Usefulness of Low Dose Topoisomerase I Inhibitor (Irinotecan) as Add on Therapy Along with Low Dose Rituximab in Lupus Nephritis Flare Patients with Deteriorating Renal Function - Our Initial Experience
- 1. Saveetha Medical College Hospital, Thandalam Tamilnadu
Abstract
Effective therapy for a lupus nephritis flare is still elusive. Topoisomerase I inhibitor irinotecan as add on therapy is shown to have favourable outcome in lupus nephritis but its use in lupus nephritis flare is not reported.
Objective: To study the usefulness, safety, and outcome of low dose irinotecan topoisomerase I inhibitor as add on therapy in a lupus nephritis flare.
Method: From January 2018 through December 2022, 4 patients with lupus nephritis who had a lupus flare and a rise in serum creatinine were administered low-dose irinotecan along with low dose rituximab (RTX). Other conventional immunosuppressants were continued. All patients were followed up minimum one year. In the event of a repeat flare, the same regimen is to be repeated. Renal function, routine clinical and blood parameters, and the urine protein to creatinine ratio (upcr) recorded at regular intervals during follow up. Kidney biopsy to be done when indicated.
Results: Patient-1 on presentation had a urine protein/creatinine ratio upcr 1.05 after 4 doses of 100 mg/week irinotecan and 200 mg of single dose RTX; upcr reduced to 0.7; it further reduced to 0.2 end of six months on follow up. Renal function improved from 2.1 mg/dl serum creatinine (s.cr) on presentation to 0.8 mg/dl end of three months and remained stable. Patient has proteinuric flare two years subsequently and received second dose of irinotecan and RTX. Upcr from 2.30 reduced to 0.50 end of four weeks and in eight weeks urine albumin was found trace. The skin rash at the presentation also disappeared. Patient 2, a lupus nephritis patient, had a rise in s.cr from 2.6 to 6.1 mg/dl and her upcr 1.56. At end of eight weeks after low dose irinotecan and RTX single dose, her s.cr came down to 2.9 with PCR of 0.85 and blood pressure well controlled. Patient-3 In spite of four doses of irinotecan given alongwith inj RTX 200mg single dose her creatinine remained at 5.89mg/dl and progressed to ESRD in one year. Patient 4, who required hemodialysis upon a lupus flare, was withdrawn from hemodialysis after receiving four doses of irinotecan and 200 mg of RTX single dose. His upcr was 0.75 in last follow up and s.cr 5.8 mg/ dl down from 8.7 mg/dl.
Conclusion: Irinotecan, a non immunosuppressive add on drug, along with low dose rituximab, was useful in our patient during a flare of lupus nephritis. Further study is needed to understand its use in lupus nephritis patients
Keywords
- Lupus Nephritis
- Lupus Flare
- Irinotecan
- Topoisomerase I Inhibitor
- ESRD
- Immunosuppressant
- Proliferative Nephropathy
Citation
Ravichandran P, Singh S, Soundararajan P (2023) Usefulness of Low Dose Topoisomerase I Inhibitor (Irinotecan) as Add on Therapy Along with Low Dose Rituximab in Lupus Nephritis Flare Patients with Deteriorating Renal Function - Our Initial Experience. J Clin Nephrol Res 10(1): 1110.
BACKGROUND
Irinotecan, a topoisomerase I inhibitor, modified the double- stranded DNA (dsDNA) and subsequently the binding of anti- dsDNA antibodies. It is used in high doses in colorectal cancer, but at very low doses, it showed a good response in reversing advanced lupus nephritis in mice [1]. The drug irinotecan, not being an immunosuppressant, was considered safe in lower doses as add on therapy for refractory lupus nephritis [2]. Role of this non immunosuppressant drug as add on therapy in lupus nephritis flare is not reported till date. Since 2018 until 2021, we used this drug on four patients with lupus nephritis, in whom there was sudden deterioration in renal function during a lupus nephritis flare, almost reaching the stage of hemodialysis.
