Influence of a Commercially Available Probiotic Pill on Gut Microbiome and Stress Response in Healthy Human Subjects
- 1. Department of Computer Science, Northeastern University, USA
- 2. Department of Chemical and Biological Sciences, Youngstown State University, USA
ABSTRACT
Animal studies have shown that dietary probiotics can modify host stress response viathe gut-brain axis. A few studies with healthy human subjects have indicated that single strain fermented milk probiotic products can alter stress response. The aim of this study was to investigate whether consumption of commercially available multispecies probiotic pills could affect the gut microbiome and stress response in healthy humans. A treated group (ingested probiotic pills) and a control group (no probiotic pills) were subjected to an elevated height ropes course (EHRC) as stressor. Stress response was measured using ELISA of salivary cortisol. Fecal bacteria DNA was sequenced by Illumina. Questionnaires assessed perceived stress and gut health. Although bacterial community structure and diversity showed no changes over time, treated subjects had significantly increased abundance of Lactobacilli and Bifidobacteria compared to controls on day 15. These two different trends would not be observed in studies that use one or the other set of markers (species specific vs. general primers). Stress response to the EHRC showed no significant differences between treated and control groups, yet cortisol values in the treated group were slightly dampened relative to controls on day 15. Overall, this study indicated that probiotic pill had a slight but significant effect on probiotic bacteria abundance, but not gut bacteria community structure, stress, or gut health. We speculate that live yogurt cultures may be needed to substantially impact both gut community structure and stress response.
KEYWORDS
Lactobacillus, Bifidobacteria, Probiotic, Stress.
CITATION
Lisko DJ, Johnston GP, Johnston CG (2022) Influence of a Commercially Available Probiotic Pill on Gut Microbiome and Stress Response in Healthy Human Subjects. JSM Gastroenterol Hepatol 9(2): 1109.
INTRODUCTION
The community of microbes within the gastrointestinal tract (gut microbiome) contain a vast genetic and metabolic diversity interacting with each other and with their host. Through evolutionary time all animals, including humans and their gut microbes have evolved multiple complex co-dependencies needed for optimal health and nutritional outcomes. Numbers of bacteria within the human colon are roughly equivalent to the total number of cells in the human body [1]. Four phyla, the Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria make up to 97% of the bacteria detected by ribosomal DNA sequencing in the gut microbiome [2]. In human adults, species composition within the gut microbiome can remain relatively stable [3]. However, environmental factors such as host age, chemical exposure (e.g., pollutants, antibiotics), social, physical or physiological stressors, all can affect gut microbiome composition, community stability, and levels of potentially beneficial microbes [4,5]. In addition, it is now well known that diet is directly associated with different gut microbiome communities [6]. Changes in relative abundances of different phyla in the gut microbiome composition can impact microbiome function [7] and are sometimes associated with host health and/or dysbiosis leading to systemic inflammation which is associated with altered intestinal permeability [8], disruptive mental behaviors [9], colitis [10], diabetes [11], and prediabetes[12].
“Probiotic” microbes are living microbes that benefit host health when consumed. By definition, probiotics are safe for their host, resistant to acidity and bile acids, adhere and colonize the intestine [13]. In recent years, modification of the gut microbiome by probiotic supplementation has been explored as a mechanism for their potential to enhance health outcomes. Many different species of probiotics have been used, the most common belonging to the Lactobacillus and Bifid bacterium genera. Extensive research has been dedicated to gastrointestinal tract (GI) and metabolic disorders [14] mostly on populations associated with common syndromes and chronic diseases (e.g., Type 2 Diabetes, Irritable Bowel Syndrome, Crohn’s Disease). However, only a handful of studies have focused on healthy adults. In the face of an exploding market for probiotics there remains great uncertainty in the knowledge of how and indeed if probiotic products benefit consumer health outcomes. Environmental stimuli to the gut and dietary sources can also influence central nervous system (CNS) development and behavior [15]. The gut-brain axis, the bi- directional signaling between the CNS and GI tract, helps maintain optimal emotional conscious health [16]. Stress response to an environmental stressor (i.e., fear, anxiety, and illness), well documented in text-books, and is a cascade series involving the CNS and the hypothalamic pituitary adrenal (HPA) pathway leading to the systemic release of cortisol. This spike in cortisol level induces the “fight or flight response” usually manifested as increased heart rate, breathing rate, and other systemic alterations [17]. Stress is an essential survival mechanism to overcome immediate crises. However, long term frequent stress signaling, common in modern western culture, is detrimental to physical health and can lead to prolonged feelings of anxiety [18]. Human stress and the HPA pathway are commonly monitored by measuring cortisol in blood or saliva [19,20].
The gut microbiome has several potential pathways to modulate the gut-brain axis such as by passing microbial products through the blood system to interact with the endocrine system (which releases cortisol via the HPA axis), spinal nerves, the Vagus nerve, and the enteric nervous system (ENS) pathways [21]. This two-way multi-component communication system concept has been termed the microbiome gut brain (MGB) axis. Indeed, host physiological, psychological, immunological, and developmental functions are enmeshed with physical, genetic and metabolic expression of the host’s microbiome [22].
