Spectral Analysis Reveals Distinct Roles of TRPM8 and TRPV1 in Autonomic Cardiovascular Regulation During Cold Stress
- 1. Department of Psychiatry, Cheng Hsin General Hospital, Taiwan
- 2. Division of Medical Research & Education, Cheng Hsin General Hospital, Taiwan
- 3. Department of Physiology, National Defense Medical Center, Taiwan
- 4. Department of Emergency Medicine, Tri-Service General Hospital, National Defense Medical Center, Taiwan
- 5. Taichung Armed Forces General Hospital, Taiwan
- †. Both authors contributed equally to the corresponding authorship.
Abstract
Cold stress (CS), triggers significant autonomic and hemodynamic disturbances, mediated in part by transient receptor potential (TRP) channels. This study investigates the distinct contributions of TRPM8 and TRPV1 in autonomic cardiovascular regulation during CS. Conscious male Sprague-Dawley rats were administered AMTB (TRPM8 antagonist) or capsazepine (CPZ; TRPV1 antagonist), while controls received either normal saline (NS) or dimethyl sulfoxide (DMSO). Hemodynamic parameters, including systolic blood pressure (SBP), heart rate (HR), and core temperature (ToC), were continuously monitored. Spectral analyses of blood pressure variability (BPV), and heart rate variability (HRV), were used to assess autonomic modulation. AMTB administration led to ToC declines and altered sympathetic activity, while CPZ attenuated cold-induced pressor (CIP) and tachycardic (CIT) responses, improved baroreflex sensitivity, and shifted autonomic balance toward parasympathetic dominance. The findings highlight TRPM8’s role in sensory-driven thermoregulation and TRPV1’s broader function in baroreflex and autonomic stability. This study provides novel insights into the interplay between TRPM8 and TRPV1 in somato-sympathetic reflex modulation and suggests potential therapeutic applications for managing cold-induced cardiovascular dysfunctions.
Highlights
TRPM8 and TRPV1 modulate pain and autonomic responses during cold stress. Cold exposure triggers nociceptors and cardiovascular autonomic reflexes.
Spectral analysis reveals effects of TRPM8 and TRPV1 antagonists on cold stress.
Both antagonists attenuate pressor responses and blood pressure variability.
KEYWORDS
- TRPM8; TRPV1; Autonomic regulation; Cold stress; Hemodynamic variability; Baroreflex; Spectral analysis
CITATION
Liu YP, Lin YC, Lin CC, Tsai SH, Tung CS (2025) Spectral Analysis Reveals Distinct Roles of TRPM8 and TRPV1 in Autonomic Cardiovascular Regulation During Cold Stress. J Cardiol Clin Res. 13(2): 1213.
INTRODUCTION
Cold exposure engages thermosensitive nociceptors, triggering autonomic reflexes that regulate cardiovascular function [1-3]. TRPM8, a cold-sensitive ion channel, detects mild cooling and menthol, while TRPV1 mediates responses to noxious heat, capsaicin, and inflammation [4-8]. Both channels are expressed in sensory neurons and vascular smooth muscle cells, linking sensory input to vascular tone modulation [9-16].
Previous studies, including our own, demonstrated that cold stress induces dynamic alterations between vasoconstriction and vasodilation, mediated by complex pathways such as α2-adrenoceptors and nitric oxide signaling [17-19]. However, the specific contributions of TRPM8 and TRPV1 in autonomic regulation during cold stress remain incompletely understood. While TRPM8 is primarily associated with vasoconstrictive responses, TRPV1 appears to play a broader role in baroreflex modulation and sympathetic outflow regulation [16,20].
Evidence suggests that α2-adrenoceptors participate in neuropathic pain, and TRPM8 and TRPV1 channels are critically involved in pain modulation processes [21- 27]. Additionally, TRPM8 is implicated in amplifying pain sensation following nerve injury and in sensing cold pain with keeping a deep body temperature [7,10,28-31], while TRPV1 channels are essential for detecting noxious heat [4]. Antagonists of these channels offer potential therapeutic benefits for analgesia and autonomic stability.
This study employs pharmacological antagonism of TRPM8 and TRPV1 to elucidate their distinct roles in autonomic cardiovascular regulation during cold stress. Using telemetry-based monitoring and spectral analysis, we aim to provide novel insights into their physiological functions and potential clinical applications in managing cold-induced cardiovascular perturbations.
MATERIALS AND METHODS
Animals
Adult male Sprague-Dawley rats (300–350 g), were housed in a temperature-controlled environment (23±1°C) with a 12-hour light/dark cycle and had ad libitum access to food and water. Before the experiment, the animals were acclimated for one week. All procedures complied with institutional guidelines and were approved by the National Defense Medical Center’s Institutional Animal Care and Use Committee (IACUC).