CASE REPORT
Here we report a series of 4 cases in whom low dose irinotecan was given as an add on therapy dose of 100 mg/wk maximum of four weeks along with an inj RTX 200 mg single dose during a flare up of lupus nephritis, as per the BILAG index, with prior written and informed consent. Dose to be repeated if there is a subsequent flare, and routine immunosuppressants, including mycophenolate mofetil and steroids, to be continued as maintenance immunosuppressants.
Case 1:
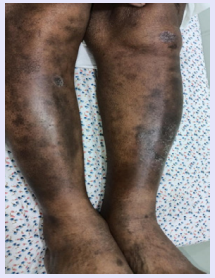
A 55 years old male in Sept 2018 presented with lupus proteinuric flare body weight increased from 64 to 84 Kgs, blood pressure 160/100 mm Hg, anasarca, skin rashes on abdomen and in both lower limbs, serum albumin 1.7 gm/dl, upcr 10.50 with s.cr 2.1mg/dl on admission. Anti-Nuclear Antibody (ANA), anti-smith and anti dsDNA antibodies were positive and PLA2R antibody was negative. A renal biopsy done at that time was consistent with class V lupus nephritis, and the immunofluorescence showed a full house pattern. The patient’s condition did not respond to pulse steroids and cyclophosphamide therapy. He was continued on oral steroids along with mycophenolic acid (MMF), three drug antihypertensives, and diuretics. With no improvement in the six-month follow up period and s.cr increasing from 2.1 mg/ dl to 3.2 mg/dl he was started on a low dose of irinotecan 100 mg per week along with RTX 200 mg as a single dose. Irinotecan was continued for 6 weeks and patient followed up every fifteen days subsequently. In April 2019 his body weight was 65 kgs, had no edema, s.cr 0.8m/dl, skin rashes disappeared and 24hrs urine protein 200gms/d. He was subsequently maintained on prednisolone 10 mg per day along with hydroxychloroquine (HCQ) 200 mg per day and MMF 500 mg twice. In October 2022, when the dose of MMF was reduced to one per day and HCQ was stopped, he again developed skin rashes in the lower limbs and a lupus proteinuric flare, with upcr increasing to 1.2 and in two weeks to 2.40. Inj RTX 200 mg single dose alongwith, irinotecan 100 mg weekly dose for 4 weeks was administered for his second time lupus proteinuric flare. The steroid dose was increased to 20 mg per day, and MMF was restarted at 500 mg bid. Two months subsequently in Dec 2022 his urine showed trace protein and serum creatinine 1.1mg/dl. He had normal lipid profile and skin lesions completely healed (Figure 1).
Figure 1: Case 1. Showing Healed lupus skin lesions After low dose irinotecan and rituximab.
His antihypertensives were stopped, and he was started on an SGLT2 inhibitor. We have planned a renal biopsy along with an electron microscopy study in the future once the patient consents for the same.
Case 2:
A 28 years old female patient presented to rheumatology department initially in the year 2018, with history of rashes, arthralgia. ANA and DsDNA were both positive. She was on HCQ and steroids. She was referred to the renal unit for evaluation and refused a kidney biopsy. She was initiated on MMF (500 mg bid alongwith HCQ and steroids. Her S Cr was 1.6mg/dl, upcr 1.25, blood pressure 140/80 mmHg controlled with calcium channel blocker. On further follow up in 2020, her urine showed active sediments and upcr 1.39 with a creatinine level of 2.1mg/dl. She was advised biopsy that she again refused and lost to follow up. In March 2022, she presented with generalised anasarca, blood pressure of 200/110 mmHg, and serum creatinine of 6.2 mg/dl. She was advised to undergo dialysis elsewhere, which she refused, and came back to us for further management. Her dose of MMF was increased, and inj RTX 200 mg single dose was given alongwith Inj irinotecan 100mg /week for four weeks. Her blood pressure was not well controlled with four drug antihypertensives, and edema persisted despite diuretics. In meantime renal biopsy was done on Oct 2022 that showed class IV lupus total modified NIH lupus nephritis activity score of 6/24 and chronicity score 6/12. After ten weeks follow up her repeat upcr was 0.96 and s.cr 3.12mg/dl. Blood pressure was well controlled, and the patient was asymptomatic. She was continued on prednisolone 20mg per day alongwith HCQ and MMF. On last follow up in Jan 2023 her serum Cr was 2.9mg/dl and 24 hrs urine protein 900 mg/d. C3 , C4 levels were normal. The patient complained of gastrointestinal symptoms and cramps on each dose of irinotecan that responded to symptomatic treatment. No other adverse effects were observed in this patient.
