The Prognostic Impact of Cyclin D1 Expression in Patients with Multiple Myeloma, an Egyptian Study
- 1. Clinical pathology Department, National Cancer Institute, Cairo University, Egypt
- 2. Clinical and chemical Pathology Department, Faculty of medicine, Cairo University
- 3. Medical oncology Department, National Cancer Institute, Cairo University
Abstract
Background: Multiple myeloma is a multifocal hematological disorder, which shows a proliferation of malignant plasma cells in the bone marrow, it represents approximately 1% of all malignancy, causing about 20% of deaths from hematological malignancies. Cyclin D1 is considered an important factor in the cell cycle control; dysregulation of cyclin D1 has an oncogenic role in multiple myeloma patients and can affect their prognosis.
Aim of the study: To determine the prognostic effect of cyclin D1 and correlate its expression with selected clinic-pathological features
Methods: This study was conducted on fifty patients diagnosed as multiple myeloma at National Cancer Institute, Cairo University to study the expression pattern of cyclin D1 by immunohistochemistry on their bone marrow biopsy specimens, patients were followed up for one year.
Results: Cyclin D1 positive expression was found in 19/50 (38%) of the patients and it was positively correlated with bone marrow fibrosis, initial serum total protein level and serum B2 microglobulin. Also, correlation of the treatment response and the outcome of the patients with cyclin D1 expression were found significant.
Conclusion: Cyclin D1 has a significant poor prognosis in multiple myeloma patients
Keywords
• Multiple myeloma
• Cyclin D1
• immunohistochemistry
CITATION
Abobakr A, Rashed RA, El-Dein El-Sayed MAM, Nasr AS, Mansour OM, et al.(2023) The Prognostic Impact of Cyclin D1 Expression in Patients with Multiple Myeloma, an Egyptian Study. J Hematol Transfus 10(2): 1116.
INTRODUCTION
Multiple myeloma (MM); one of the B-cell malignancies which had been identified by abnormal overgrowth of malignant plasma cells. Patients with M.M exhibit abnormal production of immunoglobulins with various clinical findings as anemia, renal impairment, hypercalcemia and also osteolytic bone lesions [1], it has a poor prognosis and increasing incidence with age [2]. Worldwide, MM constitutes about 1% of all the malignant diseases, it also accounts for about 10-15% of hematopoietic malignancies [3]. In Egypt, the incidence of MM is about 1.3% of all malignant disorders [4] with mean age 58.5 years and with higher incidence in males than females [5].
In recent years, with the advances in therapeutic approaches and treatment protocols of MM cases, many patients achieve durable response [6], however, the overall prognosis remains unsatisfactory. So, there is a need to discover new prognostic markers and indicators aiming to improve the patients' clinical outcome [7]. Cyclins and Cyclins Dependent Kinases (CDKs) areconsidered a pivotal regulator of the cell cycle with few cyclins–CDKs complexes were linked to the cell cycle regulation and exhibit other different cellular functions [8]. D-type cyclins (Cyclin D1, D2 and D3) which interact with CDK4 and CDK6 forming cyclin D1-CDK4 complex which translocate into the nucleus with the ability to modulate the transition from G1 to S phase [9].
Cyclin D1, is encoded by CCND1 gene on chromosome 13, it's widely expressed by most of the human cells, the higher expression level led to uncontrolled cellular proliferation, therefore promoting tumor development and considered as an oncogenic driver in many types of malignant disorder as lung cancer, renal cell carcinoma [10] ,breast cancer [11] and laryngeal carcinoma [12]. CD1 overexpressed present in lymphoid neoplasms such as multiple myeloma and mantle cell lymphoma [13]. Overexpression of cyclin D1 was found in about 50% of MM patients and can affect their prognosis [14].
The aim of this study was to identify the expression pattern of cyclin D1 in MM patients by immunohistochemistry (IHC) on their bone marrow biopsy specimens, and to correlate them with the pathological and prognostic parameters of Egyptian MM patients.