Research related to CNS responses and probiotic supplementation has been performed using rodents or humans affectedby CNS disordersand using specificprobioticformulations [21,23,24]. On the other hand, research related to CNS responses and probiotic supplementation in healthy rodents or humans is promising but limited. Administration of dietary probiotic Lactobacillus rhamnosus (JB-1) to “normal” and “healthy” mice resulted in less anxiety-like behavior and lower corticosterone levels [25] while healthy women with no gastrointestinal or psychiatric symptoms provided with fermented milk (containing:B. animalis ,Streptococcus thermophiles, Lactobacillus bulgaricus, and Lacto coccus lactis) for 4 weeks showed a significant effect on activity of brain regions that control central processing of emotion and sensation compared to the placebo group [26]. Another study on healthy individuals indicated that consumption of a formulated multiprobiotic (Bifid bacterium spp.and Lactobacillus and Lacto coccus) was associated with reduced self-reported feelings of sadness and aggressive thoughts [27]. Data from these few studies indicate that probiotics may provide potential therapeutic approaches to alleviate psychological and physical stress. However, there has not been any published reports of the effects of multispecies probiotic pill consumption on stress in healthy humans. Therefore, this study aimed to investigate the effects of a commercially available multi-species probiotic pill on1) gut microbiome community composition, 2) stress response to induced stress through analysis of salivary cortisol, 3) perceived stress and 4) bowel health. It was hypothesized that consuming probiotic pills would increase the abundance of probiotic species in the human GI tract; this in turn would impact gut health, attenuate morning cortisol production, and reduce measured stress (via salivary cortisol) and perceived stress (self-reported) following exposure to a stressor.
MATERIALS AND METHODS
Subjects
All subjects included in this study were volunteers that gave written informed consent approved by the Institutional Review Board (IRB) at Youngstown State University. Fifteen healthy individuals (males and females) between the ages of 18 to 56 were selected for the study. Individuals with irregular production of cortisol or on medication that alters adrenal function (i.e., anxiety medications or anti-inflammatory medications) were excluded from this study. Volunteer participants were divided in two groups: treatment group and control group.
Participants in both groups were asked to follow their normal diets throughout the entire study and to report any constipation, diarrhea, illness, or consumption of any antibiotics or other form of probiotics.
Experimental Design
The length of the study was 60 days. The treatment group was provided with a 30-day supply of commercially available probiotic pills containing Lactobacillus (acidophilus and rhamnosus) and Bifid bacterium (B. lactis, B. longum, B. breve, and B. bifidum) and were asked to consume 1 probiotic pill (109 CFUs per pill according to manufacturer) at the same time daily for 30 consecutive days. After the 30-day probiotic pill regime, the treatment group refrained from consuming the supplied probiotic pills, but still followed their regular eating habits for 30additional days. The control group did not consume the probiotic pill during the 60 days. To induce stress, all subjects were to perform the Elevated High Ropes Course (EHRC) (Figure 1)
Figure 1: Elevated Height Ropes Course (EHRC) at Youngstown State University gymnasium, used to induce stress to healthy subjects.
on days 0, 15, 30, and 60. The EHRC consisted of various obstacles located 6 meters above a gymnasium floor. Each subject was to perform this task to the best of their ability. In order to compensate for habituation, each subsequent performance on the EHRC was more extensive and challenging than the previous performance. A more challenging segment of the course was added each time.
Sample Collection
Fecal samples were collected on days 0, 15, 30 and 60. Each volunteer provided their fecal samples in sterile 90 mL containers (Therapak Co., Buford, GA, USA) and delivered them to the laboratory within 12 hours of collection on each sample day. Each subject collected their own saliva sample upon awakening in the morning on days 0, 15, 30 and 60. Saliva was also collected before performing the EHRC test, and 20 minutes after completing the EHRC test.Saliva was collected using the Salimetrics® Oral Swab (SOS) (Sal metrics, State College, PA USA). All samples were immediately processed and/or stored frozen for analyses.
Analyses
Viable Counts in Probiotic Pills: Serial dilutions were performed in triplicate using 1g of crushed probiotic pills, plated onBD LBS Agar (Lactobacillus Selection Agar-BD Diagnostic Systems, Heidelberg, Germany) to enumerate viable Lactobacillus and BSM-Agar (Bifid us Selective Medium Agar) (Sigma-Aldrich’s. Louis, MO, USA) to enumerate viable bifid bacteria. Plates were then incubated anaerobically using a GazPak EZ Pouch System (BD Technologies, Franklin Lakes, and NJ) at 37°C for 24 – 48 hr. for Lactobacillus and 72 hr. for bifid bacteria.
Blank dilution controls were included.
Microbiome Molecular Analyses:
• Extraction of Fecal Microbial DNA: Microbial DNA was isolated from 200-300 mg of feces using a modified bead beating method [28] to minimize DNA shearing for downstream applications. Extracted DNA was then purified using QIAamp DNA Stool Mini Kit (Qiagen, Valencia, Ca USA) and quantified using a Nano Drop (Thermo Scientific, Waltham, MA USA). Extracted microbial DNA was then normalized to 10 ng/ µl, aliquoted, and stored at -20°C until further analysis.
• PCR Amplification and Next Generation Sequencing of Microbial DNA: Fecal microbial DNA was cleaned with the Wizard DNA Clean-Up System (Promega, Madison, WI, USA) and normalized between OD260/280 = 1.8 and OD260/230 = 2 as the starting concentration. Samples were placed in ice and shipped to the Molecular and Cellular Imaging Center at the Ohio State University. Library preparation and Illumina MiSeq Sequencing was performed for bacteria ribosomal DNA, V4 -V5 region using primers (515 F 5’GAGTGCCAGCMGCCGCGGTAA 3’ and 806 R 5’ ACGGACTACHVGGGTWTCTAAT 3’) with 300 PErun cycles. In addition, normalized microbial genomic DNA was PCR amplified using genus specific primers for 16S rDNA Lac1 (5’-AGCAGTAGGGAATCTTCCA-3’) and Lac2 (5’-ATTYCACCGCTACACATG-3’) for Lactobacillus and primers G-Bifid-F (5’-CTCCTGGAAACGGGTGG-3’) and G-Bifid-R (5’-CGTGTTCTTCCCGATATCTACA-3’) for Bifid bacterium. Each 20 µl Creation contained: 0.04 µl of each primer (0.2u µM), 10 µl Go Taq Green Master Mix 1x (Promega, Madison, WI USA), 1 µl of genomic DNA (10ng/µl), and 8.2 µl of molecular grade water. PCR reactions were amplified using Px2 Thermocycler (ThermoScientific, Waltham, MA, USA) and gel electrophoresis was run to verify successful amplification of PCR products. Triplicate samples from separate PCR runs of adequate length were pooled and sent for analyses to Case Western University Genomics Core facility (Cleveland, OH) for NGS using Misses Illumina.
