T2* with Ultrashort Echo Time Can Enable the Detection of Myocardial Scarring
- 1. Department of Cardiology, University Hospital Duesseldorf, Germany
- 2. University of Luebeck, Germany
- 3. Linköping University, Sweden
- 4. UT Southwestern, USA
- 5. King’s College London, Division of Imaging Sciences, UK
Abstract
Purpose: Patients with cardiovascular disease often suffer from renal impairment.? We aimed to validate ultrashort echo time (UTE) T2* imaging without the use of any contrast agents in a phantom and patients with known cardiac fibrosis.
Methods: A phantom of porcine tissue with muscle and fibrosis was created and scanned with UTE. The imaging data were compared with histology. Thereafter, 27 patients with known CAD were prospectively included between December 2013 and July 2014. Cardiac MRI was conducted on a 1.5-T scanner with UTE imaging using a long (6 ms) and short (0.6 ms) echo time. The signal intensity of visualized scarring was compared to (LGE) images followed by graphical analysis using a t-test, Bland-Altman plots and Pearson correlation analysis.
Results: Among the 27 included patients (age: 75 ± 10 years), scar tissue areas defined by UTE and conventional LGE imaging were well correlated (r = 0.9, p<0.0001). The interobserver reproducibility of the UTE assessment was satisfactory (bias = -0.33, 95 % limits of agreement = -4.980–4.315).
Data conclusion: In vivo MRI of myocardial scarring is feasible using T2* imaging with UTE in patients with coronary artery disease. This technique detected subendocardial scarring without using a contrast agent.
Keywords
• T2*
• UTE
• Myocardial scarring
• LGE
• Renal impairment
CITATION
Elkenhans B, Vieregge I, Henningsson M, Hussain TM, Botnar R, et al. T2* with Ultrashort Echo Time Can Enable the Detection of Myocardial Scarring. J Cardiol Clin Res. 2023; 11(2): 1192.
ABBREVIATIONS
UTE: Ultrashort Echo; SNR: Signal to Noise Ratio; LGE: Late Gadolinium Enhancement; PCI: Percutaneous Coronary Intervention; CABG: Coronary Artery Bypass Graft; CAD: Coronary Artery Disease; CAO: Cerebral Artery Occlusion; COPD: Chronic Obstructive Lung Disease
INTRODUCTION
Myocardial infarction remains the leading cause of death in developed countries despite advances in medical and interventional treatment [1]. Scar tissue resulting from myocardial infarction and minor myocardial injuries such as previous PCI and previous CABG can result in impaired systolic and diastolic function and is a cause of chronic heart failure [2]. The current standard of care for identifying and assessing cardiac scarring is late gadolinium enhancement (LGE) cardiac magnetic resonance imaging (MRI) with a gadolinium-based contrast agent [3,4]. However, patients with cardiac diseases often also suffer from chronic renal impairment [5], and gadolinium-based contrast agents are not safe for use in patients with severe renal impairment (estimated glomerular filtration rate <30 ml/min) because of the risk of nephrogenic systemic fibrosis [6]. For this reason, patients who are at risk for nephrogenic systemic fibrosis would benefit from cardiac MRI sequences that can detect scarring without the application of a contrast agent, because renal insufficiency is more prevalent in patients with heart failure and is an independent prognostic factor in diastolic and systolic dysfunction [7].
Late gadolinium enhancement cardiac MRI has been used extensively in a large number of studies for measurement of myocardial scarring [8].
T2* imaging is currently in clinical use for the imaging of myocardial haemorrhage [9] in the context of microvascular obstruction and adverse remodelling [10] because of its ability to image myocardial fibre structure [11]. T2* mapping methods are broadly introduced for the characterization of myocardial diseases [12].
The typical drawback of the T2 and T2*-weighted negative contrast is its poor sensitivity when used to study areas with low background signal.
UTE imaging can be used for the detection of iron oxide nanoparticles (IONP) in mouse tumor models. It enables capturing signal enhancement from T1 effect with little influence of signal decay of the from T2 and T2* effect, allowing for obtaining positive contrast on T1 weighted UTE images [13].
The first use of UTE for the visualization of cardiac fibrosis was reported in rats in 2011 [14].