MATERIALS AND METHODS
Our patients were recruited from the Medical Oncology clinic, National Cancer Institute, Cairo University in the period between January 2020 till December 2020 then followed up for a year till December 2021. Local institutional research board approval was taken prior to the study. All patients were Egyptians, their age range between 35 to 76 years. We established the diagnosis through examination of wright –Giemsa-stained BM aspirate smears to assess the percentage of plasma cells (≥ 10% of clonal plasma cells) is required for diagnosis, also through serum protein electrophoresis for detection of monoclonal bands (M. Band) and serum immunofixation.
Other laboratory investigations including: complete blood picture, kidney function tests (urea and creatinine), total serum calcium, total serum protein, Lactate dehydrogenase (LDH), β2 microglobulin and serum albumin as described by International Myeloma Working Group [15].
Cyclin D1 Immunohistochemistry
Immunohistochemical staining was performed on core bone marrow biopsy, on paraffin embedded 2-3μ-thick tissue sections using anti-cyclin D1 (rabbit monoclonal IgG. Genemed, Biotechnologies). Preliminary preparation of slides was done through routine de-paraffinization and rehydration, antigen retrieval was required for the 1ry antibody in PH 9 in pressure cooker for 20 minutes. Endogenous peroxidase was blocked by Peroxidase Blocking Solution, and then anti-Cyclin D1 was added to cover the tissue completely. Ready to use Poly horseradish peroxidase (HRP) was used as enzyme conjugate. DAB as chromogen solution was added to DAB Buffer Solution and was added to cover the slides. This was followed by Counterstain with diluted hematoxylin for 3 minutes. Finally mounting the slides was done using DPX mountant.
Interpretation of the Results
Examining the stained slides for cyclin D1, using positive internal control as the staining of the endothelium in the tested slides by cyclin D1, we evaluated the staining nuclear pattern, the positive reaction was also graded semi-quantitatively according to number of positive nuclei to grade1 (10-19% nuclei positive) and grade 2 (20-50% nuclei positive) and finally grade 3 (> 50%nuclei positive) [16].
Treatment protocol
Patients either received VAD protocol (Vincristine, Doxorubcin, Dexamethasone), Thalidomide, Alkeran, DCEP protocol (Dexamethasone, Cyclophosphamide, Etoposide, Cisplatin) or Velcade and they were followed up for one year to assess their response of treatment. Patients were divided according to their response to treatment after 1 year into two groups: responded cases and refractory or relapsed cases [17].
STATISTICAL ANALYSIS
Statistical analysis was done using IBM© SPSS© Statistics version 22 (IBM© Corp., Armonk, NY, USA). The numerical data were expressed as mean and standard deviation or median and range as appropriate. While qualitative data were expressed as frequency and percentage. Chi-square test or Fisher’s exact test was used to examine the relation between qualitative variables. Also, for not normally distributed quantitative data, comparison between two groups was done using Mann-Whitney test (non- parametric t-test). All tests were two-tailed. A p-value < 0.05 was considered statistically significant.
RESULTS
This study was carried out on 50 newly diagnosed patients with MM; they were 25 males and 25 females with male: female ratio 1:1. Patients' characteristic and initial laboratory findings were shown in Table 1.