• Quantitative PCR of Lactobacillus 16s rDNA genes:Relative abundance of Lactobacillus genes was determined by quantitative PCR (qPCR) using primers Lac1 (5’-AGCAGTAGGGAATCTTCCA3’) and Lac2 (5’- ATTTCACCGCTACACATG-3’).Standard curves were constructed from DNA purified from Lactobacillus isolated from probiotic pills grown on selective media (LBS). Standards were constructed by performing serial dilutions of 10-3- 10-9 of Lactobacillus DNA. Real-time PCR was performed using the IQ™5 real-time detection system (Bio-Rad Inc., Hercules, CA, USA). The qPCR reaction was performed using a total volume of 25 μl of the following reagents: 12.5 μl of SYBR Green buffer (Qiagen, Valencia, CA, and USA), 9.0 μl of RNase free water, 25 μM of primer, and 25 ng of DNA. The reaction conditions for amplification were 95°C for 3 min, 40 cycles of 95°C for 10s and 55?C for 30s. To determine specificity of amplification, a melt curve was performed after the last cycle, which consisted of 81 cycles by slowly heating from 55°C to 95°C.
205 Measured Stress Using Salivary Cortisol:
levels were determined using the High Sensitive Salivary Enzyme Immunoassay Kit (Salimetrics, State College, PA USA) according to the manufacturer’s instructions.
Questionnaires
Perceived Stress Questionnaire: At each sample date, all subjects completed a perceived stress questionnaire (PSQ) [29]. The PSQ is an instrument for assessing stressful life events and the circumstances that can trigger disease-like symptoms that may alter one’s psychological state. The questionnaire consisted of 30 questions pertaining to stressful feelings and experiences that individuals may Have felt during a two-week period on a scale from 1 (almost never) to 4 (usually or very frequently). In addition, the PSQ included one single question related to feelings of perceived stress before the EHRC on a scale from 1 (minimal stress) to 10 (maximum stress) (SI-1). For calculations and comparison daily, perceived stress was calculated as the sum value from 30 questions (4 points maximum) divided by 120 to give a final value out of 1. Perceived stress before the EHRC data was divided by 10 to give a final value out of 1.
Bowel Health Questionnaire:
Each subject answered a Bowel Health Questionnaire which consisted of 11 questions, each sampling day (SI-2). This questionnaire was given to determine whether the probiotic pills affected self-reported gastrointestinal health-related functions (i.e., bowel movement, flatulence, etc.). Seven questions on a scale of 1 (almost never) to 4 (usually) were related to bowel related issues. Four questions asked subjects to record how frequently they were constipated, had diarrhea, passed a stool, or had taken a stool softener.
Statistical Analyses
Statistical analysis of cortisol concentrations was determined by a 4-parameter nonlinear regression analysis of collected EIA 450 nm absorbance readings using My Assays Inc. (www. myassays.com). Statistical analyses were performed using IBM SPSS Statistics 20 for Windows (IBM Corp., Armonk, NY, USA) and R package [30].
RESULTS AND DISCUSSION
Microbiome
Optimal interactions among different taxa in the gut microbiome can provide a balanced ecosystem of commensal bacteria essential for host health by metabolizing nutrients, synthesizing vitamins, maintaining intestinal barrier functions, and preventing invasion by pathogenic bacteria [7, 8]. Results from animal studies also indicate that dietary probiotic consumption can modulate stress [31, 24]. Thus, it is of interest to determine whether consumption of probiotics disrupts or alters the gut microbiome community structure and whether probiotic consumption can affect the including stress response in healthy human subjects. In this study the treated group received an average daily dose of 1.4x109 (n=3, SD =4.5x108) CFUs of Lactobacillus and 3.3x108 (n=3, SD=2.7x108) of Bifid bacteria from consumption of a single pill. Viable counts of other probiotic bacteria within the multispecies pill were not determined.
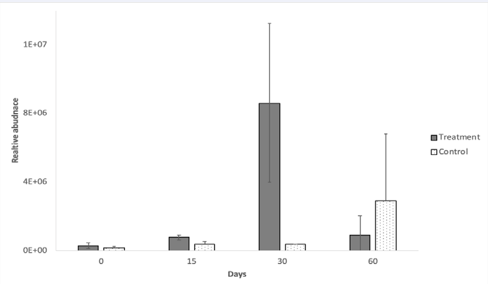
Consumption of the multispecies probiotic pill appeared to slightly impact relative abundance of probiotic bacteria. A clear indication was the higher relative abundance of Lactobacillus in the treatment group compared to the control group over time as measured by qPCR of amplicons generated from genus specific primers (Figure 2).
Figure 2: Average relative abundance of fecal Lactobacillus using qPCR in subjects who Consumed a probiotic pill daily for the first 30 days (treatment group, n=8), and in subjects who did not consume the probiotic pill (control group, n=7). Error bars represent standard deviation.
The highest relative abundance of Lactobacillus in the treatment group was found after 30 days of probiotic consumption, which dropped drastically after 30 days of no probiotic pill consumption (day 60)as expected if the probiotic pill consumption indeed supplemented the gut Lactobacillus population. The other indication that probiotic pill consumption affected the gut Lactobacillus population was from Illumina analyses of amplicons generated from genus specific primers.