Experimental Design
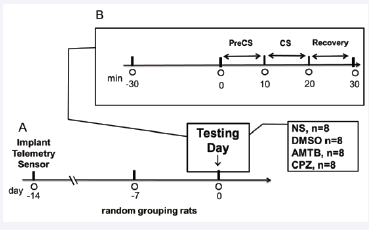
The experimental timeline is shown in Figure 1.
Figure 1: Timeline of experimental procedures across treatment groups. Following a one-week acclimation period, rats underwent telemetric transmitter implantation two weeks before testing. Each group received a designated treatment—normal saline (NS), dimethyl sulfoxide (DMSO), AMTB (TRPM8 antagonist), or capsazepine (CPZ, TRPV1 antagonist)—30 minutes before the cold stress (CS) trial. Data collection encompassed the pre-cold stress (PreCS), CS, and recovery phases.
Rats underwent surgical implantation of telemetric transmitters (TL11M2-M2-C50-PXT, DSI, Minnesota, USA) via the femoral artery, positioning the catheter in the descending aorta for continuous monitoring of systolic blood pressure (SBP), heart rate (HR), and core temperature (ToC). Transmitters were secured within the abdominal cavity, and the rats were allowed a two-week postoperative recovery period to ensure stable baseline recordings [17,18].
After recovery, animals were randomly assigned to one of four treatment groups (n = 8 per group):
- Normal Saline (NS): Rats received a slow bolus injection of 0.9% NaCl (1.5 ml), via the tail vein over 5 minutes, serving as a baseline reference for hemodynamic responses.
- DMSO: Rats were administered 10% dimethyl sulfoxide (DMSO) in 0.9% NaCl (1.5 ml) via the tail vein over 5 minutes to evaluate vehicle effects.
- AMTB (TRPM8 antagonist): Rats received a bolus injection of 1.6 mM AMTB dissolved in 10% DMSO + saline (1.5 ml) via the tail vein over 5 minutes. The dose (0.972 mg) was selected based on prior studies demonstrating its efficacy in modulating cold-induced responses [7,8,24].
- Capsazepine (CPZ, TRPV1 antagonist): Rats received an intraperitoneal injection of CPZ (1 mg/kg; 0.3 mg), dissolved in 10% DMSO + saline (1.5 ml), 30 minutes before cold stress to account for pharmacokinetic differences between intravenous and intraperitoneal administration [5,6,25,26].
Each animal underwent a 10-minute cold stress (CS) trial, during which their glabrous forepaws and hindpaws were immersed in ice water maintained at 4°C. Baseline measurements (PreCS), were recorded at ambient room temperature (23°C). Hemodynamic parameters were continuously recorded across PreCS, CS, and the recovery phase. For spectral analysis, data from minutes 3 to 8 of the CS phase were used to ensure stability and minimize artifacts from initial stress responses [17,18].
Spectral Signal Acquisition and Processing
Oscillatory blood pressure and inter-beat interval- derived HR signals were digitized at 1 kHz using LabChart Pro software (ADInstruments, Sydney, Australia). Spectral analysis was performed using a fast Fourier transform (FFT) to derive the power spectral density of BPV and HRV in predefined frequency bands: very low frequency (VLF: 0.02–0.2 Hz), low frequency (LF: 0.2–0.6 Hz), and high frequency (HF: 0.6–3 Hz). BPV components were normalized and expressed as absolute power (mmHg²/ Hz), while HRV was analyzed based on inter-beat interval data (ms²/Hz). Cross-spectral analysis was conducted to evaluate BPV-HRV coherence across frequency bands, with K²IBI/SBP values > 0.58 indicating significant BPV-HRV covariation.
Statistical Analysis
Data were analyzed using two-way repeated-measures ANOVA, with TRIAL (PreCS, CS) as the within-subject factor and GROUP (NS, DMSO, AMTB, CPZ) as the between- subject factor. Tukey’s post hoc test was performed to identify significant inter-group differences. Correlations between BPV and HRV components were assessed using Pearson’s correlation coefficient. All statistical analyses were performed using SPSS 18.0 software (SPSS Inc., Chicago, IL, USA), with significance set at p < 0.05.
RESULTS
Primary data on hemodynamic parameters and spectral variables are presented in Tables 1,2 and Figures 2-5, with supplementary information available in Figures S1-S2.