UTE has its application mainly for cardiac imaging at high magnetic fields [15], because of challenges like long acquisition times, low signal to noise ratios and cardiac motion [16].
In this study, we aimed to develop a competitive method to LGE for the assessment of myocardial scarring without the use of contrast agent.
To this end, we first established a phantom of porcine tissue with muscle and fibrosis.
Then, we compared the abilities of UTE T2* imaging with conventional LGE MRI to detect myocardial scarring in patients with known coronary artery disease (CAD), such as myocardial infarction, after PCI and CABG, because in these patients there should be an increased rate of myocardial scarring.
We did not include patients with severe renal impairment, although this technique is intended to better serve those patients.
We wanted to establish UTE as clinical indicator for myocardial scarring in patients with renal impairment without the need of contrast application.
MATERIALS AND METHODS
Study population
This study prospectively included 27 patients with known CAD and myocardial scarring from the University of Duesseldorf. The study was approved by the Institutional Review Board (IRB) approval of the University of Duesseldorf. Informed consent was obtained from the patients, and patients were referred for assessments and treatment of CAD between December 2013 and July 2014. The inclusion criteria were as follows: known artery disease and myocardial scarring, hypertension, peripheral artery disease (PAD), diabetes, and atrial fibrillation. The exclusion criteria were any contraindications to cardiac MRI, such as implanted pacemakers or claustrophobia. There were no exclusion criteria regarding age.
Cardiac MRI protocol
Patients were scanned as a part of CAD assessment using a 1.5-T scanner (Achieva, Philips Healthcare, Best, The Netherlands) with a 32-channel coil in supine position. First, multislice, multiphase cine imaging was performed using a standard, steady-state, free precession pulse sequence in the short axis (voxel size 1.9 × 1.86 × 8.0 mm3, TE 3.2, TR 1.59, flip angle 60°) to image the entire left and right ventricles to analyse their functional parameters.
Then, Three-dimensional UTE imaging was performed in the midventricular region with a single breath-hold and an iterative technique (voxel size 2.95 × 2.95 × 10.0 mm3, echo time (TE) either 6 ms or 0.6 ms, repetition time (TR) 10 ms, flip angle 20° as shown in [Figure 1].
At last, LGE imaging (voxel size 1.37 × 1.37 × 10 5 mm3, TE 1.8, TR 3.65, flip angle 15°), was performed using a Look-Locker sequence 10 min after the administration of the contrast agent gadoterate meglumine (Dotarem) (0.2 mmol/l/kgKG; Guerbet, Villepinte, France).
Cardiac MRI analysis
Images with a long TE (6ms) were subtracted from images with a short TE (0.6ms) using Osirix software (version 5.9, Geneva, Switzerland), and UTE areas were measured on the resulting subtracted image. The signal-to-noise ratio of the subtracted image was calculated from the measurements of signal intensity in Osirix using the formula (SBlood -SMyocardium )/(0.5 × [NBlood +NMyocardium ]), for which the mean value was used for each variable.
Endocardial and epicardial borders of the subtracted image were manually contoured at end-diastole and end-systole by two different researchers with at least five years of cardiac MRI experience to allow for the calculation of ventricular volumes and mass (epicardial volume – endocardial volume × myocardial density [1.05 g/cm3]), and the values were indexed to body surface area. LGE areas were measured by two experienced researchers and indexed to normal myocardium on the scanner workstation (EWS, release 3.2.2, Philips). Midventricular slices were manually chosen using EWS, and LGE images were visually compared of signal intensity with UTE images by two experienced researchers regarding infarct size.
Consensus reading was performed for image quality scoring by two readers using the image quality scores defined in Table 3. The readers analysed all images independently in a blinded and random order. Disagreements were discussed before a single final grade was given by using the average score.
Statistical analysis
Statistical analysis was performed using GraphPad Prism (version 5, La Jolla, CA 92037, USA). The signal intensity and infarcted areas on UTE images were compared to those on LGE images using a t-test and Pearson correlation analysis. Furthermore, Bland-Altman analysis and Pearson correlation analysis were performed to assess interobserver variability. P-values less than 0.05 were treated as statistically significant.