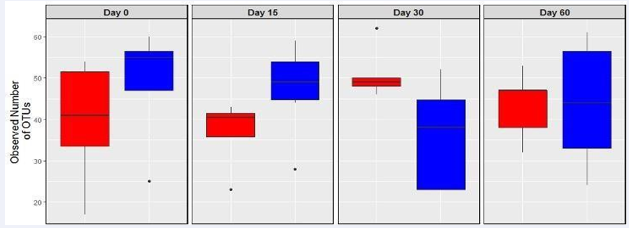
These data revealed that OTUs representing L. casein andL. rhamnosus OTUs were the most abundant Lactobacillus in the treatment group while OTU’s identified as an uncultured Lactobacillus were the most abundant in the control group. Illumina analyses of amplicons generated from genus specific primers also showed that consumption of the multispecies probiotic pill appeared to have an effect on abundance and diversity of bifid bacteria. Numbers of observed bifid bacteria OTUs were significantly higher in the treatment group compared to the controls at day 15 (Figure 3).
Figure 3: Number of unique OTUs belonging to the Bifidobacteria genus normalized to 10ng/ul fecal DNA from subjects who consumed a probiotic pill daily for the first 30 days (treatment group, n=8 in blue), and in subjects who did not consume the probiotic pill (control group, n=7 in red). Error bar represents standard deviation.
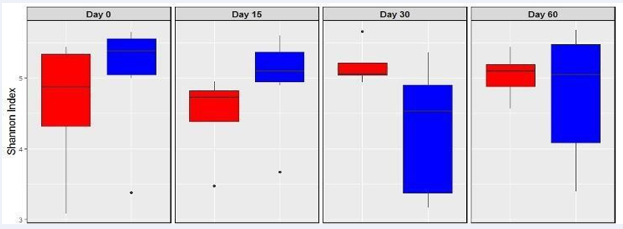
In the treatment group relative abundance of Bifid bacteria was represented mainly byB. longum and B. angulatum while in the control group relative abundance was mostly uncultured bifid bacterium and some B. longum and B. angulatum (data not shown). Illumina sequencing of genus specific primers revealed that Bifid bacteria diversity was lowest at day 30 in the experimental group compared to days 0, 15, and day 60 (after 30 days without probiotic pill) using two different statistical metrics (i.e., Chao1 and Shannon).
Shannon-Wiener index of bifid bacteria, was higher but not statistically different from the control on day 15 (Figure 4)
Figure 4: Shannon-Wiener diversity index of Bifidobacteria (based on Illumina sequences) from subjects who consumed a probiotic pill for the first 30 days (treatment group, n =8 in blue) and 673 for subjects who did not consume the probiotic pill (control group, n=7 in red). Error bar represents standard deviation.
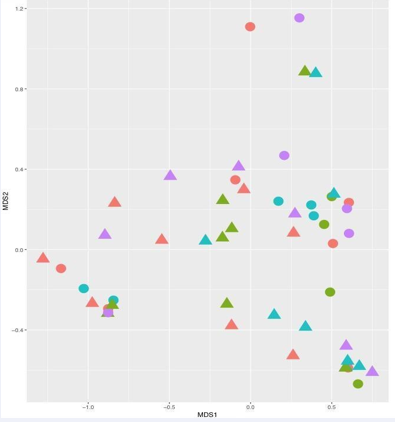
while Shannon diversity remained somewhat constant for subjects who did not consume the probiotic pill. NMDs analyses of Bifid bacteria OTU’s revealed closer clustering on day 15 (Figure 5).
Figure 5: Non-metric multidimensional scaling (MDS) showing distribution of Bifidobacteria (based on Illumina sequences) from subjects who consumed a probiotic pill for the first 30 days (treatment group =8) in triangles and for subjects who did not consume the probiotic pill (control group n=7) in circles. Sampling days are colored coded: day 0 red, day 15 green, day 30 686 blue and day 60 purple.
These findings suggest that probiotic pill consumption did affect both groups of probiotic bacteria in the gut microbiome.
CFU’s of Lactobacillus and Bifid bacteria were present in relatively high numbers in the probiotic pill (109 and 108 respectively) yet they were not detected in feces by Illuminasequencing of amplicons generated using V4-V5 rDNA primers for bacteria. Although present and functionally important as probiotic bacteria, Lactobacillus and Bifid bacteria were detected as amplicons generated using genus specific primers (discussed above). This may be explained by a relatively low abundance of Lactobacillus and Bifid bacteria in the gut bacteria community, as has been shown in other studies [32-34]. Another explanation is selective bias in the primers chosen (either in the general primers or in the genus specific primers), since DNA sequence amplification using different primer sets frequently result in different genera and Species detected [35].
Consumption of the multispecies probiotic pill did not affect overall gut microbiome community structure nor did microbiome diversity show any particular trend when examined using V4 -V5 general bacteria primers followed by Illumina sequencing. Thus, measured changes in Lactobacillus relative abundance and bifid bacteria species distribution were not reflected in the gut microbiome bacteria community structure with the methods used in this present study. These findings are in agreement with some other studies that have also indicated probiotics had no measurable change in human gut microbiome community structure. For example, probiotic consumption did not change the human host microbiome composition, although metabolic activity of the microbiota was altered [36]. Yet other studies [37] have indicated that, in contrast, probiotics can alter the gut microbiome community structure when human subjects consumed relatively large quantities of Greek yogurt.
