The Role and Mechanism of Pyroptosis in Abdominal Aortic Aneurysm
- 1. Department of Thoracic and Cardiovascular Surgery, Nanjing First Hospital, Nanjing Medical University, China
ABSTRACT
Abdominal aortic aneurysm (AAA) is a life-threatening disease associated with chronic inflammation in the vascular wall while its specific pathogenesis is not fully understood. Recently, a growing number of studies have indicated that pyroptosis, which is a pro-inflammatory kind of programmed cell death, might play a vital role in AAA. In this review, we first summarize the role of pyroptosis in AAA progression by not only providing a literature review on the expression changes of NLRP3 inflammasome components and effector mediators in clinical and experimental AAAs, but also discussing the effects of genetic defects or pharmacological inhibition of NLRP3 inflammasome components on experimental AAAs. Next, we introduce the mechanism of canonical and non-canonical pathway of pyroptosis and its activation and execution process. Finally, we discuss several pyroptosis-related drug targets for treating AAA by inhibiting the assembly of NLRP3 inflammasome and its effector mediators. In conclusion, we believe that pyroptosis might be a new treatment target of AAA.
KEYWORDS
Abdominal aortic aneurysms, Pyroptosis, Inflammation, Drug targets.
CITATION
Chen X (2022) The Role and Mechanism of Pyroptosis in Abdominal Aortic Aneurysm. JSM Gastroenterol Hepatol 9(1): 1104.
INTRODUCTION
In recent years, abdominal aortic aneurysm (AAA) has become a severe threat to public health with smoking, male sex, age (>60), family history and other cardiovascular diseases being possible risk factors for its development [1,2]. The aortic wall is composed of endothelial cells (ECs), vascular smooth muscle cells (VSMCs), fibroblasts and extracellular matrix (ECM) proteins and is divided into three layers: intima, media and adventitia [3]. Previous studies revealed that AAA is a chronic vascular complication with pathological features of VSMCs depletion [4], accelerated ECM degradation [5], activation of the renin–angiotensin system (RAS)[6-8], accumulation of reactive oxygen species (ROS)[9] and infiltration of inflammatory cells [10], which lead to weakening of the aortic wall. However, although inflammation and cell death (CD) are key factors in the development of this disease, the specific regulatory mechanism remains unclear. Currently, except for risky open surgery or endovascular intervention, there is no effective drug therapy to prevent the progression of AAA [11].
For many years, atherosclerosis was thought to be a major cause of the progression of AAA. However, in recent decades more and more evidence has supported the notion that CD and sterile inflammation across the tunica media/adventitia junction contributes to the pathological process of AAA in pivotal ways [12,13]. In this review, we summarize the current knowledge of pyroptosis, which is a pro-inflammatory kind of programmed CD and its role in AAA progression and treatment.
Cell Death (CD)
CD is of great importance in maintaining homeostasis and normal physiological functions and its changes are vital in the pathology of various kinds of diseases. Until now increasing forms of CD have been defined according to different characteristics of morphology and biological changes [14]. Basically, CDs can be divided into programmed cell death (PCD) and non-programmed cell death (NPCD) with apoptosis and necrosis being typical representatives of both. Apoptosis is an autonomous and ordered process of CD only influencing single cell without any inflammatory response morphologically characterized by cell shrinkage and integrated cell membrane [15]. On the contrary, necrosis is a passive process of CD that affects a large area of adjacent cells and often occurs with a marked inflammatory response, morphologically characterized by cell swelling and membrane rupture [16]. With the deepening of research, more and more forms of CD have been discovered, such as pyroptosis, autophagy, necroptosis and ferroptosis [17-20]. (Table 1)
Table 1: Differences among various types of cell death.
|
|
Apoptosis |
pyroptosis |
autophagy |
ferroptosis |
necroptosis |
necrosis |
|
Type |
programmed cell death |
programmed cell death |
programmed cell death |
programmed cell death |
programmed cell death |
non-programmed cell death |
|
Predisposing factors |
gene regulation |
pathological stimulus |
nutrient deprivation, oxidative stress and protein aggregates |
overwhelming lipid peroxidation causing complete cell failure |
the stimulation of TNFR1, TLRs and certain other receptors |
overwhelming stimuli from outside the cell |
|
Morphology |
cell shrinkage, plasma membrane blebbing with integrity, nuclear fragmentation, formation of apoptotic bodies and phagocytosis by neighbouring cells |
cell swelling, rupture of plasma membrane and release of cell contents |
autophagosome formation, vacuolization of the cytoplasm, no chromatin condensation |
mitochondrial volume shrinkage, increased density and rupture of mitochondrial membrane but with normal nucleus |
cell swelling, loss of plasma membrane integrity, swelling of cytoplasmic organelles. |
plasma membrane rupture, swelling of cytoplasmic organelles, lack of inter-nucleosomal DNA fragmentation |
|
Mechanism |
caspase family proteins (caspase-3,6, and 7) |
caspase-1, caspase-11/4/5, caspase-3, caspase-8, gasdermin family proteins |
ATGs |
GPX4, System Xc |
RIPK1, RIPK3, MLKL |
— |
|
Peripheral reaction |
no inflammatory response |
inflammatory response |
no inflammatory response |
inflammatory response |
inflammatory response |
inflammatory response |
Although pyroptosis shares several features with apoptosis, such as chromatin condensation and annexin V positive staining, there are some differences in detail. Compared with apoptosis, pyroptosis is often accompanied with an inflammatory response and the nucleus remains intact without karyorrhexis. Apart from that, cells tend to swell in pyroptotic cells due to pore formation on cell membrane but shrink in apoptosis [21]. Moreover, the caspase involved in apoptosis and pyroptosis is different. Apoptosis-related caspases mainly include caspase-2,8,9,10 in the
initiation process and caspase-3,6,7 in the execution process [22] while caspases participating in pyroptosis include caspase-1 in the canonical inflammasome pathway and caspase-4/5/11 in the non-canonical inflammasome pathway [23]. Recently, a growing number of studies have indicated the specific role of pyroptosis and related inflammasome activation in AAA development.
