Anatomically Corrected Malposition of Great Arteries or DORV with Malposed Great Arteries: A Case Study
- 1. Department of Cardiology, Sri Jayadeva institute of cardiovascular sciences and research, Bangalore
- 2. Department of Pediatric Cardiology, Sri Jayadeva institute of cardiovascular sciences and research, Bangalore
Abstract
Anatomically corrected malposition is characterized by atrioventricular and ventriculoarterial concordance. However, there is an abnormal spatial relationship of the great arteries. Here, we describe the case of a 22-year-old male with anatomically corrected malposition of the great arteries. The patient was diagnosed witha double outlet right ventricle, a closed large ventricular septal defect and malposed great arteries. This case is reported due to the rare occurrence and to highlight the importance of using a segmental approach to diagnose and differentiate the corrected malposition from DORV with malposition.
Keywords
Anatomically corrected malposition of great arteries (ACMGAs), Subaortic conus; Double outlet right ventricle (DORV), Ventricular septal defect (VSD), Abnormal spatial relation
Citation
Mahla H, Mallikarjun K, Usha, Mahimarangaiah J, Manjunath CN (2015) Anatomically Corrected Malposition of Great Arteries or DORV with Malposed Great Arteries: A Case Study. J Cardiol Clin Res 3(2): 1050.
ABBREVIATIONS
DORV: Double Outlet Great Vessels; AV: Atrio Ventricular; VA: Ventriculo Arterial; ACMGAs; Anatomically Corrected Malposition of Great Arteries; VSD: Ventricular Septal Defect; LV: Left Ventricle/Ventricular; RV: Right Ventricle/Ventricular; CCTGA: Corrected Transposition of Great Arteries; TGA: Transposition of Great Arteries; LVOT: Left Ventricular Outflow Tract; RVOT: Right Ventricular Outflow Tract
CASE REPORT
A 22-year-old male was referred to our tertiary care institute with a diagnosis of double outlet right ventricle with a ventricular septal defect and malposed great arteries. The patient was evaluated in the referring institute for atypical chest pain for ten days without any significant past history. A clinical examination, including cardiac testing, was normal, and the resting oxygen saturation was 98% on room air. Although the transthoracic echocardiographic window was poor, it revealed the patient had situs solitus, and there was AV and VA concordance. The aorta was located to the left and anterior to the pulmonary artery (Figures 1,2, video 1,2)
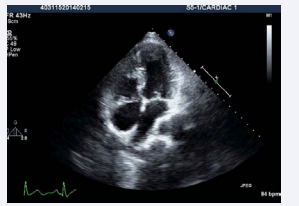
Figure 1: Transthoracic echocardiogram apical 4 chamber view showing AV and VA concordance
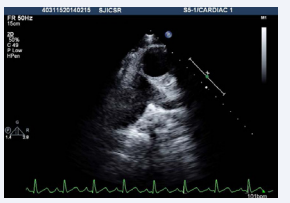
Figure 2: Transthoracic echocardiogram basal short axis view showing aorta to the left and anterior of pulmonary artery
Video 1: Transthoracic echocardiogram apical 4 chamber view showing AV and VA concordance
Video 2: Transthoracic echocardiogram basal short axis view showing aorta to the left and anterior of pulmonary artery
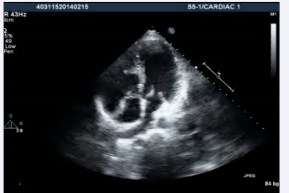
. Additionally, the patient had a subaortic conus and aortomitral discontinuity with a possible ventricular septal defect (Figure 3, video3)
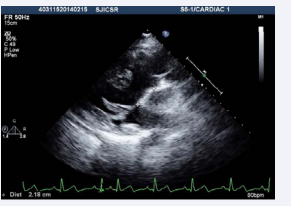
Figure 3: Transthoracic echocardiogram high parasternal view showing presence of subaortic conus with aortomitral discontinuity.