Salivary cortisol
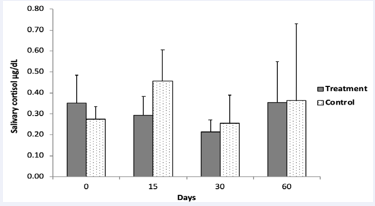
There were no statistical differences between the daily salivary cortisol levels upon awakening in the probiotic treatment group vs. the control group. However, average daily salivary cortisol upon awakening (µg/dL) in the treatment group decreased from 0.35 at day 0to 0.29 after 15 days, and to the lowest value of only 0.21 after 30 days and then returned to 0.35 at day 60 (Figure 6).
Figure 6: Average daily salivary cortisol measured by ELISA in subjects that consumed the probiotic pill daily for the first 30 days (treatment group, n=8) and subjects that did not (control 694 group, n=7). Error bar represents standard deviation.
In contrast, in the control group, salivary cortisol levels shifted greatly, especially from 0.27 at day 0 to 0.45 after 15 days and were higher than the treatment group. Lower cortisol over time in the treatment group compared to controls, with a rebound in cortisol levels on day 60 (after 30 days of no probiotic pill) indicated that perhaps probiotics affected waking stress levels, but differences were not statistically significant. High standard deviations could be due in part to variable time lapse upon awakening before sample collection, since it is known that even 30 minutes after waking can increase salivary cortisol levels [38]. In fact, salivary cortisol levels follow circadian fluctuation; concentrations in the morning are much higher than those in the evening [39]. Subjects were instructed to collect saliva samples immediately upon awakening, but compliance was not independently verified (such as by motion monitoring).
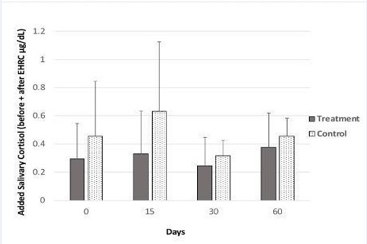
It was originally planned to calculate the induced stress response due to activity onv their using the differences between saliva cortisol levels (after-before stressor). However, some subjects showed higher levels prior to participating in the EHRC while other subjects showed higher cortisol levels following their participation in the EHRC activity. This was explained by some, but not all subjects stating that they experienced high stress just observing the EHRC while waiting and watching other participants prior to their actual activity on their. Thus, instead, salivary cortisol data obtained both before and after induced stress were evaluated as totals (before + after). Average values of salivary cortisol (both before plus after EHRC activity) were greater in the treatment group than in the control group on each sample day (Figure 7),
Figure 7: Salivary cortisol concentration in subjects that consumed the probiotic pill daily for the first 30 days (treatment group, n=8) and in subjects that did not (control group, n=7). Solid bars represent the sum of salivary cortisol sampled just before and 10 minutes after EHRC stressor. Error bars represent standard deviation.
with day 15 showing the greatest difference between the groups. However, variability was high and all differences
were not statistically significant. The finding of anticipatory stress in this present study is in agreement with findings from a previous study of induced stress in humans involving patients having dental extractions [40]. In that study 41% of the patients expressed more stress (higher cortisol concentrations) before the extraction while54% had higher cortisol concentrations 10 minutes after the extraction. Additionally, all patients had higher cortisol concentrations (both before and after) compared to a control group that did not undergo dental extraction. There is evidence in humans and other animals that dietary consumption of transient microbes can modulate brain chemistry and observed behavior, but in this present study, there were no clear indications that altering the resident microbiota affected the CNS response to stress. However, it is important to note that anxiety-prone organisms are usually more disposed to anxiety- like behaviors than “normo-anxious”ones [41] which could have been an additional underlying factor in this present study.
Perceived Stress
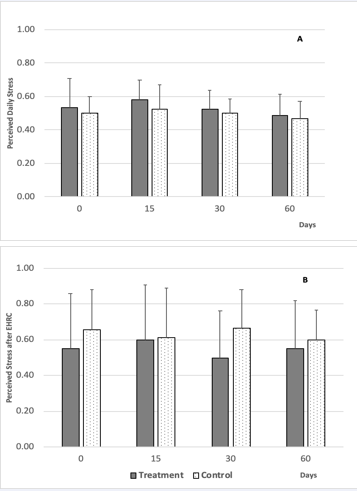
Perceived stress takes into account personal feelings and the impact of daily external conditions on one’s ability to manage and overcome stress [42]. Perceived stress is routinely measured via questionnaires as a scale [43], using easy to understand general questions. As shown in Figure 8,
Figure 8: Panel A: Average perceived daily stress in subjects that consumed the probiotic pill daily for the first 30 days (treatment group, n=8) and in subjects that did not (control group, n=7). Calculated values were from self-reported answers to a questionnaire (SI-1). Higher values represent more stress. Panel B: Average perceived stress calculated from self-reported answers to a questionnaire (SI-1) after subjects were exposed to the stressor (EHRC) in the treatment group and control group. Error bars represent standard deviation.
in the treatment group the lowest average relative reported perceived daily stress was found after 30 days of probiotic pill. No significant changes over time were observed. In the control group perceived daily stress did not change from day 0 to day 30 and no differences in reported values were statistically significant. The perceived stress questionnaire relied on recollection of stress-related feelings and experiences over the course of the previous week and poor recollection of each subject may have influenced the reported data. Average relative perceived stress following EHRC also showed no statistically significant differences with time nor between treatment and controls.