Table 1: Systolic blood pressure (SBP) and heart rate (HR) across experimental groups during PreCS and CS.
|
Blood Pressure |
|||||
|
|
|
NS |
DMSO |
AMTB |
CPZ |
|
PreCS |
SBP (mmHg) |
122.12±3.68 |
118.12±3.27 |
132.97±5.53a3b3 |
116.21±4.58c3 |
|
CS |
SBP (mmHg) |
149.87±2.61### |
140.87±2.61###a3 |
140.41±5.61###a1 |
122.84±4.56a3b3c3 |
|
Heart Rate |
|||||
|
|
|
NS |
DMSO |
AMTB |
CPZ |
|
PreCS |
HR (beats/min) |
301.91±9.27 |
311.91±6.27 |
325.55±8.02a1 |
|
|
CS |
HR (beats/min) |
478.24±11.34### |
466.24±10.34### |
452.96±11.46### |
366.44±12.50###a3b3c3 |
Table 2: Power spectral density of cardiovascular variability across treatment groups.
|
Blood Pressure Variability |
|||||
|
|
|
NS |
DMSO |
AMTB |
CPZ |
|
PreCS |
VLF (mmHg2) |
1.33±0.30 |
1.73±0.30 |
2.54±0.49a1 |
0.90±0.11b1c1 |
|
LF (mmHg2) |
1.26±0.23 |
2.56±0.63a1 |
3.04±0.61a1 |
0.92±0.04b1c1 |
|
|
HF (mmHg2) |
0.88±0.17 |
1.88±0.27a1 |
0.93±0.2b1 |
1.30±0.30 |
|
|
TP (mmHg2) |
6.76±1.68 |
7.97±1.68 |
13.12±2.6a1b1 |
4.31±0.54b1c1 |
|
|
CS |
VLF (mmHg2) |
8.28±1.56### |
10.28±1.56### |
7.27±2.71# |
2.52±0.43#a1b1c1 |
|
LF (mmHg2) |
12.93±3.91### |
12.93±2.71### |
10.66±1.99# |
4.69±1.24#a1b1c1 |
|
|
HF (mmHg2) |
20.06±8.05### |
20.37±5.38### |
5.41±1.62#a2b2 |
8.01±3.31#a1b1 |
|
|
TP (mmHg2) |
88.51±35.18### |
92.71±3.82### |
47.83±10.55##a1b1 |
29.31±9.78##a1b1 |
|
|
Heart Rate Variability |
|||||
|
|
|
NS |
DMSO |
AMTB |
CPZ |
|
PreCS |
VLF (ms2) |
15.86±1.87 |
17.86±1.8 |
16.88±3.40 |
6.21±3.17a1b1c1 |
|
LF (ms2) |
1.30±0.32 |
1.42±0.32 |
1.87±0.52 |
0.72±0.55c1 |
|
|
HF (ms2) |
6.76±0.82 |
7.76±0.85 |
9.06±1.31 |
7.87±2.33 |
|
|
LF/HF |
0.19±0.03 |
0.18±0.02 |
0.24±0.06 |
0.09±0.04a1b1c1 |
|
|
TP (ms2) |
40.25±7.44 |
44.95±7.08 |
55.42±12.38 |
28.23±9.43c1 |
|
|
CS |
VLF (ms2) |
7.13±2.66### |
7.23±2.66## |
9.78±4.81 |
4.49±1.51 |
|
LF (ms2) |
0.93±0.20 |
1.23±0.20 |
1.37±0.42 |
1.01±0.10 |
|
|
HF (ms2) |
5.03±1.07 |
5.03±3.49 |
4.21±1.39 |
11.29±3.00a1c1 |
|
|
LF/HF |
0.18±0.07 |
0.24±0.01# |
0.44±0.09#a1b1 |
0.08±0.03a1b1c1 |
|
|
TP (ms2) |
25.33±6.56 |
28.33±4.6# |
24.91±6.57# |
35.64±9.49 |
|
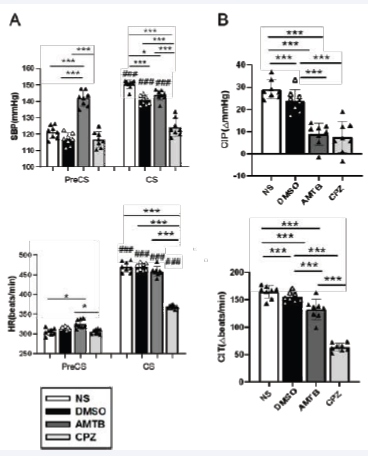
Figure 2: Hemodynamic responses during PreCS and CS across treatment groups. (A) Systolic blood pressure (SBP) and heart rate (HR) changes. Statistical significance is indicated for comparisons between PreCS and CS phases (*p < 0.05, **p < 0.01, ***p < 0.001) and between groups (#p < 0.05, ##p< 0.01, ###p < 0.001). (B) Cold-induced pressor (CIP) and tachycardia (CIT) responses, calculated by comparing PreCS and CS values (*p < 0.05, **p < 0.01,***p < 0.001). CPZ significantly attenuated CIP and CIT during CS (p < 0.001). Data are presented as mean ± SEM. Abbreviation: ?, difference.