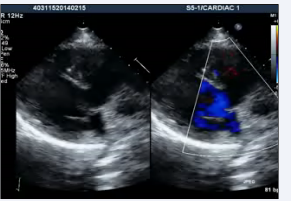
Video 3: Transthoracic echocardiogram modified parasternal view showing anterior aorta and doubtful VSD.
The aortic arch was left sided. A transthoracic echocardiogram suggested a possible closed VSD of the DORV (which is rare) with malposed great arteries.
We performed transesophageal echocardiogram to define the anatomy and confirmed AV and VA concordance (video 4)
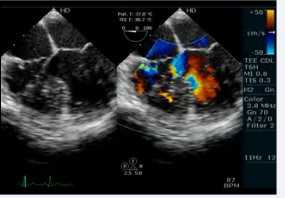
Video 4 :Transesophageal echocardiogram 4 chamber view showing AV and VA concordance with a doubtful VSD.
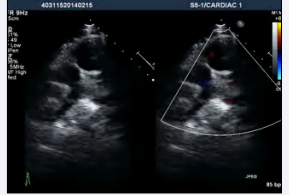
. The echocardiogram showed an elongated left ventricular outflow tract due to subaortic conus (Figure 4, video 5).
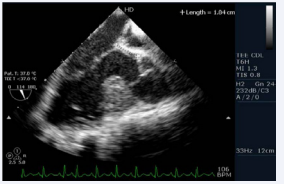
Figure 4: Transesophageal echocardiogram long axis view showing presence of subaorticconus with aortomitral discontinuity
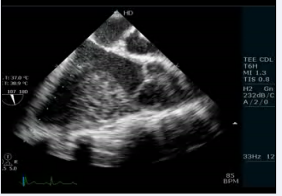
Video 5: Transesophageal echocardiogram long axis view showing subaortic conus with aortomitral discontinuity.
There were also sub pulmonary conus with pulmonary tricuspid discontinuity (video 6)
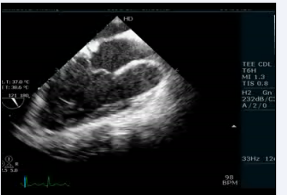
Video 6: Transesophageal echocardiogram long axis view showing subpulmonary conus with pulmonary tricuspid discontinuity
and a small ostium secundum atrial septal defect (left to right shunt). Furthermore, there was thickening of the interventricular septum that appeared as a pouch. The patient underwent cardiac catheterization to define the anatomy and determine the complexity because the VSD was not confirmed. The QP:Qs was 1:1. The left ventriculography revealed a normal contractile ventricle connected to the left sided aorta. There was no evidence of VSD. However, there was opacification of a small pouch just medial to the septum. There was no opacification of the pulmonary artery (Figure 5; video7)
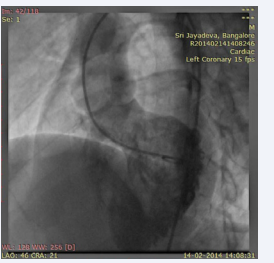
Figure 5: LV angiogram in LAO view showing opacification of left sided aorta and a pouch medial to septum and no evidence of VSD.
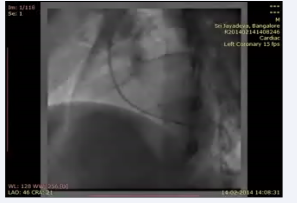
Video 7: LV angiogram in LAO view showing opacification of the left sided aorta and a pouch medial to septum.
. The right ventriculography revealed a trabeculated normally contractile ventricle connected to the right sided pulmonary artery (Figure 6; video 8)
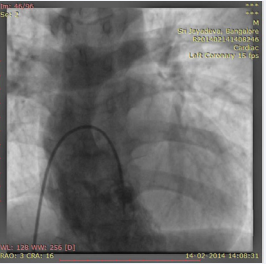
Figure 6: RV angiogram in AP cranial view showing opacification of right sided pulmonary artery.
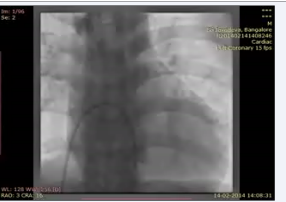
Video 8: RV angiogram in AP cranial view showing opacification of the right sided pulmonary artery.