Pyroptosis in AAA
Previous studies have shown that pyroptosis plays a vital role in the progression of AAA by promoting the destruction of the elastic layer of the vascular wall [24]. A study found that the mRNA levels for NLRP3, caspase-1 and IL-1β in circulating blood leukocytes were elevated in AAA patients compared with non- AAA patients while the protein levels for NLRP3 indicated the opposite result [25]. Another study also found increased protein levels of caspase-1 and mRNA levels of NLRP3 in the abdominal aorta of AAA patients and this increase was also proved by immunohistochemical staining of NLRP3 and caspase-1 in the following study [26,27]. In experimental AAAs induced by Ang II or calcium chloride, NLRP3 and caspase-1 were also highly expressed in the AAA group than in the WT group [28]. As for the in vitro study, VSMCs from patients with AAA showed increased ability to express NLRP3 and caspase-1 under the stimulation of IFN-γ [29].
To further analyze the role of pyroptosis in AAA pathogenesis, we summarize several studies using mice with genetic knockout of NLRP3, caspase-1 or apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC). In the Ang II- infused ApoE -/- mice, we found that depletion of NLRP3, ASC, or caspase-1 in mice alleviated the severity of AAA and reduced the infiltration of inflammatory cells in the aortic wall with decreased serum levels of IL-1β, MMP2, MMP9 [30]. In addition, application of siRNAs-mediated NLRP3 silencing to aortic wall could also attenuate AAA formation and reduce aortic MMP9 activity in
calcium chloride-induced AAA model [31]. Except the gene knockout method, specific inhibitors were also used in exploring influence of pyroptosis on experimental AAAs. Treatment with MCC950, a selective NLRP3-inflammasome inhibitor, could prevent aortic aneurysms and dissections by inhibiting NLRP3–caspase-1 inflammasome activation and diminishing the N-terminal cleavage of MMP-9 in Ang II-infused WT mice with a high-fat, high-cholesterol diet [28]. Apart from that, Q-Vd-OPh, a pan-caspase inhibitor, was also found to inhibit the enlargement of abdominal artery and reduce AAA incidence in Ang II-infused ApoE -/- mice by alleviating aortic macrophage infiltration and apoptosis of smooth muscle cell [32]. Moreover, administration of many anti-diabetic drugs, including glyburide, glucagon-like peptide receptor agonists and sitagliptin have also been proved to suppress AAA formation by decreasing ROS production and inflammatory response [33-36].
As the effect mediator of pyroptosis, IL-1β and IL-18 also participate in the progress of AAA. In clinical AAAs, serum IL-1β levels were higher in AAA patients than healthy controls, as well as its mRNA and protein levels in aortic tissues [25]. Similarly, the mRNA and protein levels of IL-18 in the aorta of patients with AAA were also increased compared with non-aneurysm controls [37]. In IL-1β knockout mice or mice treated with anakinra, an IL-1 receptor blocker, we found reduction of aortic dilatation and less macrophage and neutrophil accumulation after intra- aortic infusion of elastase [38]. In Ang II- infused mice with treatment of β-aminopropionitrile, genetic depletion of IL-18 lowered AAA incidence and the maximum diameter of AAA by reducing aortic CD68-positive macrophage infiltration. This mechanism was further illustrated that IL-18 deficiency caused a switch of macrophage from M1 phenotype associated with pro- inflammatory response toward M2 phenotype associated with anti-inflammatory response in aneurysmal aorta [39].