Case 3:
A 56 year old female presented to us in December 2019, she was a diagnosed case of class-IV lupus nephritis as per the renal biopsy report, with advanced glomerulosclerosis, interstitial fibrosis. She presented with a creatinine level of 6.4 mg/dl. She was advised hemodialysis elsewhere and she did not want to undergo the procedure and wanted second opinion and be on medical therapy that could postpone hemodialysis. Her blood pressure was 200/110 mmHg with four drug antihypertensives, and she had symptoms of cardiac failure and pulmonary edema. She was started on sacubitril valsartan (ARNI) and SGLT2 inhibitors, along with diuretics. Her cardiac failure improved and her blood pressure was controlled. Subsequently she was started on Inj irinotecan 100mg per week for five week alongwith MMF 500mg bid and she refused steroids. HCQs was stopped as she had adverse reaction. Her upcr was 1.2 and her urine showed active sediments. On follow up her blood pressure was on the higher side, the edema persisted, and in a span of six months, she had presented with pulmonary edema three times as ARNI was withdrawn due to hyperkalaemia. She was lost to follow-up, but after one year she returned for further treatment, and her s.cr was 8.9 mg/dl, and she was initiated on hemodialysis. She is still on maintenance dialysis with no urine output.
Case 4:
A 34 year old male who was diagnosed with lupus nephritis presented with sudden onset breathlessness, and s.cr 8.7 mg/dl and a blood pressure of 180/120 mmhg. He was admitted and hemodialysis was initiated. After four dialysis sessions, he was stable and was discharged with antihypertensives and an A-V fistula created. His serum creatinine at discharge was 9.0 mg/ dl. He had been on hemodialysis for six months, and on routine evaluation, he gave a history of passing a good amount of urine. Hemodialysis was continued, serum creatinine remained at 8.9 mg/dl, and serology evaluation showed dsDNA and ANA positivity; his upcr was 2.25; a kidney biopsy was not done due to a high risk of bleeding. He was initiated on irinotecan 100 mg per week for four weeks and a single dose of RTX 200mg iv, along with MMF 500mg bid and steroids at 10mg per day. At end of six mo. follow up his creatinine was 4.5 mg/dl when he was weaned away from hemodialysis. Subsequently, one month after stopping hemodialysis, his creatinine remained at 5.8 mg/dl. He remained asymptomatic subsequently with well controlled blood pressure with one drug hypertensive drug. His upcr was 0.75 and ANA 1+ dsDNA negative in last follow done on Jan 2023.
DISCUSSION
Relapses or flares of systemic lupus erythematosus (SLE) are frequent and observed in 27–66% of patients, and more than a quarter of those already on hemodialysis also experience a disease flare [3-5]. Increased morbidity results from damage due to lupus nephritis as well as drug side effects. Prevention of renal flares might, therefore, also decrease long-term morbidity and mortality [6]. Higher s.cr >2mg/dl, at the time of flare carries worse prognosis.
Induction and/or immunosuppressive therapy are recommended in such patients, yet there is always a need for newer interventions [7]. In the present study, we selected only those patients in whom the disease had progressed and almost reached a stage where they were on the verge of hemodialysis, as seen in cases 2, 3, and 4. Whereas in Case No. 1, the routine regimen was not working for him, and he was frequently coming back with side effects from drugs that all patients could not afford, like full dose rituximab.