RESULTS
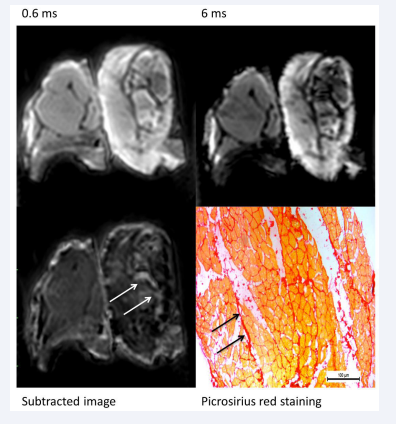
Phantom study results In our phantom study with tissue of porcine muscle and fibrosis the imaged areas accorded with the histological stainings of picrosirius red (Supplementary Figure 1).
Supplementary Figure 1: The upper left image described UTE imaging of a phantom created of porcine muscle and fibrosis tissue acquired with 0.6ms.
The upper right image showed UTE imaging of a phantom created of porcine muscle and fibrosis tissue acquired with 6ms.
The left image below exhibited the subtracted image with Osirix software (white arrows: fibrotic tissue).
The right image below contained the histological staining with Picrosirius red of the phantom (black arrows: fibrotic tissue).
Study population
Of the 45 patients (age: 75 ± 10 years) recruited, 18 were excluded because of poor image quality on T2* MRI; the imaging results for 27 patients were therefore included in this study. These patients suffered despite of myocardial scarring caused by myocardial infarction, previous CABG and previous PCI also of comorbidities such as PAD, CAO, stroke, atrial fibrillation, hypertension, COPD and diabetes mellitus (Table 1).
The mean values of these patients regarding functional parameters included an ejection fraction of 53%, an end diastolic volume of 168ml, an end systolic volume of 99ml, a myocardial mass of 158g, a stroke volume of 70 l/min and a cardiac output of 5,1l (Table 2).
UTE imaging findings
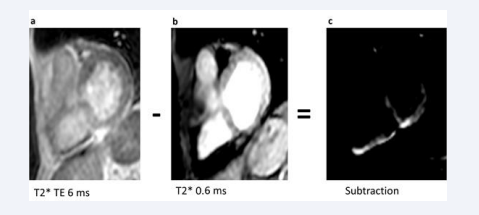
We acquired the UTE images with a TE of 6 ms and a TE of 0,6 ms. Afterwards, we conducted the subtraction with Osirix software and investigated the areas with SNR (Figure 1).
Figure 1: Representative UTE images a) Short axis slice with T2* sequence and 6 ms echo time b) Short axis slice with T2* sequence and 0.4 ms echo time c) Subtracted UTE image (6 ms minus 0.6 ms)
We successfully detected fibrotic tissue with UTE MRI in all patients, similar to in our previous muscle fibrosis model and histology study with picrosirius red staining.
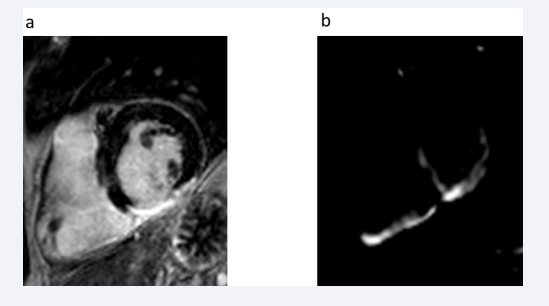
We compared our UTE findings with conventional late gadolinium enhancement images (Figures 2, 3).
Figure 2: Comparison of a) LGE MRI-identified myocardial scarring and b) subtracted UTE-identified myocardial scarring
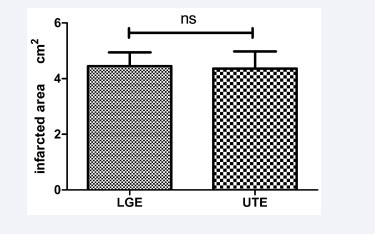
Figure 3: Scar size (cm2), as measured by LGE and subtracted UTE imaging in 28 patients with a history of coronary artery disease and myocardial scarring. The measured scar areas were not significantly different (ns) between imaging methods.