Mechanism of pyroptosis
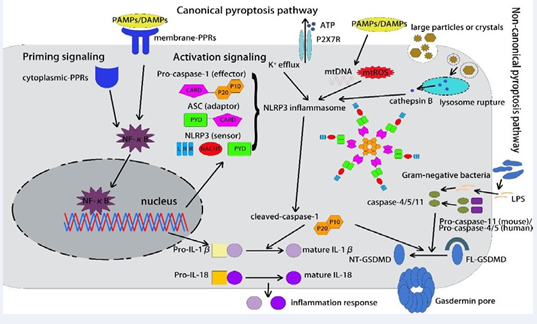
Pyroptosis can be initiated by the activation of inflammasomes, including NLRP3 inflammasome, AIM2 inflammasome and pyrin inflammasome, which act as molecular signal platforms leading to CD and inflammatory response [40]. The NLRP3 inflammasome comprises three parts: NLRP3, pro-caspase-1 and ASC [41]. Upon triggered by pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), the pyrin domain in NLRP3 combines with the pyrin domain in ASC, which in turn binds to pro-caspase-1 through a card-card interaction, resulting in the assembly of NLRP3 inflammasome [42]. Basically, pyroptosis is mainly divided into the canonical pathway depending on caspase-1 and the non-canonical pathway depending on caspase-4,5 in human or caspase-11 in mice. Apart from that, recent studies have found that caspase-3 and caspase-8 could also induce pyroptosis via gasdermin E (GSDME) and gasdermin D (GSDMD) respectively. In the canonical pathway, PRRs recognize pathogenic stimuli and bind to pro- caspase-1, triggering the assembly of a multi-protein complex, further activating the auto-cleavage of pro-caspase-1 to initiate pyroptosis [43]. In the non-canonical pathway, caspase-11 in mice and caspase-4 and caspase-5 in human could recognize the lipopolysaccharide (LPS) component of Gram-negative bacterial to initiate pyroptosis [44]. Once inflammatory caspases are activated, IL-1β and IL-18 are produced as precursor proteins without biological activity and are cleaved as bioactive cytokines prior to secretion [45]. The secretion and maturation of IL-1β and IL-18 are essential for the cleavage of GSDMD, which is the executor of pyroptosis [46], leading to formation of membrane pore and secretion of inflammatory mediators to trigger pyroptosis [47].
Recent studies have shown that gasdermin family proteins are direct executors of pyroptosis, including GSDMD and GSDME [46]. The GSDMD protein can be divided into a lipophilic N-terminal domain and a hydrophilic C-terminal domain [48]. N-terminal GSDMD shows a lipid-binding preference and thus binds with the lipid components of plasma membrane and intracellular organelle membranes, leading to its oligomerization. Then nonselective pores are formed, resulting in the afflux of cytokines, ions and free extracellular water [49]. The cells swell and rupture rapidly, causing the release of cell contents and thus inflammatory response. Apart from GSDMD, GSDME can also be cleaved into N-terminal and C-terminal fragments by caspase-3 and the N-terminal fragment of GSDME is similar to that of GSDMD [50]. Caspase-3 was considered to be a vital activator of apoptosis previously and now proved to also participate in the process of pyroptosis by cleaving and activating GSDME [51].
NLRP3 inflammasome activation requires a two-step process: priming signaling through pattern recognition receptors (PRRs) to activate NF-κB pathway, leading to up-regulation of transcriptional responses to pro-inflammatory mediators and activation signaling to form a molecular platform triggering the assembly of NLRP3 inflammasome. Generally, there are three types of NLRP3 activators: ROS, lysosome rupture and ion efflux [40,52,53]. ROS is considered to be the most important mechanism among these three activators, which mainly derived from the byproducts of oxygen metabolism in the electron transport
chain of mitochondria [54]. Under physiological conditions, the production and clearance of ROS in vivo are in equilibrium. However, this balance could be broken when electrons escaping from the electron transport chain under mitochondrial dysfunction, resulting in the accumulation of excessive ROS [55]. Apart from that, ATP-induced mtROS production could lead to the synthesis of oxidative mtDNA and mitochondrial dysfunction, which has also been reported to promote activation of NLRP3 inflammasome [56]. In addition to ROS, lysosome membrane rupture and release of lysosomal protease cathepsin B caused by phagocytosis of large particles or crystals also lead to NLRP3 activation [57]. Cathepsins, particularly cathepsin B, have been reported to be involved in the NLRP3 inflammasome activation by using CA-074-Me, the mostly used cathepsin B-specific inhibitor which was reported to significantly inhibit NLRP3 inflammasome activation in microvascular ECs under the stimulation of cell wall fragments [58]. In addition, ion flux, especially K+ efflux, was reported to serve as triggers in NLRP3 inflammasome activation. K+ efflux and intracellular ionic contents alteration can be induced by nigericin, a K+/H+ ionophore, or ATP, thus triggering NLRP3 inflammasome activation and promoting IL-1β maturation [59] (Figure 1).
Figure 1: The mechanism of canonical and non-canonical pyroptosis pathway. In the canonical pathway, NLRP3 inflammasome activation requires a two-step process: priming signaling and activation signaling. In the priming signaling process, membrane PRRs and cytoplasmic PRRs first recognize extracellular and intracellular danger signals, including DAMPs and PAMPs, and then activate the translocation of NF-κB to nucleus, further inducing the transcription of NLRP3 and pro-IL-1β. In the activation signaling process, several activation signals promote assembly of the NLRP3 inflammasome, including K+ efflux, mitochondrial reactive oxygen species (mtROS) and mitochondrial DNA (mtDNA), cathepsin B released by lysosomal rupture. The NLRP3 inflammasome is a protein complex consisting of three parts: the sensor NLRP3, the adaptor ASC and the effector pro-caspase-1. Once activated, NLRP3 recruits and binds ASC through PYD-PYD interactions. In turn, ASC recruits and binds pro-caspase-1 through CARD-CARD interactions, leading to auto-cleavage of pro-caspase-1 and release of its active subunits p20/10. Active cleaved-caspase-1 converts pro-IL-1β and pro-IL-18 to mature IL-1β and IL-18, leading to inflammation. The active pro-caspase-1 also converts FL-GSDMD to NT-GSDMD, which oligomerizes and forms pores on the plasma membrane in turn. In the non-canonical pathway, pro-caspase-11 in mouse cells and pro-caspase-4/5 in human cells could recognize the LPS component of Gram-negative bacteria through a direct binding between LPS and the caspases, inducing the oligomerization and activation of the inflammatory caspases. The active caspase-4/5/11 subsequently cleaves FL-GSDMD to NT-GSDMD and induces pyroptosis.