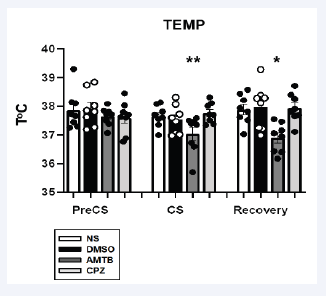
Figure 3: Core temperature (ToC) responses across treatment groups during PreCS and CS. Data are expressed as mean ± SEM, with significant differences indicated by *p < 0.05, **p < 0.01. AMTB administration led to a reduction in ToC.
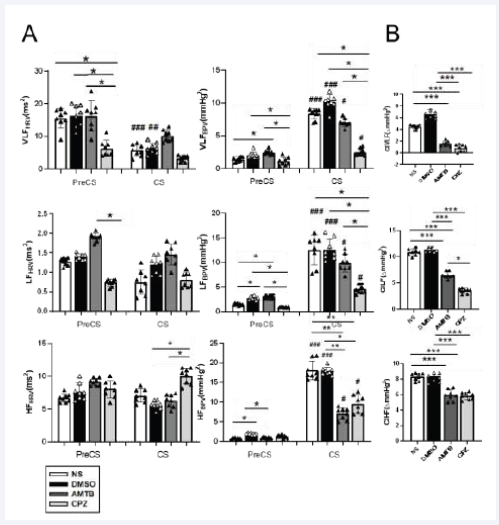
Figure 4 Spectral analysis of blood pressure variability (BPV) and heart rate variability (HRV) during PreCS and CS. (A) Power spectral density of BPV and HRV across very low-frequency (VLF), low-frequency (LF), and high-frequency (HF) bands. (B) Cold-induced BPV changes (?) across frequency bands, denoted as CIVLFBPV, CILFBPV, and CIHFBPV. CPZ reduced LF and VLF BPV components, suggesting diminished sympathetic activity, while AMTB modulated LFBPV during baseline conditions. Abbreviations: CIVLFBPV, cold-induced very low-frequency BPV; CILFBPV, cold- induced low-frequency BPV; CIHFBPV, cold-induced high-frequency BPV
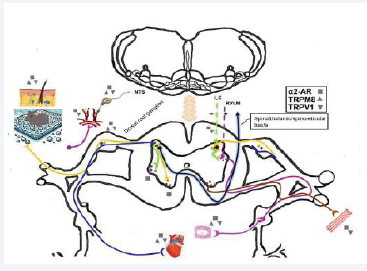
Figure 5: Diagram illustrating TRPM8, TRPV1, and α2-adrenoceptor integration within the somato-sympathetic neuroaxis during cold- and pain-induced cardiovascular modulation. The schematic highlights sensory afferent pathways, second-order neurons, spinal pain and autonomic circuits, and efferent systems. Sensory afferents (blue lines: sympathetic; yellow lines: somatic) are depicted on the left, while efferent motor fibers appear on the right. Sympathetic neurons originating from the rostral ventrolateral medulla (RVLM) and locus coeruleus (LC) project to the spinal intermediolateral column (IML) and dorsal horn pain circuits. The purple line represents IML neurons synapsing onto sympathetic preganglionic neurons, which extend to the heart and vasculature via sympathetic ganglia. The brown line denotes anterior horn motor neurons projecting to skeletal muscles.