Lupus nephritis and irinotecan LUNIRI study done in mice showed topoisomerase I inhibitor irinotecan role in reversing lupus nephritis and prolonging the survival of NZB/W F1 mice. This effect was accompanied by the induction of ssDNA breaks, the inhibition of renal cell apoptosis, and the prevention of subendothelial IgG deposits. Irinotecan also had a different mechanism from what is known about its anticancer therapy [1,2]. Though the study provided evidence of the role of a topoisomerase I inhibitor in lupus nephritis, no major trial has been done to date to see its benefit as a non-immunosuppressive agent in lupus nephritis patients.
DNA topoisomerase expression was shown to be high in chronic proliferative glomerulonephritis and in lupus nephritis. It was shown to be increasingly expressed in glomeruli, tubules, and interstitial monocyte infiltrates in lupus nephritis compared to other types of nephritis [8]. This study also raised the possibility of using a topoisomerase I inhibitor in the treatment of glomerulonephritis [8]. These reasons made us believe that the non-immunosuppressive drug irinotecan may be of benefit to our patient, who had no other option left.
A lupus flare is usually precipitated by environmental factors that may trigger the disease. These include exposure to UV light, infections, certain hormones, and drugs that may activate the innate and adaptive immune systems, resulting in inflammation, cytotoxic effects, and clinical symptoms. Increased antigen load into the circulation is one reason for such flare that triggers autoimmune antibody response [6]. Thus, timely control of lupus flares can be achieved by suppressing excessive immune system activation. Irinotecan’s ability to prevent subendothelial basement membrane deposit is a possible action that may be one reason why, in our patients, we saw a good response in the recovery of renal function as well as an improvement in proteinuria. In order to further prove this, we have planned further trials, whereas in the present study, conclusive concrete evidence could not be drawn as to the role of irinotecan and the improvement seen in the patients.
Two types of renal flares are described: (i) nephritic flares and (ii) `proteinuric flares, characterised by an increase in proteinuria of at least 2 g per day or a doubling of proteinuria in a nephrotic patient without modification of plasma creatinine [7]. Whenever a nephritic or proteinuric flare is diagnosed, aggressive treatment is recommended for better ten year survival. Even though the prognosis of lupus flares has improved with early intervention, The main problem for the physician still remains as compromise between the efficacy and the toxicity of therapy. In developing countries, patients present themselves late, as seen in our patients. The frequency of lupus flares by BILAG index [9] increases and so does the renal failure progression. Refractory lupus nephritis indicates an inadequate response to lupus nephritis therapy. It implies persisting or worsening disease activity despite therapy, but the definition is complicated by the parameters of response, proteinuria, and renal function, which do not clearly discriminate between activity and irreversible damage [10-12].
Administration of low-dose irinotecan as an add-on medication for the treatment of refractory lupus nephritis was shown to be safe in a case report where the author hypothesised that low-dose irinotecan could be a potential new treatment for lupus nephritis, using the DNA modification mechanism of irinotecan rather than immunosuppression as a mechanism [2]. Though there was beneficial effect on proteinuria conclusion could not be drawn if it will be useful in lupus flares. In the present study, we had Case No. 1, who had a proteinuric flare, and it responded rapidly to low doses of irinotecan and low single dose RTX. Similar responses were seen during his subsequent flare. A rapid reversal of proteinuria was seen subsequent to low dose irinotecan and RTX administered during the second flare episode. No pulse steroid or cyclophosphamide was used, which makes us believe that combination therapy of irinotecan and RTX is an alternate option when steroids are to be avoided or less toxic drugs are to be used. A kidney biopsy is contemplated, along with an electron microscopy study, in this patient once he gives his consent to see the changes in the basement membrane.
The patient in Case 2 had a nephritic flare, and his creatinine jumped to 6.6 mg/dl. The patient’s renal function stabilised at 2.9 mg/dl after six months, though the urine still shows the presence of pus cells and 2+ urine protein. In both cases, blood pressure was better controlled following the therapy with irinotecan, and no side effects were observed. Case 3 presented with renal and cardiac failure and, within six months, had to be on hemodialysis. Case 4 responded well in that he was weaned off hemodialysis and remains stable on MMF and steroids. The only side effects noticed during irinotecan administration were diarrhoea and a mild headache that were self-limited and responded to symptomatic therapy.