Pyroptosis-related drug targets for treating AAA
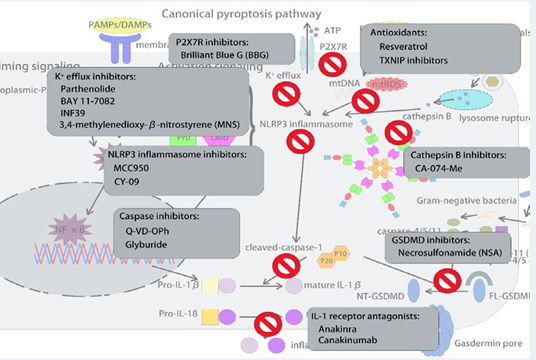
Recently, more and more studies have shown that the progression of AAA can be prevented by many inhibitors of pyroptosis by alleviating inflammation response and degeneration of the aortic wall. In general, the complicated mechanism of pyroptosis provides several targets for inhibiting its activation, including blocking of assembly of NLRP3 inflammasome, suppression of caspase-1 activation and GSDMD cleavage and other traditional cardiovascular drugs.
Inhibitors of the NLRP3 inflammasome: NLRP3 inflammasome can be activated by ROS, lysosome rupture and ion efflux, so application of ROS scavenger, blockage of P2X7 signaling and inhibition of K+ efflux may prevent pyroptosis by blocking the assembly of NLRP3 inflammasome. MCC950, a diarylsulfonylurea- containing compound, selectively inhibited the NLRP3 inflammasome without influencing other inflammasomes and immune responses while the specific molecular mechanism remains unclear. MCC950 may bind to NLRP3 and affect key steps in its activation, which involves post-translational modifications [60]. A recent study indicated that MCC950 was effective at lowering the blood pressure of mice and inhibiting secretion of pro-inflammatory cytokines [61], which might prevent the progression of AAA. In vitro studies, we observed that MCC950 could reduce caspase-1 activity and further downregulate the expression of IL-1β and IL-18 by inhibiting NLRP3 inflammasome [62]. Recent years, another specific NLRP3 inhibitor, CY-09 was found to directly bind to the NACHT domain of NLRP3, limiting its oligomerization and assembly of the inflammasome [63]. CY- 09 could significantly reduce platelet aggregation by affecting the threshold concentration of collagen and the contraction of damaged clots in platelets [64]. P2X7 receptor (P2X7R), which is located on cell membrane, can activate NLRP3 inflammasome by forming nonselective pores and mediating K+ efflux in the progression of AAA [65]. Brilliant blue G (BBG) is a specific P2X7R inhibitor and was proved to inhibit endoplasmic reticulum stress
and reduce pyroptosis in PHN rats [66]. In addition, parthenolide, BAY 11-7082, INF39, and 3,4-methylenedioxy-β-nitrostyrene (MNS) were all reported to prevent K+ efflux by inhibiting the ATPase activity [67-69].
Caspase inhibitors: Quinoline-Val-Asp- difluorophenoxymethylketone (Q-VD-OPh) is a broad-spectrum caspase inhibitor that can reduce Ang II-induced AAAs in Apo E-/- mice with profoundly diminished levels of medial apoptosis and inflammation [32]. In addition, pharmacologic blockade of the NLRP3–caspase-1 inflammasome cascade by glyburide, which is used as a type of anti-diabetic drugs could also attenuate AAA formation in WT mice [33].
GSDMD inhibitors: Necrosulfonamide (NSA) which is a chemical inhibitor of GSDMD binds to cysteine 191 to block oligomerization of GSDMD and pore formation, thus suppressing IL-1β release and pyroptotic killing without GSDMD cleavage [70]. Another study also indicated that NSA could inhibit the accumulation of the long chain of GSDMD and pore formation through physical binding [71].
IL-1β inhibitors: Inhibition of cytokine IL-1β was another strategy used to prevent pyroptosis in AAA. IL-1 receptor antagonist (IL-1Ra) which is produced by ECs, SMCs and
macrophages could significantly suppress AAA formation after Ang II infusion through diminishing the inflammatory level [72]. Anakinra, another IL-1R antagonist, was also proved to attenuate experimental AAA formation by disrupting IL-1β signaling pathway[38]. Interestingly, a recently completed clinical trial CANTOS indicated that canakinumab which is an IL-1β-neutralizing antibody could decrease the rate of recurrent cardiovascular events by targeting the IL-1β innate immunity pathway, demonstrating that targeting inflammation in AAA is effective [73].
Others: Dipeptidyl Peptidase-4 (DPP-4) inhibitors, such as sitagliptin and teneligliptin, have been demonstrated to play a protective role in AAA [74]. Treatment with DPP-4 inhibitors suppressed AAA formation in Apo E-/- mice with HFD and Ang ? infusion by reducing macrophage infiltration, levels of gelatinolytic activity and apoptotic cells [35,75,76]. Additionally, this suppression was associated with increased levels of plasma active glucagon-like peptide-1 (GLP-1) [77], which is another type of anti-diabetic drugs and was also proved to prevent AAA development in rats with the use of GLP-1 receptor analog (lixisenatide) [34].