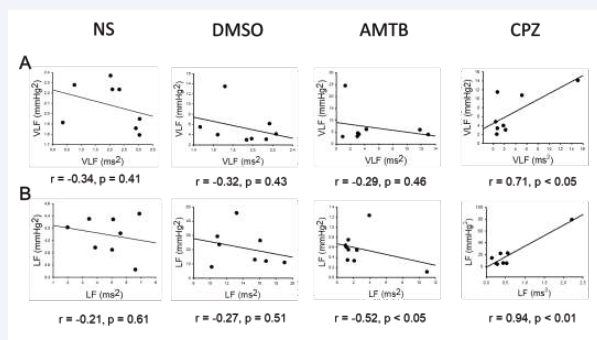
Figure S1: Correlation between spectral power components of blood pressure variability (BPV) and heart rate variability (HRV) during cold stress (CS). (A) Correlation between very low-frequency BPV (VLF, mmHg²) and HRV (VLF, ms²). (B) Correlation between low-frequency BPV (LF, mmHg²) and HRV (LF, ms²) across treatment groups
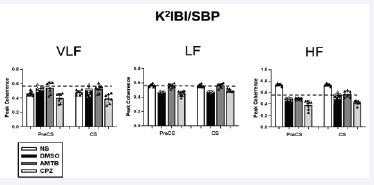
Figure S2: Correlation between systolic blood pressure (SBP) and inter-beat interval (IBI) oscillations across frequency bands. Correlation analysis was performed using peak coherence values (K²IBI/SBP) for the different treatment groups throughout the experiment. Frequency bands analyzed include very low-frequency (VLF), low-frequency (LF), and high-frequency (HF). Abbreviations: SBP, systolic blood pressure; IBI, inter-beat interval; K²IBI/SBP, peak coherence value.
Hemodynamic Responses and Core Temperature to Cold Stress
During the CS trial, SBP, HR, and ToC exhibited significant inter-group variations. Two-way ANOVA revealed significant main effects of TRIAL, GROUP, and their interaction on SBP and HR. As shown in Figure 2A, SBP was significantly lower in the DMSO group than in the NS group during CS (p < 0.001). AMTB-treated rats exhibited significantly elevated SBP during PreCS compared to the NS and DMSO groups (p < 0.001), while CPZ-treated rats showed no significant differences in baseline SBP or HR. However, CPZ significantly attenuated cold-induced pressor (CIP) and tachycardic (CIT) responses during CS (p < 0.001). Figure 2B illustrates that both AMTB and CPZ significantly reduced CIP and CIT responses relative to NS and DMSO, highlighting their roles in autonomic modulation during cold stress.
AMTB significantly lowered ToC during CS (p < 0.01), reinforcing the role of TRPM8 in cold-induced thermoregulation (Figure 3).
Spectral Analysis of Autonomic Modulation
Cold stress induced BPV and HRV changes across all frequency bands (Table 2, Figure 4). Two-way ANOVA revealed significant effects of TRIAL, GROUP, and their interaction on BPV and HRV frequency bands.
To further assess cold-induced BPV changes (CIVLFBPV, CILFBPV, and CIHFBPV), CS and PreCS values were compared across groups. Both AMTB and CPZ significantly reduced these parameters (p < 0.001, Figure 4B), indicating their potential to mitigate cold-induced hemodynamic disturbances.
Baroreflex Sensitivity and Autonomic Stability
Correlation analysis revealed that CPZ reversed the negative BPV-HRV relationships observed in the NS and DMSO groups (Figure S1). CPZ restored positive correlations between BPV and HRV parameters, suggesting improved baroreflex sensitivity. Furthermore, BPV-HRV coherence was evaluated using peak coherence values (K²IBI/SBP) across frequency bands (Figure S2). In the LF region during both PreCS and CS phases, AMTB exhibited higher coherence than DMSO and CPZ. However, CPZ showed lower coherence values than AMTB, suggesting a reduction in sympathetic dominance and enhanced cardiovascular stability (Figure S2).
DISCUSSION
The relevant mechanisms of the somatic-sympathetic neuroaxis in cold-induced nociception and cardiovascular responses are illustrated in Figure 5. TRPM8 and TRPV1 interact at multiple levels of the autonomic regulatory pathway, including primary sensory afferents, spinal cord pain-autonomic circuits, descending bulbospinal sympathetic neurons, and vascular smooth muscle regulation. This integrated framework highlights the intricate role of TRP channels in cardiovascular homeostasis under cold stress.
This study elucidates the distinct yet interrelated roles of TRPM8 and TRPV1 in autonomic cardiovascular regulation during cold stress. TRPM8 antagonism primarily influenced thermoregulation and sympathetic activity, whereas TRPV1 antagonism had broader effects on baroreflex function and autonomic stability. These findings enhance our understanding of how sensory-driven mechanisms integrate with autonomic circuits to modulate cardiovascular responses.
DMSO as a Solvent Control
Since this study is based on systemic peripheral drug administration, neither AMTB nor CPZ is expected to cross the blood-brain barrier and directly affect central TRP channels. Therefore, our analysis focuses on the primary sensory neurons and vascular smooth muscle cells as potential target sites for these antagonists [10,14,30,31,37,38].