In this present study, consumption of multispecies probiotic pills did not affect relative daily perceived stress. Daily perceived stress did not correlate with morning salivary cortisol. However, the lowest perceived stress was recorded after 30 days of probiotic pill consumption which coincided with low cortisol measurements after the EHRC in the treatment group. Perhaps these two findings represented a small signal that may indicate an influence of probiotic pill consumption. In fact, conflicting findings from different studies on the effects of probiotics on perceived stress and stress response all use different probiotic regimes with very different groups of human subjects so perhaps it should not be surprising that the results also differ. For example, a study of healthy women receiving a multispecies probiotic treatment [44] showed similar results to this present study wherein probiotic consumption had no significant effect, compared to controls, on both self-reported stress and salivary cortisol concentrations following induced stress; yet the authors did find that their probiotic treatment provided stress-dependent beneficial effects on cognition. On the other hand, a different study showed that healthy women and men that consumed specifically probiotics formulated with L. helveticusand B. longum for 4 weeks, had slightly lower but significantly less self- reported perceived stress, anxiety and depression [22]. Similarly, daily consumption of L. gasser for 4 weeks improved anxiety, depressive mood and lowered salivary cortisol in healthy male medical students [21]. Metabolic functions vary within species and among strains within a species [45] of probiotic bacteria. Particular probiotic species and strains appear to affect human hosts differently depending on host genetics, immunity, and environmental exposures [46].
Bowel Health
Questionnaires addressing bowel symptoms are considered good indicators of bowel disease [47]. In this study, the bowel health questionnaire was general and did not ask for specific outcomes. Inter-individual variability in intensity, severity and fluctuation of digestive symptoms [48] over time was not included. Nevertheless, higher scores in the gut health questionnaire responses were considered indicatorsofpoorer intestinal health.
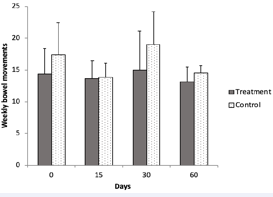
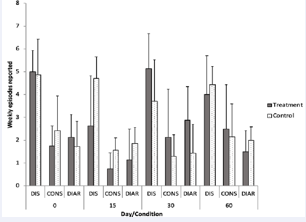
Overall weekly self-reported numbers of bowel movements during the length of the study, frequencies of diarrhea, and bowel discomfort Figure 9.
Figure 9: Weekly number of bowel movements in subjects that consumed the probiotic pill daily for the first 30 days (treatment group, n=8) and in subjects that did not (control group, n=7).Values were calculated from self-reported answers in a bowel health questionnaire (SI-2). Error bars represent standard deviation.
Figure 10.
Figure 10: Weekly number of bowel discomfort, constipation, and diarrhea episodes in subjects that consumed the probiotic pill daily for the first 30 days (treatment group, n=8) and in subjects that did not (control group, n=7). Values were calculated from self-reported answers in a bowel health questionnaire (SI-2). Error bars represent standard deviation.
respectively) were similar in both groups during the length of the study. Average frequency of both constipation, diarrhea and bowel discomfort was lowest after 15 days of treatment, compared to initial and later values in the treatment group, suggesting that perhaps probiotics may have provided a mild effect on bowel regularity. A systematic review of trials [49] indicated that effects of supplementation of probiotics on stool frequency and bowel transit were too heterogeneous to measure positive outcomes (e.g., less constipation). In addition, a double-blind placebo study revealed that healthy adults suffering constipation did not benefit from probiotic supplementation [50].
CONCLUSIONS
There remain major gaps in our knowledge regarding how the brain receives signals from gut bacteria. Exact mechanisms of if and how intestinal microbes affect host CNS function and behavior are complex and remain largely unknown. Moreover, research on probiotic consumption has relied mostly on animal studies, which should be extrapolated to humans only with caution. Multiple studies suggest that the yogurt/cultured milk matrix can have an important supplemental effect on the host gut microbial community resulting in beneficial health outcomes. Indeed, the nutritional matrix provided in fermented products may play an important role supporting growth and activity of probiotic bacteria in the gut providing fiber-rich or milk- associated oligosaccharides that are preferentially metabolized by certain gut microbes.
However, most probiotic research has utilized specialized laboratory formulations and has not studied current commercially probiotic pills available to any consumer who believes unverified health claims. Since this study did not take
placebo effect into account, discounting placebo effect would imply that probiotics within the pill would have even less effect on reported outcomes. Although consumption of a commercially widely available probiotic pill did show a slight significant effect on Lactobacillus, probiotic pill consumption showed no effect on human gut microbial community diversity, measured stress, stress response, perceived stressor self-reported bowel health.
Certainly, more research is needed in healthy human subjects to determine whether particular dietary probiotic strains or mixes, with or without prebiotics, in a fermentation media such as yogurt or in pill form can affect host stress response or augment host health in any way. Different environmental factors (nutrition, contaminant exposure, external stressors), host factors (epigenetics, genetics, immunity, health history), and gut microbiome composition all need to be studied to determine if/ how particular probiotic bacteria impact human stress response. Aspects of microbiology, bioinformatics, and neuroscience all must be integrated to elucidate interactions between multiple complex systems underlying the MGB. Lastly, much more basic research is needed before reliable personalized dietary probiotics could be offered to allow consumers to achieve particular health outcomes.
LIMITATIONS OF THE STUDY
We acknowledge the need to be cautious in interpreting the results as a consequence of the probiotic consumption pill. Placebo controls were not included since we did not have the means to provide a sterile pill in the same form as the probiotic pill. Participants were allowed to decide their wake-up time. Thus, time of daily salivary cortisol measurement was neither monitored nor enforced. In addition, anxiety-prone individuals were not determined ahead of the study. More importantly, this current study was indeed limited by the number of human subjects and number of samples provided. It was extremely difficult to recruit volunteers.
Similar studies would benefit if conducted with more subjects to provide clearer signals relating to probiotic pill consumption, gut microbial community changes, stress, and cortisol. No culturing of Lactobacilli or Bifid bacteria species recovered from fecal material were conducted in this study since investigators thought to minimize potential health risk to undergraduate research students involved in the project.