Resveratrol, which is extracted from red wine and grape
skin, is reported to have an antioxidant and anti-inflammatory
effect on preventing cardiovascular events [78,79]. Recently, resveratrol has been shown to activate silent sirtuin 1 (Sirt1), which is a regulator of aging [80]. H. Kaneko et al found that treatment with resveratrol prevents the development of AAAs in mice and is associated with reduced angiogenesis, oxidative stress, inflammatory response, matrix metalloproteinase activity, and extracellular matrix destruction [81]. Another study also indicated the protective role of resveratrol in AAA progression with upregulation of angiotensin-converting enzyme 2 (ACE2), which is important in maintaining the balance of the RAS [82]. (Figure 2)
Figure 2 :Possible pharmacological approaches targeting the NLRP3 inflammasome and pyroptosis pathway to reduce AAA.
(Table 2).
Table 2: Potential pharmacological approaches targeting the NLRP3 inflammasome and pyroptosis to reduce AAA.
|
Drugs |
Mechanism of Action |
Effects on AAA |
Organism |
References |
|
MCC950 |
selectively inhibits the NLRP3 inflammasome |
— |
— |
60-62 |
|
CY-09 |
directly binds to the NACHT domain, limiting oligomerization of NLRP3 and assembly of the NLRP3 inflammasome |
— |
— |
63,64 |
|
Brilliant blue G (BBG) |
specific P2X7R inhibitor |
— |
— |
66 |
|
Q-VD-OPh |
broad-spectrum caspase inhibitor |
reduce Ang II-Induced AAAs with profoundly diminished levels of inflammation |
Apo E-/- mice |
32 |
|
Glyburide |
blockade of the NLRP3–caspase-1 inflammasome cascade |
reduce Ang II-Induced AAAs |
WT mice |
33 |
|
Necrosulfonamide (NSA) |
inhibitor of GSDMD, binding to cysteine 191 to block its oligomerization and pore formation |
— |
— |
70,71 |
|
Anakinra |
IL-1R antagonist |
attenuate experimental AAA formation |
Apo E-/- mice |
38 |
|
Canakinumab |
IL-1R antagonist |
— |
— |
73 |
|
Sitagliptin and teneligliptin |
Dipeptidyl Peptidase-4 (DPP-4) inhibitors |
suppress AAA formation in Apo E-/- mice with HFD and Ang ? infusion by reducing macrophage infiltration, levels of gelatinolytic activity and apoptotic cells |
Apo E-/- mice |
35,75,76 |
|
Lixisenatide |
Glucagon-like peptide-1 (GLP-1) receptor analog |
prevent AAA development |
rat |
34 |
|
Resveratrol |
antioxidant which can activate silent sirtuin 1 (Sirt1) |
prevent AAA development by reducing angiogenesis, oxidative stress, inflammatory response, matrix metalloproteinase activity, and extracellular matrix destruction |
Apo E-/- mice |
78-82 |
|
CA-074-Me |
cathepsin B-specific inhibitor |
— |
— |
58 |
FUNDING
This study was supported by grants from The Young Program of National Natural Science Foundation of China (No. 81900417).
REFERENCES
- Aggarwal S, Qamar A, Sharma V, Sharma A. Abdominal aortic aneurysm: A comprehensive review. Exp Clin Cardiol. 2011; 16: 11-5.
- Umebayashi R, Uchida HA, Wada J. Abdominal aortic aneurysm inaged population. Aging (Albany NY). 2018; 10: 3650-1.
- Jana S, Hu M, Shen M, Kassiri Z. Extracellular matrix, regional heterogeneity of the aorta, and aortic aneurysm. Exp Mol Med. 2019; 51: 1-15.
- Liu Z, Fitzgerald M, Meisinger T, Batra R, Suh M, Greene H, et al. CD95- ligand contributes to abdominal aortic aneurysm progression by modulating inflammation. Cardiovascular Research. 2019; 115: 807- 18.
- Adams L, Brangsch J, Hamm B, Makowski MR, Keller S. Targeting the Extracellular Matrix in Abdominal Aortic Aneurysms Using Molecular Imaging Insights. Int J Mol Sci. 2021; 22.
- Iida Y, Xu B, Schultz GM, Chow V, White JJ, Sulaimon S, et al. Efficacy and mechanism of angiotensin II receptor blocker treatment in experimental abdominal aortic aneurysms. PloS one. 2012; 7: e49642.
- Xuan H, Xu B, Wang W, Tanaka H, Fujimura N, Miyata M, et al. Inhibition or deletion of angiotensin II type 1 receptor suppresses elastase-induced experimental abdominal aortic aneurysms. J Vasc Surg. 2018; 67: 573-84.
- Xu B, Xuan H, Iida Y, Miyata M, Dalman RL. Pathogenic and Therapeutic Significance of Angiotensin II Type I Receptor in Abdominal Aortic Aneurysms. Curr Drug Targets. 2018; 19: 1318-26.
- Sánchez-Infantes D, Nus M, Navas-Madroñal M, Fité J, Pérez B, Barros- Membrilla AJ, et al. Oxidative Stress and Inflammatory Markers in Abdominal Aortic Aneurysm. Antioxidants (Basel, Switzerland). 2021; 10: 602.