Dimethyl sulfoxide (DMSO), was used as a solvent control due to its high solubility for lipophilic compounds like AMTB and CPZ. Importantly, DMSO has known pharmacological effects, including mild vasodilation and anti-inflammatory properties, which could influence cardiovascular dynamics. Previous studies have indicated that DMSO may exert modest hemodynamic effects, including altering blood pressure variability and vascular tone [39]. Thus, its use as a control group ensures that observed effects of AMTB and CPZ are attributable to TRP channel modulation rather than solvent-related influences. The significant differences observed between the DMSO and TRPM8/TRPV1 antagonist groups further support the specificity of TRP channel involvement in cardiovascular regulation.
TRPM8-Mediated Thermoregulation and Sympathetic Control
TRPM8 inhibition led to significant reductions in core body temperature and modifications in sympathetic tone, supporting its role as a primary sensor of mild cooling and its involvement in thermoregulatory vasoconstriction [7,9-12]. The observed decrease in temperature of the core (ToC) following AMTB administration suggests that TRPM8 plays a critical role in the thermoregulatory response to cold, particularly by modulating cutaneous vasoconstriction and metabolic heat production. This aligns with findings that TRPM8 activation is associated with cold-induced vasoconstriction and sympathetic excitation, processes essential for maintaining core temperature homeostasis [7,10,28-31]. However, its impact on baroreflex function was comparatively less pronounced than TRPV1 antagonism, suggesting a more localized effect within peripheral thermosensory circuits. The reduction in ToC further supports the hypothesis that TRPM8 modulates cold sensation primarily through afferent pathways influencing thermoregulatory and autonomic reflex arcs [22,31].
TRPV1 and Its Role in Baroreflex and Autonomic
Stability
TRPV1 inhibition with CPZ significantly attenuated cold-induced hemodynamic disturbances, reducing blood pressure and heart rate fluctuations while restoring baroreflex sensitivity. The observed increase in baroreflex gain highlights TRPV1’s pivotal role in autonomic integration, consistent with studies demonstrating its involvement in cardiovascular reflex arcs [4,11,13,15,16,21,40]. Furthermore, the ability of CPZ to counteract negative BPV-HRV correlations suggests its potential as a therapeutic target for baroreflex dysfunction- related disorders.
Comparative Implications of TRPM8 and TRPV1 Antagonism
Both TRPM8 and TRPV1, similar to α2-adrenergic receptors [27], play key roles in thermal pain regulation. The co-expression of TRPV1 and α2-adrenoceptors in primary sensory neurons contributes to pain modulation [21]. Similarly, TRPM8 activity can be modulated by α2a-adrenergic receptor activation [19], suggesting that these channels are critical components of both sensory processing and autonomic responses, particularly in cardiovascular regulation during cold stress.
The differential effects of TRPM8 and TRPV1 antagonists reveal their distinct yet complementary roles in autonomic regulation. TRPM8 primarily mediates peripheral sensory- driven vascular and thermoregulatory responses, while TRPV1 exerts broader effects on baroreflex modulation and systemic autonomic adjustments [3,9,10,12,15,16,23]. These findings align with the functional organization of TRP channels in nociceptive and autonomic pathways, where TRPM8 channels primarily function as peripheral cold sensors, while TRPV1 integrates systemic reflex responses [4].
Limitations and Future Directions
Although our findings provide new insights, certain limitations should be acknowledged. First, the pharmacokinetics of AMTB and CPZ were not directly assessed, necessitating further studies incorporating pharmacokinetic profiling to optimize dosing regimens. Second, our systemic drug administration approach precludes precise localization of TRP channel effects, highlighting the need for selective, tissue-targeted delivery methods in future research.
Further investigations should explore the interactions between TRP channels and other autonomic regulatory pathways, including α2-adrenoceptor signaling and nitric oxide-mediated vascular modulation [17-19]. The application of advanced imaging techniques could help elucidate regional hemodynamic changes, thereby refining our understanding of TRPM8 and TRPV1 functions in cold stress responses.
CONCLUSION
Our study implicates that TRPM8 and TRPV1 play distinct yet interconnected roles in modulating cardiovascular responses to cold stress. TRPM8 primarily mediates thermosensory-driven sympathetic responses, while TRPV1 governs baroreflex function and autonomic homeostasis. These findings provide mechanistic insights into sensory-autonomic integration and suggest potential therapeutic strategies for managing cold-induced autonomic dysfunctions. Future research should further clarify the interplay between TRP channels and autonomic regulatory networks to enhance translational applications in clinical settings.
Ethics Approval and Consent to Participate
All the experiments were approved by the National Defense Medical Center (NDMC) animal care committee (IACUC-17-097). Experiments were carried out following the guidelines laid down by the animal ethics/welfare committee and conforming to the principles and regulations of this Journal.
Consent for Publication
All the authors read and approved the final version of the manuscript.