AUTHOR CONTRIBUTIONS
Conceptualization, C. G. J. and G. P. J.; methodology, C. G. J and G. P. J.; validation, D. J. L. and G. P. J.; formal analysis, D. J. L. and G. P. J.; investigation, D. J. L.; resources, C. G. J.; data curation, D. J. L. and G. P. J; writing-original draft preparation, C. G. J. and G. P. J.; writing-review and editing, C. G. J. and G. P. J.; project administration, C. G. J.; visualization, D. J. L. and G. P. J.; supervision, C. G. J. and G. P. J. All authors have read and agreed to the published version of the manuscript.
INSTITUTIONAL REVIEW BOARD STATEMENT
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Youngstown State University (Reference protocol #044- 15 approved).
INFORMED CONSENT STATEMENT
Informed consent was obtained from all subjects involved in the study.
DATA AVAILABILITY STATEMENT
Samples will be deposited in NCBI Genbank upon acceptance.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
SUPPLEMENTAL INFORMATION
Perceived Stress and Bowel Health Questionnaires are submitted as supplemental materials.
ACKNOWLEDGEMENTS
We thank Youngstown State University for providing financial support. Thanks to Mr. Ed Budde and Mr. Justin Waldern for their laboratory technical assistance.
ACKNOWLEDGEMENTS
We thank Youngstown State University for providing financial support. Thanks to Mr. Ed Budde and Mr. Justin Waldern for their laboratory technical assistance.
REFERENCES
- Sender R, Fuchs R, Milo R. Revised estimates for the number ofhuman and bacteria cells in the body. PLoS Biol. 2016; 14: e1002533.
- Khanna S, Tosh P. A clinician’s primer on the role of the microbiome in human health and disease. Mayo Clin Proc. 2014; 89: 107-114.
- Jeremiah J Faith, Janaki L Guruge, Mark Charbonneau, Sathish Subramanian, Henning Seedorf, Andrew L Goodman, et al. The long- term stability of the human gut microbiota. Science. 2013; 341: 12377439.
- De Palma G, Collins S, Bercik P, Verdu E. The microbiota-gut–brain axis in gastrointestinal disorders: stressed bugs, stressed brain or both? J Physiol. 2014; 592: 2989–97.
- Jeffrey D Galley, Michael C Nelson, Zhongtang Yu, Scot E Dowd, Jens Walter, Purnima S Kumar, et al. Exposure to a social stressor disrupts the community structure of the colonic mucosa-associated microbiota. BMC Microbiol. 2014; 14: 189.
- Hannelore Daniel, Amin Moghaddas Gholami, David Berry, Charles Desmarchelier, Hannes Hahne, Gunnar Loh, et al. High-fat diet alters gut microbiota physiology in mice. ISME J. 2014; 8: 295-308.
- Albenberg L,Wu G. Diet and the intestinal microbiome: associations, functions, and implications for health and disease. Gastroenterology. 2014; 146: 1564–1572.
- Shin, N, Whon T, Bae T. Proteobacteria: microbial signature ofdysbiosis in gut microbiota. Trends Biotechnol. 2015; 33: 496–503.
- Alexandra C Vaughn, Erin M Cooper, Patricia M DiLorenzo, Levi J O’Loughlin, Michael E Konkel, James H Peters, et al. Energy-dense diet triggers changes in gut microbiota, reorganization of gut brain vagal communication and increases body fat accumulation. Acta Neurobiol Exp (Wars). 2017; 77: 18–30.
- Thirumahal Selvanantham, Qiaochu Lin, Cynthia Xinyi Guo, Anuradha Surendra , Stephanie Fieve, Nichole K Escalante, et al. NKT cell-deficient mice harbor an altered microbiota that fuels intestinal inflammation during chemically induced colitis. J Immunol. 2016; 197: 4464–72.
- Tanusree Sen, Carolina R Cawthon, Benjamin Thomas Ihde, Andras Hajnal, Patricia M DiLorenzo, Claire B de La Serre , et al. Diet-driven microbiota dysbiosis is associated with vagal remodeling and obesity. Physiol Behav. 2017; 173: 305–317.
- Stacey M Lambeth, Trechelle Carson, Janae Lowe, Thiruvarangan Ramaraj, Jonathan W Leff, Li Luo, Callum J Bell, et al. Composition, diversity and abundance of gut microbiome in prediabetes and type 2 Diabetes. J Diabetes Obes. 2015; 2: 1-7.
- Konstantinos Papadimitriou, Georgia Zoumpopoulou, Benoit Foligné, Voula Alexandraki, Maria Kazou, Bruno Pot, et al. Discovering probiotic microorganisms: in vitro, in vivo genetic and omics approaches. Front Microbiol. 2015; 6: 58.
- Rasnik K Singh, Hsin-Wen Chang, Di Yan, Kristina M Lee, Derya Ucmak, Kirsten Wong, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017; 15: 1-17.
- Colpitts S, Kasper L. Influence of the gut microbiome on autoimmunityin the central nervous system. J Immunol. 2017; 198: 596-604.
- Chen, X, D’Souza R, Hong, S. The role of gut microbiota in the gut- brain axis: current challenges and perspectives. Protein Cell. 2013; 4: 403-14.
- Smith S, Vale W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006; 8: 383–395.
- Dhabhar F. The short-term stress response – Mother nature’s mechanism for enhancing protection and performance under conditions of threat, challenge, and opportunity. Front Neuroendocrinol. 2018; 49: 175-192.
- Daisuke Sawada, Tomoko Kawai, Kensei Nishida, Yuki Kuwano, Shigeru Fujiwara, Kazuhito Rokutan, et al. Daily intake of Lactobacillus gasseri CP2305 improves mental, physical, and sleep quality among Japanese medical students enrolled in a cadaver dissection course. J Functional Foods. 2017; 31: 188-197.