- Kuivaniemi H, Ryer EJ, Elmore JR, Tromp G. Understanding the pathogenesis of abdominal aortic aneurysms. Expert Rev Cardiovasc Ther. 2015; 13: 975-87.
- Schmitz-Rixen T, Böckler D, Vogl TJ, Grundmann RT. Endovascular and Open Repair of Abdominal Aortic Aneurysm. Dtsch Arztebl Int. 2020; 117: 813-9.
- Jagadesham VP, Scott DJ, Carding SR. Abdominal aortic aneurysms:an autoimmune disease? Trends Mol Med. 2008; 14: 522-9.
- Tilson MD. Decline of the atherogenic theory of the etiology of the abdominal aortic aneurysm and rise of the autoimmune hypothesis. J Vasc Surg. 2016; 64: 1523-5.
- Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH,Blagosklonny MV, et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012; 19: 107-20.
- Moujalled D, Strasser A, Liddell JR. Molecular mechanisms of cell death in neurological diseases. Cell death and differentiation. 2021; 28: 2029-44.
- Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018; 25: 486-541.
- Lamb CA, Yoshimori T, Tooze SA. The autophagosome: origins unknown, biogenesis complex. Nature reviews Molecular cell biology. 2013; 14: 759-74.
- Yu Y, Yan Y, Niu F, Wang Y, Chen X, Su G, et al. Ferroptosis: a cell death connecting oxidative stress, inflammation and cardiovascular diseases. Cell Death Discov. 2021; 7: 193.
- Seo J, Nam YW, Kim S, Oh DB, Song J. Necroptosis molecular mechanisms: Recent findings regarding novel necroptosis regulators. Exp Mol Med. 2021; 53: 1007-17.
- Yu J, Wang Q, Zhang X, Guo Z, Cui X. Mechanisms of Neoantigen- Targeted Induction of Pyroptosis and Ferroptosis: From Basic Research to Clinical Applications. Front Oncol. 2021; 11: 685377.
- Bertheloot D, Latz E, Franklin BS. Necroptosis, pyroptosis and apoptosis: an intricate game of cell death. Cellular & Molecular Immunology. 2021; 18: 1106-21.
- Jeng MJ, Soong WJ, Lee YS, Tsao PC, Yang CF, Chiu SY, et al. Meconium exposure dependent cell death and apoptosis in human alveolar epithelial cells. Pediatr Pulmonol. 2010; 45: 816-23.
- Wang Q, Wu J, Zeng Y, Chen K, Wang C, Yang S, et al. Pyroptosis: A pro-inflammatory type of cell death in cardiovascular disease. Clinica chimica acta; International Journal of Clinical Chemistry. 2020; 510: 62-72.
- Ji N, Qi Z, Wang Y, Yang X, Yan Z, Li M, et al. Pyroptosis: A New Regulating Mechanism in Cardiovascular Disease. J Inflamm Res. 2021; 14: 2647-66.
- Wu X, Cakmak S, Wortmann M, Hakimi M, Zhang J, Böckler D, et al. Sex- and disease-specific inflammasome signatures in circulating blood leukocytes of patients with abdominal aortic aneurysm. Mol Med. 2016; 22: 505-18.
- Erhart P, Cakmak S, Grond-Ginsbach C, Hakimi M, Böckler D, Dihlmann S. Inflammasome activity in leucocytes decreases with abdominal aortic aneurysm progression. Int J Mol Med. 2019; 44: 1299-308.
- Gonzalez-Hidalgo C, De Haro J, Bleda S, Cañibano C, Michel I, AcinF. Differential mRNA expression of inflammasome genes NLRP1 and NLRP3 in abdominal aneurysmal and occlusive aortic disease. Ther Adv Cardiovasc Dis. 2018; 12: 123-9.
- Ren P, Wu D, Appel R, Zhang L, Zhang C, Luo W, et al. Targeting the NLRP3 Inflammasome With Inhibitor MCC950 Prevents Aortic Aneurysms and Dissections in Mice. J Am Heart Assoc. 2020; 9: e014044.
- Wortmann M, Skorubskaya E, Peters AS, Hakimi M, Böckler D, Dihlmann S. Necrotic cell debris induces a NF-κB-driven inflammasome response in vascular smooth muscle cells derived from abdominal aortic aneurysms (AAA-SMC). Biochem Biophys Res Commun. 2019; 511: 343-9.
- Usui F, Shirasuna K, Kimura H, Tatsumi K, Kawashima A, KarasawaT, et al. Inflammasome activation by mitochondrial oxidative stress in macrophages leads to the development of angiotensin II-induced aortic aneurysm. Arterioscler Thromb Vasc Biol. 2015; 35: 127-36.
- Sun W, Pang Y, Liu Z, Sun L, Liu B, Xu M, et al. Macrophage inflammasome mediates hyperhomocysteinemia-aggravated abdominal aortic aneurysm. J Mol Cell Cardiol. 2015; 81: 96-106.
- Yamanouchi D, Morgan S, Kato K, Lengfeld J, Zhang F, Liu B. Effects of caspase inhibitor on angiotensin II-induced abdominal aortic aneurysm in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2010; 30: 702-7.
- Wu D, Ren P, Zheng Y, Zhang L, Xu G, Xie W, et al. NLRP3 (Nucleotide Oligomerization Domain-Like Receptor Family, Pyrin Domain Containing 3)-Caspase-1 Inflammasome Degrades Contractile Proteins: Implications for Aortic Biomechanical Dysfunction and Aneurysm and Dissection Formation. Arterioscler Thromb Vasc Biol. 2017; 37: 694-706.