FUNDING
Ministry of Science and Technology and CHGH-NDMC cooperative research project, Taiwan, granted this study with MOST 103-2320-B-350-001, MOST 108-2314-B-016- 047-MY3, CH-NDMC-110-6.
ACKNOWLEDGMENT
The authors thank Ms. Chan-Fan Young and Ms. Huei- Yin Siao for their technical assistance.
REFERENCES
- Benarroch EE. Pain-autonomic interactions: a selective review. Clin Auton Res. 2001;11: 343-349.
- Cortelli P, Giannini G, Favoni V, Cevoli S, Pierangeli G. Nociception and autonomic nervous system. Neurol Sci. 2013; 34: S41-46.
- Schlereth T, Birklein F. The sympathetic nervous system and pain. Neuromolecular Med. 2008; 10: 141-147.
- Vay L, Gu C, McNaughton PA. The thermo-TRP ion channel family: properties and therapeutic implications. Br J Pharmacol. 2012; 165: 787-801.
- Cabral LD, Giusti-Paiva A. The Transient Receptor Potential Vanilloid 1 Antagonist Capsazepine Improves the Impaired Lung Mechanics during Endotoxemia. Basic Clin Pharmacol Toxicol. 2016; 119: 421-427.
- Walker KM, Urban L, Medhurst SJ, Patel S, Panesar M, Fox AJ, et al. The VR1 antagonist capsazepine reverses mechanical hyperalgesia in models of inflammatory and neuropathic pain. J Pharmacol Exp Ther. 2003; 304: 56-62.
- Gonzalez-Muniz R, Bonache MA, Martin-Escura C, Gomez-Monterrey I. Recent Progress in TRPM8 Modulation: An Update. Int J Mol Sci. 2019; 20: 2767.
- Pham DT, Hsu RM, Sun MF, Huang CC, Chen YH, Lin JG. TRPM8’s Role in the Shift Between Opioid and Cannabinoid Pathways in Electroacupuncture for Inflammatory Pain in Mice. Int J Mol Sci. 2024; 25: 12901.
- Johnson CD, Melanaphy D, Purse A, Stokesberry SA, Dickson P, Zholos AV. Transient receptor potential melastatin 8 channel involvement in the regulation of vascular tone. Am J Physiol Heart Circ Physiol. 2009; 296: H1868-1877.
- Reimundez A, Fernandez-Pena C, Garcia G, Fernandez R, Ordas P, Gallego R, et al. Deletion of the Cold Thermoreceptor TRPM8 Increases Heat Loss and Food Intake Leading to Reduced Body Temperature and Obesity in Mice. J Neurosci. 2018; 38: 3643-3656.
- Phan TX, Ton HT, Gulyas H, Porszasz R, Toth A, Russo R, et al. TRPV1 in arteries enables a rapid myogenic tone. J Physiol. 2022; 600: 1651-1666.
- Zholos A, Johnson C, Burdyga T, Melanaphy D. TRPM channels in the vasculature. Adv Exp Med Biol. 2011; 704: 707-29.
- Baylie RL, Brayden JE. TRPV channels and vascular function. Acta Physiol (Oxf). 2011; 203: 99-116.
- Minic Z, O’Leary DS, Reynolds CA. Spinal Reflex Control of Arterial Blood Pressure: The Role of TRP Channels and Their Endogenous Eicosanoid Modulators. Front Physiol. 2022; 13: 838175.
- Toth A, Czikora A, Pasztor ET, Dienes B, Bai P, Csernoch L, et al. Vanilloid receptor-1 (TRPV1) expression and function in the vasculature of the rat. J Histochem Cytochem. 2014; 62: 129-144.
- Sun H, Li DP, Chen SR, Hittelman WN, Pan HL. Sensing of blood pressure increase by transient receptor potential vanilloid 1 receptors on baroreceptors. J Pharmacol Exp Ther. 2009; 331: 851-859.
- Lin YH, Liu YP, Lin YC, Lee PL, Tung CS. Cooling-evoked hemodynamic perturbations facilitate sympathetic activity with subsequent myogenic vascular oscillations via alpha2-adrenergic receptors. Physiol Res. 2017; 66: 449-457.
- Lin YH, Liu YP, Lin YC, Lee PL, Tung CS. Characterization of the role of endogenous nitric oxide in myogenic vascular oscillations during cooling- evoked hemodynamic perturbations of rats. Can J Physiol Pharmacol. 2017; 95: 803-810.
- Bailey SR, Eid AH, Mitra S, Flavahan S, Flavahan NA. Rho kinase mediates cold-induced constriction of cutaneous arteries: role of alpha2C- adrenoceptor translocation. Circ Res. 2004; 94: 1367-1374.