- Michaël Messaoudi, Robert Lalonde, Nicolas Violle, Hervé Javelot, Didier Desor, Amine Nejdi, Jean-François Bisson, et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr. 2011; 105: 755–64.
- Cryan, J.; Dinan, T. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012; 13: 701-712.
- Gilbert S, Sapp J, Tauber A. A symbiotic view of life: we have neverbeen individuals. Q Rev Biol. 2012; 87: 325-341.
- Grenham S, Clarke G, Cryan J, Dinan T. Brain-gut-microbe communication in health 568 and disease. Front Physiol. 2011; 2: 94.
- A Venket Rao, Alison C Bested, Tracey M Beaulne, Martin A Katzman, Christina Iorio, John M Berardi, et al. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathog. 2009; 1: 6.
- Javier A Bravo, Paul Forsythe, Marianne V Chew, Emily Escaravage, Hélène M Savignac, Timothy G Dinan, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. PNAS. 2011; 108: 16050- 16055.
- Tillisch K, Labus, J, Kilpatrick L, Jiang Z, Stains, J, Ebrat B, et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013; 44: 1394–401.
- Steenbergen L, Sellaro R, van Hemert S, Bosch J, Colzato L. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav Immun. 2015; 48: 258-64.
- Yu Z, Morrison M. Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques 2004; 36: 808- 812.
- Levenstein, S, Prantera, C, Varvo, V, Scribano, M, Berto, E, Luzi, C, Andreoli A. Development of the Perceived Stress Questionnaire: a new tool for psychosomatic research. J Psychosom Res. 1993; 37: 19-32.
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2013.
- Barreau F, Ferrier L, Fioramonti J, Bueno L. Neonatal maternal deprivation triggers long term alterations in colonic epithelial barrier and mucosal immunity in rats. 2011; 53: 501-506.
- Arboleya, S, Watking C, Stanton, C, Ross, P. Gut Bifidobacteria populations in human health and aging. Front Microbiol. 2016; 7: 2014.
- Odamaki T, Kato, K, Sugahara H, Hashikura, N, Takahashi, S, Xiao J Z, et al. Age- related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol. 2016; 16: 90.
- Gueimonde M, Debor L, Tölkkö S, Jokisalo E, and Salminen S. Quantitative assessment of faecal bifidobacterial populations by real-time PCR using lanthanide probes. JAppl Microbiol. 2007; 102: 1116–1122.
- Fiona Fouhy, Adam G Clooney, Catherine Stanton, Marcus J Claesson, Paul D Cotter, et al. 16S rRNA gene sequencing of mock microbial populations- impact of DNA extraction method, primer choice and sequencing platform. BMC Microbiol. 2016; 16: 123.
- McNulty N, Yatsunenko T, Hsiao A, Faith J, Muegge B, Goodman A, et al. The impact of a consortium of fermented milk strains on the gut microbiome of gnotobiotic mice and monozygotic twins. Sci Transl Med. 2011; 3: 106.
- Lisko D, Johnston, G, Johnston C. Effects of dietary yogurt on the healthy human gastrointestinal (GI) microbiome. Microorganisms. 2017; 5: 6.
- Il-Young Shin, Ryun-Sup Ahn, Sae-Il Chun, Young-Jin Lee, Min-Soo Kim, Chea-Kwan Lee, Simon Sung, et al. Cortisol awakening response and nighttime salivary cortisol levels in healthy working Korean subjects. Yonsei Med J. 2011; 52: 435-444.
- Sjörs A, Ljung T, Jonsdottir I. Long-term follow-up of cortisol awakening response in patients treated for stress-related exhaustion. BMJ Open. 2012; 2: 1-8.
- Umeanuka, O, Saheeb, B, Uguru, C, Chukwuneke F. Evaluation of cortisol concentrations in saliva as a measure of stress in patients having routine dental extractions. Br J Oral Maxillofac Surg. 2015; 53: 557-560.
- Nam H, Clinton S, Jackson, N, Kerman I. Learned helplessness and social avoidance in 616 the Wistar-Kyoto rat. Front Behav Neurosci. 2014; 8: 109.
- Phillips A C. Perceived stress. In Encyclopedia of behavioral medicine; Gellman, M., Turner, J. Eds.; Springer, New York, USA, 2013; 1453-1454.
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Social Behavior, 1983; 24: 385–396.
- Papalini S, Michels F, Kohn N, Wegman N, van Hemert S, Roelofs K, et al. Stress matters: Randomized controlled trial on the effect of probiotics on neurocognition. Neurobiol Stress. 2016; 10: 100141.
- Lima-Ojeda JI. Am I and My Bacterial Circumstances: Linking Gut Microbiome, Neurodevelopment, and Depression. Front Psychiatry. 2017; 8: 153.
- Theresa L Montgomery, Axel Künstner , Josephine J Kennedy, Qian Fang, Lori Asarian, et al. Interactions between host genetics and gut microbiota determine susceptibility to CNS autoimmunity. Proc Natl Acad Sci U S A. 2020; 117: 27516-27527.
- Adelstein B, Irwig L, Macaskill P, Katelaris P, Jones D, Bokey L. A self-administeredreliable questionnaire to assess lower bowel symptoms. BMC Gastroenterol. 2008; 8: 8.
- Fernando Azpiroz, Denis Guyonnet, Yves Donazzolo, David Gendre, Jérôme Tanguy, Francisco Guarner, et al. Digestive symptoms in healthy people and subjects with irritable bowel syndrome: validation of symptom frequency questionnaire. J Clin Gastroenterol. 2015; 49: 64-70.
- Miller LE, Ouwehand AC, Ibarra A. Effects of probiotic-containing products on stoolfrequency and intestinal transit in constipated adults: systematic review and meta-analysis of randomized controlled trials. Ann Gastroenterol. 2017; 30: 629-639.