- Yu J, Morimoto K, Bao W, Yu Z, Okita Y, Okada K. Glucagon-like peptide-1 prevented abdominal aortic aneurysm development in rats. Surg Today. 2016; 46: 1099-107.
- Lu HY, Huang CY, Shih CM, Chang WH, Tsai CS, Lin FY, et al. Dipeptidyl peptidase-4 inhibitor decreases abdominal aortic aneurysm formation through GLP-1-dependent monocytic activity in mice. PLoS One. 2015; 10: e0121077.
- Lu HY, Huang CY, Shih CM, Lin YW, Tsai CS, Lin FY, et al. A potential contribution of dipeptidyl peptidase-4 by the mediation of monocyte differentiation in the development and progression of abdominal aortic aneurysms. J Vasc Surg. 2017; 66: 1217-26.e1.
- Liu CL, Ren J, Wang Y, Zhang X, Sukhova GK, Liao M, et al. Adipocytes promote interleukin-18 binding to its receptors during abdominal aortic aneurysm formation in mice. Eur Heart J. 2020; 41: 2456-68.
- Johnston WF, Salmon M, Su G, Lu G, Stone ML, Zhao Y, et al. Genetic and pharmacologic disruption of interleukin-1β signaling inhibits experimental aortic aneurysm formation. Arterioscler Thromb Vasc Biol. 2013; 33: 294-304.
- Suehiro C, Suzuki J, Hamaguchi M, Takahashi K, Nagao T, Sakaue T, et al. Deletion of interleukin-18 attenuates abdominal aortic aneurysm formation. Atherosclerosis. 2019; 289: 14-20.
- Gong T, Yang Y, Jin T, Jiang W, Zhou R. Orchestration of NLRP3 Inflammasome Activation by Ion Fluxes. Trends Immunol. 2018; 39: 393-406.
- Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019; 19: 477-89.
- Amin J, Boche D, Rakic S. What do we know about the inflammasomein humans? Brain Pathol. 2017; 27: 192-204.
- Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes.Cell. 2014; 157: 1013-22.
- Yi YS. Caspase-11 Noncanonical Inflammasome: A Novel Key Player in Murine Models of Neuroinflammation and Multiple Sclerosis. Neuroimmunomodulation. 2021: 1-9.
- Lamkanfi M, Dixit VM. Modulation of inflammasome pathways bybacterial and viral pathogens. J Immunol. 2011; 187: 597-602.
- He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015; 25: 1285-98.
- Liu X, Lieberman J. A Mechanistic Understanding of Pyroptosis: The Fiery Death Triggered by Invasive Infection. Adv Immunol. 2017; 135:
- 81-117.Liu Z, Wang C, Yang J, Chen Y, Zhou B, Abbott DW, et al. Caspase-1 Engages Full-Length Gasdermin D through Two Distinct Interfaces That Mediate Caspase Recruitment and Substrate Cleavage. Immunity. 2020; 53: 106-14.
- Wang K, Sun Q, Zhong X, Zeng M, Zeng H, Shi X, et al. Structural Mechanism for GSDMD Targeting by Autoprocessed Caspases in Pyroptosis. Cell. 2020; 180: 941-55.e20.
- Wang Y, Peng J, Xie X, Zhang Z, Li M, Yang M. Gasdermin E-mediated programmed cell death: An unpaved path to tumor suppression. J Cancer. 2021; 12: 5241-8.
- Feng S, Fox D, Man SM. Mechanisms of Gasdermin Family Members in Inflammasome Signaling and Cell Death. J Mol Biol. 2018; 430: 3068-80.
- Liu Q, Zhang D, Hu D, Zhou X, Zhou Y. The role of mitochondria in NLRP3 inflammasome activation. Molecular Immunol. 2018; 103: 115-24.
- Rajamäki K, Lappalainen J, Oörni K, Välimäki E, Matikainen S, Kovanen PT, et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One. 2010; 5: e11765.
- Sugamura K, Keaney JF Jr. Reactive oxygen species in cardiovascular disease. Free Radic Biol Med. 2011; 51: 978-92.
- Desoti VC, Lazarin-Bidóia D, Sudatti DB, Pereira RC, Alonso A, Ueda-Nakamura T, et al. Trypanocidal action of (-)-elatol involves an oxidative stress triggered by mitochondria dysfunction. Mar Drugs. 2012; 10: 1631-46.
- Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012; 36: 401-14.
- Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008; 9: 847-56.
- Chen Y, Li X, Boini KM, Pitzer AL, Gulbins E, Zhang Y, et al. Endothelial Nlrp3 inflammasome activation associated with lysosomal destabilization during coronary arteritis. Biochim Biophys Acta. 2015; 1853: 396-408.
- Pétrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007; 14: 1583-9.
- Coll RC, Robertson AA, Chae JJ, Higgins SC, Muñoz-Planillo R, Inserra MC, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015; 21: 248-55.
- Krishnan SM, Ling YH, Huuskes BM, Ferens DM, Saini N, Chan CT, et al. Pharmacological inhibition of the NLRP3 inflammasome reduces blood pressure, renal damage, and dysfunction in salt- sensitive hypertension. Cardiovascular Research. 2019; 115: 776- 87.