. The patient was diagnosed withDORV with VSD and L-malposed great vessels. The patient is currently receiving medical follow-up.
DISCUSSION
Anatomically corrected malposition of the great arteries was first reported by Theremin [1] in 1895and was first characterized by Van Praagh et al. in 1975 [2,3]. Anatomically corrected malposition ofthe great arteries are a rare form of congenital heart disease. The disease is characterized as having great arteries that are abnormally related to each other and to the ventricles. However, the arteries arise above the anatomically correct ventricles. Van Praagh [4] classified this rare congenital heart disease into four types using a segmental approach.
Type 1disease is SDL (situs solitus, D-loop ventricles with left and anterior aorta).This is most common type of ACMGAs and occurs in 88% of patients.Type 2 is SLD (situs solitus, L-loop ventricles with right and anterior aorta) and it is observed in 6% of cases. Type 3 is ILD (situs inversus, L-loop ventricles with right and anterior aorta). Type 3 disease has not been reported in the literature.Type 4 is IDL (situs inversus, D-loop ventricles with left and anterior aorta). It is also observed in 6% of cases.Our case belongs to type 1 disease because the patient wasSDL. Types 1 and 3 disease are physiologically corrected, but types 2 and 4 have transposition physiology. The most commonly associated anomalies include the following: VSD, subaortic obstruction, right ventricular hypoplasia, RVOT obstruction, juxtaposed atrial appendage, and right aortic arch.Our patient did not present any of the associated anomalies. A subaortic obstruction can develop because the posteriorly placed left ventricle is connected to the anterior aorta (elongated LVOT). The embryonic basis for this condition was described by Goor and Edwards [5,6] as an isolated inversion of conus. This indicates the inversion of conal musculature together with the arteries in the reverse direction. During embryo development, the rotations at the conoventricular and conotruncal junctions bring the aorta and PA in relation to LV and RV, respectively. The next important step is the absorption of the conus. In the normal heart the subaortic conus is completely absorbed. The persistence of bilateral coni results in both great arteries arising from the RV, which results in DORV. The presence of bilateral coni in the present case led to the diagnosis of DORV with VSD and L-malposed great vessels. The VSD must have closed due to the formation of a trabecular pouch. The LV blood was directed into the aorta, which is an extremely rare occurrence.
In our adult patient, the transthoracic echocardiographic window was poor. Thus, we demonstrated the anatomy more precisely using a transesophageal echocardiogram. Although the transesophageal echocardiogram clarified the diagnosis, there was still some doubt regarding the presence of VSD, and the pouch near the ventricular septum needed to be defined. These issues were addressed by cardiac catheterization, which proved no associated VSD. In general, catheterization with assessment of hemodynamics is indicated only in patients who are scheduled to undergo surgery due to associated anomalies. MRI and CT can also be used to delineate anatomy. The prognosis depends on associated anomalies.Our patient is on medical follow- up because there were no associated anomalies.
CONCLUSION
ACMGAs are a rare entity among congenital heart diseases. The condition should be considered when differentiating congenital cardiac conditions with malposition of the great arteries. The diagnosis becomes difficult in adult patients with a poor echo window. These patients can easily be misdiagnosed with common conditions such as CCTGA, DORV, or transposition of the great arteries (TGA). All of these conditions are associated with malposition of the great arteries. Conversely, DORV with spontaneously closed VSD (a rare occurrence) and malposed great arteries can be misdiagnosed as ACMGAs.Thus, a segmental approach is required for a proper diagnosis with multimodality imaging (especially in adults).
ACKNOWLEDGEMENTS
We are thankful to Dr Neha Godara for the kind support.








































































































