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002; 108: 705-715.
- Matsushita Y, Manabe M, Kitagawa I, Higuchi M, Hosaka YZ, Kitamura N. Inhibition of transient receptor potential vanilloid type 1 through alpha (2) adrenergic receptors at peripheral nerve terminals relieves pain. J Vet Med Sci. 2021; 83: 1570-1581.
- Bavencoffe A, Gkika D, Kondratskyi A, Beck B, Borowiec AS, Bidaux G, et al. The transient receptor potential channel TRPM8 is inhibited via the alpha 2A adrenoreceptor signaling pathway. J Biol Chem. 2010; 285: 9410-9419.
- Bahari Z, Meftahi GH. Spinal alpha (2)-adrenoceptors and neuropathic pain modulation; therapeutic target. Br J Pharmacol. 2019; 176: 2366-2381.
- Lashinger ES, Steiginga MS, Hieble JP, Leon LA, Gardner SD, Nagilla R, et al. AMTB, a TRPM8 channel blocker: evidence in rats for activity in overactive bladder and painful bladder syndrome. Am J Physiol Renal Physiol. 2008; 295: F803-810.
- Yang MH, Jung SH, Sethi G, Ahn KS. Pleiotropic Pharmacological Actions of Capsazepine, a Synthetic Analogue of Capsaicin, against Various Cancers and Inflammatory Diseases. Molecules. 2019; 24: 925.
- Maggi CA, Bevan S, Walpole CS, Rang HP, Giuliani S. A comparison of capsazepine and ruthenium red as capsaicin antagonists in the rat isolated urinary bladder and vas deferens. Br J Pharmacol. 1993;108: 801-805.
- Donello JE, Guan Y, Tian M, Cheevers CV, Alcantara M, Cabrera S, et al. A peripheral adrenoceptor-mediated sympathetic mechanism can transform stress-induced analgesia into hyperalgesia. Anesthesiol. 2011;114: 1403-1416.
- Cao S, Li Q, Hou J, Li Z, Cao X, Liu X, Qin B. Intrathecal TRPM8 blocking attenuates cold hyperalgesia via PKC and NF-kappaB signaling in the dorsal root ganglion of rats with neuropathic pain. J Pain Res. 2019; 12: 1287-1296.
- Iraci N, Ostacolo C, Medina-Peris A, Ciaglia T, Novoselov AM, Altieri A, et al. In Vitro and In Vivo Pharmacological Characterization of a Novel TRPM8 Inhibitor Chemotype Identified by Small-Scale Preclinical Screening. Int J Mol Sci. 2022; 23: 1879.
- de Oliveira C, Garami A, Lehto SG, Pakai E, Tekus V, Pohoczky K, et al. Transient receptor potential channel ankyrin-1 is not a cold sensor for autonomic thermoregulation in rodents. J Neurosci. 2014; 34: 4445-4452.
- Winchester WJ, Gore K, Glatt S, Petit W, Gardiner JC, Conlon K, et al. Inhibition of TRPM8 channels reduces pain in the cold pressor test in humans. J Pharmacol Exp Ther. 2014; 351: 259-269.
- Schumacher MA. Transient receptor potential channels in pain and inflammation: therapeutic opportunities. Pain Pract. 2010; 10: 185-200.
- Janig W. Systemic and specific autonomic reactions in pain: efferent, afferent and endocrine components. Eur J Anaesthesiol. 1985; 2: 319- 346.
- Dembowsky K, Czachurski J, Amendt K, Seller H. Tonic descending inhibition of the spinal somato-sympathetic reflex from the lower brain stem. J Auton Nerv Syst. 1980; 2: 157-182.
- Sato A, Schmidt RF. Somatosympathetic reflexes: afferent fibers, central pathways, discharge characteristics. Physiol Rev. 1973; 53: 916-947.
- Schramm LP. Spinal sympathetic interneurons: their identification and roles after spinal cord injury. Prog Brain Res. 2006; 152: 27-37.
- Macefield VG, Burton AR, Brown R. Somatosympathetic Vasoconstrictor Reflexes in Human Spinal Cord Injury: Responses to Innocuous and Noxious Sensory Stimulation below Lesion. Front Physiol. 2012; 3: 215.
- Iftinca M, Altier C. The cool things to know about TRPM8! Channels (Austin). 2020; 14: 413-420.
- Hameroff SR, Otto CW, Kanel J, Weinstein PR, Blitt CD. Acute cardiovascular effects of dimethylsulfoxide. Crit Care Med. 1981; 9: 855-857.
- Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. 1999; 51: 159-212.