safety
MELD and CPT Scores: Median week one MELD score 20 (13,34) was significantly elevated compared to baseline MELD score of 16 (8,31) (p=0.003) (Figure 2).
Figure 2.
The effect of PSAE on MELD score
Boxplot depicting median and range of MELD Score (Model for End Stage Liver Disease) scores to compare baseline to post procedure findings in week time interval (n = number of patients at each time interval).
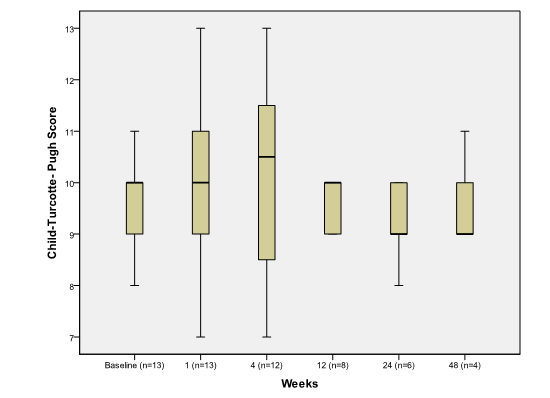
The major cause of elevation in MELD score was related to worsening azotemia during the first week post procedure. CPT score, however, remained unchanged when comparing pre and post embolization values (p>0.05) (Figure 3).
Figure 3.
The effect of PSAE on CPT score
Boxplot depicting median and range of child-turcotte-pugh scores to compare baseline to post procedure findings in week time interval (n = number of patients at each time interval).
Side effects and Complications: The most reported symptoms post PSAE were abdominal pain, low grade fever and nausea. These symptoms were reported by almost all patients and resolved by the time of discharge. Three patients developed left side pleural effusion as a response to splenic infarction. Effusions were successfully treated with diuretics. Two patients developed spontaneous bacterial peritonitis (SBP) and were treated effectively with antibiotics. One patient developed a splenic abscess, which was treated successfully with organism specific antibiotics as well as percutaneous drainage. Only one patient died from esophageal variceal bleeding six weeks after the procedure.
Four patients had worsening decompensation and underwent liver transplant within four months of PSAE. Of these patients, three are still alive and one patient died two years after transplantation from non-procedure related complications. None of the patients had worsening ascites or hepatic encephalopathy. Spleen size was stable within the first three months after the procedure; however, it decreased significantly by six and twelve months after the procedure (Table 2).
Table 2: Spleen Size Before and After PSAE: Size is measured in centimeter. Comparison is performed between spleen size at baseline and 3,6 and 12 months after PSAE. P<0.05* statistically significant.
| Time Interval | Mean ± SD (cm) | P value |
| Baseline (n=13) | 19.9±4.5 | |
| 3 Months (n=9) | 18.3±5 | 0.206 |
| 6 Months (n=6) | 14.8±4.8 | 0.0005* |
| 12 Months (n=3) | 15.3±5.5 | 0.002* |