The current objective unmet need in the management of lupus nephritis is an improved remission rate, indicated by improvements in proteinuria, improvement in GFR, reduction of flares, reduced side effects, and low cost [13]. The lunar trial that used rituximab for lupus flare as an induction agent showed that complete elimination of CD-19 cells correlated with better remission. The dose used and cost of such therapy limit its routine use [14]. Current evidence, though, supports the off-label use of RTX to induce remission of refractory LN, but in our case we observed better results with irinotecan as add on therapy. Our own previous experience in use of rituximab in low dose in high risk renal transplant has shown that in India patients 200mg single dose rituximab had profound effect on CD-19 count and also cost effective for us to use this low dose rituximab in the study [15]. If similar or better results could have been achieved even without rituximab can be answered only if larger controlled trial is done and in this limited case series it is difficult to analyse if combination of irinotecan and ritux feared better or not, though clinically we found this as a better combination. Lupus flares are shown to arise through immune pathways involving activation of naive B cells, monocytes or both [16]. Thus, it is understandable that the use of irinotecan alone, without ritux or steroids, would not have shown better results. Improvement in proteinuria and GFR, with s.cr failing to reach more than 50% of baseline, was observed in three patients. The case no 3 patient did not respond as she also had other comorbid conditions. Though this patient’s cardiac failure and fluid overload status responded better to diuretic and heart failure therapy, the patient still required dialysis as her potassium levels prevented us from maintaining her on ARNI resulting in frequent episodes of fluid overload that required hemodialysis.
In the present series, patient no. 3 did not respond to the irinotecan and ritux therapy.She was also not willing to take steroids for fear of aggravation of hypertension and cardiac disease. If adding steroids to her regimen would have been better or not, it cannot be concluded unless further study is done to see if steroids are crucial in any regimen or until newer drugs show proven benefits without steroids.
Most of patients, as seen in the study, come to renal unit late into their disease and lupus being a chronic disease are referred to nephrology unit only when lupus nephritis progresses with deterioration in renal function. All the patients in this study did not have a proper medication history, nor are the data on SLEDAI or BILAG made available. All patients that were selected were those who were in lupus flare and long term follow up will provide more insight if lupus flare incidence can also be reduced with this regimen. Further study is also needed to see if the same regimen is useful in other proliferative diseases where irinotecan can be given as a non immunosuppressive add on drug for its role in the inhibition of topoisomerase I which is expressed in glomeruli, interstitial spaces, and tubules in various types of glomerulonephritis and in proliferative immune cells [8].
Skin rashes in lupus is manifested in variety of ways and various therapy given for healing of lesions. In case no. 1, the patient had subacute cutaneous lupus erythematosus (SCLE) and skin lesions simulating a psoriatic type pattern. The skin lesions on both occasions disappeared in one week as soon as Irinotecan and rituximab were given on both occasions. Figure 1 shows the healed skin lesion in the patient. A similar disappearance of skin lesions was seen in an animal study using irinotecan but not reported in a human study [17,18].
CONCLUSION
Irinotecan low dose combined with low dose rituximab was found to be safe and cost effective in treating lupus nephritis flare. Further controlled trial is needed to see if it can be used in routine as non immunosuppressive add on agent in lupus nephritis patients.
REFERENCES
- Manuela Frese-Schaper, Jakob Zbaeren, Mathias Gugger, Marc Monestier, Steffen Frese. Reversal of Established Lupus Nephritis and Prolonged Survival of New Zealand Black × New Zealand White Mice Treated with the Topoisomerase I Inhibitor Irinotecan. J Immunol. 2010; 184: 2175-2182.