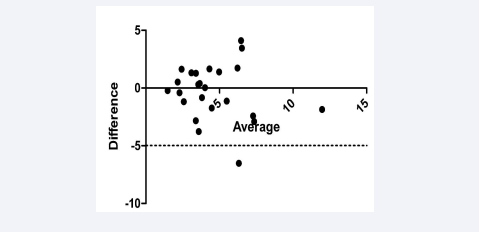
The subtracted images were analysed by two experienced viewers with more than five years of experience. The interobserver reproducibility of UTE analysis (bias = -0.33, 95 % limits of agreement = -4.980–4.315; Figure 5) and variability (r = 0.9, p<0.0001) were both low.
Figure 5: Bland-Altman plot of interobserver variability in UTE analysis (r = 0.9, p<0.0001) (bias = -0.33, 95 % limits of agreement = -4.980–4.315)
In summary, we observed no differences in signal intensity based on scar tissue location (e.g., inferior, lateral, or anterior). UTE imaging revealed a significantly higher SNR in infarcted areas than in healthy myocardium (p<0.02). UTE imaging is able to detect cardiac fibrosis during daily clinical routine.
Correlation between UTE and LGE imaging
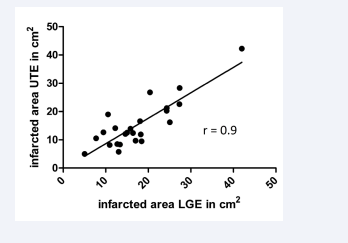
We identified significantly lower signal intensity on LGE images than on UTE images (p<0.0001). However, no significant difference in the size of the infarcted areas was observed between LGE and UTE (Figure 3), and the infarcted area size in UTE imaging correlated closely to those calculated by LGE imaging (r = 0.9, p<0.0001; Figure 4).
Figure 4: Correlation of the infarcted area assessed by LGE and UTE imaging (r = 0.9, p<0.0001)
UTE imaging is a competitive method to conventional LGE for the identification of myocardial scarring without the application of a contrast agent.
DISCUSSION
We aimed to validate ultrashort echo time (UTE) T2* imaging without the use of any contrast agents in a phantom of porcine tissue with muscle and fibrosis and in patients with known myocardial scarring. In our phantom study, the fibrotic areas seen in UTE imaging were identical to histological stainings with picrosirius red.
Our results were in line with the findings of Siu et al, who created a phantom model using collagen solutions. They also confirmed an excellent correlation between UTE collagen signal fraction obtained with 7T MR and collagen [17].
According to de Jong et a l[14]., who first described a pre clinical model the feasibility of using UTE of post-infarcted myocardial fibrosis in rats, little is known so far about the ability of scanning human myocardium and myocardial scarring with UTE imaging.
We were the first group who implemented UTE in a clinical setting [18].
In our study, we documented a high level of agreement between UTE and LGE imaging.
Our findings were in line with the group of Schuijf et al, who performed recently clinical studies with UTE at 3 T. They showed promising results in a patient with previous MI regarding the comparison of UTE to conventional LGE images [19].
Finally, we observed good interobserver reliability between the comparison of UTE imaging and conventional LGE.
These results also matched with the outcome of Schuijf et al., who compared myocardial scarring with UTE imaging and LGE [19].
There are several limitations to this study. We performed UTE imaging with one slice due to long scan times. Non-cartesian k-space sampling schemes might be the solution for reducing scanning time.
We excluded eight patients due to poor image quality. Short readout times are needed to reduce T2* blurring. Here, MR reconstruction changes might be an option.
Nevertheless, as bulk cardiac motion is still challenging, recently techniques have been proposed for the acquisition of accelerated, ECG-triggered radial k-space data [20,21,22], which might be transferred to UTE imaging.
Our findings fit into the bigger picture of scanning options without the use of contrast agent for patients with myocardial scarring and renal failure.
DECLARATIONS
Ethics approval: This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the University of Duesseldorf.
Consent to participate: Informed consent was obtained from all individual participants and from their parents or guardians included in the study.
Consent for publication: The patients provided signed informed consent regarding publication of their data.
Author’s contribution statement
Britta Elkenhans wrote the main manuscript text and conducted the experiments. Ingmar Vieregge and Florian Boenner edited the manuscript. Malte Kelm and Tienush Rassaf supervised the experiments.
ACKNOWLEDGEMENTS
To Juliane Geisler and Bernhard Schnackenburg