- Gao R, Shi H, Chang S, Gao Y, Li X, Lv C, et al. The selective NLRP3-inflammasome inhibitor MCC950 reduces myocardial fibrosis and improves cardiac remodeling in a mouse model of myocardial infarction. International Immunopharmacology. 2019; 74: 105575.
- Jiang H, He H, Chen Y, Huang W, Cheng J, Ye J, et al. Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders. J Exp Med. 2017; 214: 3219-38.
- Qiao J, Wu X, Luo Q, Wei G, Xu M, Wu Y, et al. NLRP3 regulates platelet integrin αIIbβ3 outside-in signaling, hemostasis and arterial thrombosis. Haematologica. 2018; 103: 1568-76.
- Xing X, Bai Y, Sun K, Chen Q, Huang H, Qiu W, et al. Identification of Candidate Genes Associated with Postherpetic Neuralgia Susceptibility. Pain Physician. 2020; 23: E281-e8.
- Yang D, He Y, Muñoz-Planillo R, Liu Q, Núñez G. Caspase-11 Requires the Pannexin-1 Channel and the Purinergic P2X7 Pore to Mediate Pyroptosis and Endotoxic Shock. Immunity. 2015; 43: 923-32.
- Cocco M, Pellegrini C, Martínez-Banaclocha H, Giorgis M, Marini E, Costale A, et al. Development of an Acrylate Derivative Targeting the NLRP3 Inflammasome for the Treatment of Inflammatory Bowel Disease. Journal of Medicinal Chemistry. 2017; 60: 3656-71.
- He Y, Varadarajan S, Muñoz-Planillo R, Burberry A, Nakamura Y, Núñez G. 3,4-methylenedioxy-β-nitrostyrene inhibits NLRP3 inflammasome activation by blocking assembly of the inflammasome. J Biol Chem. 2014; 289: 1142-50.
- Juliana C, Fernandes-Alnemri T, Wu J, Datta P, Solorzano L, Yu JW, et al. Anti-inflammatory compounds parthenolide and Bay 11-7082 are direct inhibitors of the inflammasome. J Biol Chem. 2010; 285: 9792- 802.
- Rathkey JK, Zhao J, Liu Z, Chen Y, Yang J, Kondolf HC, et al. Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis. Sci Immunol. 2018; 3.
- Rashidi M, Simpson DS, Hempel A, Frank D, Petrie E, Vince A, et al. The Pyroptotic Cell Death Effector Gasdermin D Is Activated by Gout- Associated Uric Acid Crystals but Is Dispensable for Cell Death and IL-1β Release. J Immunol. 2019; 203: 736-48.
- Isoda K, Akita K, Kitamura K, Sato-Okabayashi Y, Kadoguchi T, Isobe S, et al. Inhibition of interleukin-1 suppresses angiotensin II-induced aortic inflammation and aneurysm formation. Int J Cardiol. 2018; 270: 221-7.
- Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. The New England Journal of Medicine. 2017; 377: 1119-31.
- Scheen AJ. Cardiovascular Effects of New Oral Glucose-LoweringAgents: DPP-4 and SGLT-2 Inhibitors. Cir Res. 2018; 122: 1439-59.
- Takahara Y, Tokunou T, Ichiki T. Suppression of Abdominal Aortic Aneurysm Formation in Mice by Teneligliptin, a Dipeptidyl Peptidase-4 Inhibitor. J Atheroscler Thromb. 2018; 25: 698-708.
- Ngetich E, Lapolla P, Chandrashekar A, Handa A, Lee R. The role of dipeptidyl peptidase-IV in abdominal aortic aneurysm pathogenesis: A systematic review. Vascular medicine (London, England). 2021: 1358863x211034574.
- De Nigris V, Prattichizzo F, Mancuso E, Spiga R, Pujadas G, CerielloA. Teneligliptin enhances the beneficial effects of GLP-1 in endothelial cells exposed to hyperglycemic conditions. Oncotarget. 2018; 9: 8898- 910.
- Hsu CN, Hou CY, Tain YL. Preventive Aspects of Early Resveratrol Supplementation in Cardiovascular and Kidney Disease of Developmental Origins. Int J Mol Sci. 2021; 22.
- Chang X, Zhang T, Zhang W, Zhao Z, Sun J. Natural Drugs as a Treatment Strategy for Cardiovascular Disease through the Regulation of Oxidative Stress. Oxidative medicine and cellular longevity. 2020; 2020: 5430407.
- Csiszar A, Labinskyy N, Podlutsky A, Kaminski PM, Wolin MS, Zhang C, et al. Vasoprotective effects of resveratrol and SIRT1: attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. Am J Physiol Heart Circ Physiol. 2008; 294: H2721-35.
- Kaneko H, Anzai T, Morisawa M, Kohno T, Nagai T, Anzai A, et al. Resveratrol prevents the development of abdominal aortic aneurysm through attenuation of inflammation, oxidative stress, and neovascularization. Atherosclerosis. 2011; 217: 350-7.
- Moran CS, Biros E, Krishna SM, Wang Y, Tikellis C, Morton SK, et al. Resveratrol Inhibits Growth of Experimental Abdominal Aortic Aneurysm Associated With Upregulation of Angiotensin-Converting Enzyme 2. Arterioscler Thromb Vasc Biol. 2017; 37: 2195-203.