- R Biesen, M Frese-Schaper, P Enghard, Q Cheng, F Hiepe, S Frese. Refractory mixed proliferative and membranous lupus nephritis treated with the topoisomerase I inhibitor irinotecan as add-on therapy. Scand J Rheumatol. 2022; 51: 237-240.
- Sprangers B, Monahan M, Appel GB. Diagnosis and treatment of lupus nephritis flares—an update. Nat Rev Nephrol. 2012; 8: 709-717.
- Illei GG, Takada K, Parkin D, Austin HA, Crane M, Yarboro CH, et al. Renal flares are common in patients with severe proliferative lupus nephritis treated with pulse immunosuppressive therapy: long-term follow up of a cohort of 145 patients participating in randomized controlled studies. Arthritis Rheum. 2002; 46: 995-1002.
- Young-Eun Kim, Su Jin Choi, Doo-Ho Lim, Hyosang Kim, Soo Min Ahn, Ji Seon Oh, et al. Disease Flare of Systemic Lupus Erythematosus in Patients with End Stage Renal disease on dialysis. J Rheumatol. 2022; 49: 1131-1137.
- Fernandez D, Kirou KA. What Causes Lupus Flares? Curr Rheumatol Rep. 2016; 18: 14.
- Ponticelli C, Moroni G. Flares in lupus nephritis: Incidence, impact on renal survival and management. Lupus. 1998; 7: 635-638.
- Ivanova LV, Rudolph P, Kellner U, Jürgensen A, Tareeva IE, Alm P, et al. Expression of DNA topoisomerases in chronic proliferative kidney disease. Kidney Int. 2000; 58: 1603-12.
- C Gordon, N Sutcliffe, J Skan, T Stoll, DA Isenberg. Definition and treatment of lupus flares measured by the BILAG index. Rheumatology. 2003; 42: 1372-1379.
- Kronbichler A, Brezina B, Gauckler P, Quintana LF, Jayne DRW. Refractory lupus nephritis: When, why and how to treat. Autoimmun Rev. 2019; 18: 510-518.
- Yu KH, Kuo CF, Chou IJ, Chiou MJ, See LC. Risk of end-stage renal disease in systemic lupus erythematosus patients: a nationwide population-based study. Int J Rheum Dis. 2016; 19: 1175-1182.
- Samir Parikh, Lee Hebert & Brad Rovin. Protecting the kidneys in lupus nephritis, Int. J. Clin. Rheumatol. 2011; 6: 529-546.
- Kapsia E, Marinaki S, Michelakis I, Liapis G, Sfikakis PP, Boletis J, et al. Predictors of Early Response, Flares, and Long-Term Adverse Renal Outcomes in Proliferative Lupus Nephritis: A 100-Month Median Follow-Up of an Inception Cohort. J Clin Med. 2022; 11: 5017.
- Weidenbusch M, Römmele C, Schröttle A, Anders HJ. Beyond the LUNAR trial. Efficacy of rituximab in refractory lupus nephritis. Nephrol Dial Transplant. 2013; 28: 106-11.
- Ravichandran P, Natrajan T, Jaganathan R. Combination treatment of low dose Anti-Thymocyte Globulin (ATG), Rituximab and high dose Sirolimus as induction agents in immune-conditioned recipients. Int Immunopharmacol. 2006; 6: 1973-6.
- Lu R, Guthridge JM, Chen H, Bourn RL, Kamp S, Munroe ME, et al. Immunologic findings precede rapid lupus flare after transient steroid therapy. Sci Rep. 2019; 9: 8590.
- Luís Uva, Diana Miguel, Catarina Pinheiro, João Pedro Freitas, Manuel Marques Gomes, Paulo Filipe. “Cutaneous Manifestations of Systemic Lupus Erythematosus”. Autoimmune Diseases. 2012; 15.
- Keil A, Hall SR, Körner M, Herrmann M, Schmid RA, Frese S. Suppression of lupus nephritis and skin lesions in MRL/lpr mice by administration of the topoisomerase I inhibitor irinotecan. Arthritis Res Ther. 2016; 18: 243.