Table 1: Correlation of cyclin D1 expression with demographic data and initial laboratory investigations
|
Initial lab data (n=50) |
Cyclin D1 –ve (%) |
Cyclin D1 +ve (%) |
P value |
|
Age group |
|
|
|
|
? 60 years (no=28) |
18 (64.3%) |
10 (35.7%) |
0.707 |
|
≥ 60 years (no=22) |
13(59.1%) |
9 (40.9%) |
|
|
Sex |
|
|
|
|
Male (n=25) |
16 (64%) |
9(36%) |
0.771 |
|
Female(n=25) |
15(60%) |
10(40%) |
|
|
Initial Hb (gm/dl) |
|
0.183 |
|
|
< 10 gm/dl (n=31) |
17(54.8%) |
14(45.2%) |
|
|
≥ 10 gm/dl (n=19) |
14(73.3%) |
5(26.3%) |
|
|
Initial TLC |
|
0.089 |
|
|
< 4 (×103/cm3) (n=7) |
2(28.6%) |
5(71.4%) |
|
|
≥ 4 (×103/cm3) (n=43) |
29(67.4%) |
14(32.6%) |
|
|
Initial Plt count |
|
0.334 |
|
|
? 150(×103/cm3) (n=17) |
9(52.9%) |
8(47.1%) |
|
|
≥ 150(×103/cm3) (n=33) |
22(66.7%) |
11(33.3%) |
|
|
Bone marrow fibrosis |
|
0.033 |
|
|
No fibrosis (n=17) |
14(82.4%) |
3(17.6%) |
|
|
Fibrosis (n=33) |
17(51.5%) |
16(48.5%) |
|
|
Initial Urea |
|
0.425 |
|
|
≤ 45mg/dl (n=22) |
15(68.2%) |
7(31.8%) |
|
|
>45mg/dl (n=28) |
16(57.1%) |
12(42.9%) |
|
|
Initial creatinine |
|
0.879 |
|
|
≤ 1.2 mg/dl (n=27) |
17(63%) |
10(37%) |
|
|
> 1.2 mg/dl (n=23) |
14(60.9%) |
9(39.1%) |
|
|
Initial total calcium |
|
0.075 |
|
|
>10.2 mg/dl (n=29) |
21(72.4%) |
8(27.6%) |
|
|
≤10.2 mg/dl (n=21) |
10(47.6%) |
11(52.4%) |
|
|
Initial LDH |
|
0.5 |
|
|
Up to 250 u/l (n=16) |
11(68.75%) |
5 (31.5%) |
|
|
> 250 u/l (n=34) |
20(58.8%) |
14 (41.2%) |
|
|
Class of Ig |
|
0.61 |
|
|
Ig A (n=18) |
12 (66.7%) |
6 (33.3%) |
|
|
Ig G (n=32) |
19 (59.4%) |
13(40.6%) |
|
|
Light chain |
|
0.336 |
|
|
Kappa (n=28) |
19 (67.9%) |
9 (32.1%) |
|
|
Lambda (n=22) |
12 (54.6%) |
10 (45.4%) |
|
|
Initial Albumin |
|
0.693 |
|
|
<3.5 g/dl (n=42) |
25 (59.5%) |
17 (40.5%) |
|
|
3.5-5.2 g/dl (n=8) |
6 (75%) |
2 (25%) |
|
|
Initial total protein |
|
0.041 |
|
|
≤ 8.7 g/dl (n=10) |
9 (90%) |
1(10%) |
|
|
> 8.7 g/dl (n=40) |
22 (55%) |
18 (45%) |
|
|
Initial ß2 microglobulin |
|
0.05* |
|
|
≤ 5.2µg/ml (n=15) |
12 (80%) |
3 (20%) |
|
|
> 5.2µg/ml (n=35) |
19 (54.3%) |
16 (45.7%) |
|
|
International staging system (ISS) |
|
|
|
|
Stage 1& 2 (n=15) |
12 (80%) |
3(20%) |
0.086 |
|
Stage 3 (n=35) |
19(54.3%) |
16(45.7%) |
|
*Significant P value < 0.05
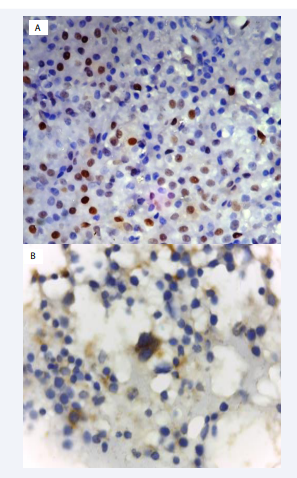
All our patients showed an increase in marrow plasma cells as shown in Figure (1).
Figure 1: A: trephine biopsy section shows positive cyclin D1 nuclear staining in plasma cells by IHC B: trephine biopsy section shows cyclin D1 negative in plasma cells by IHC
All our patients showed M band in serum Protein electrophoresis, 35/50 (70%) of the patients were in gamma region and 15/50 (30%) were in beta region. By immunofixation, 32/50 (64%) were Ig G and 18/50 (36%) were Ig A, as for the light chain 28/50 (56 %) showing Kappa light chain, and 22/50 (44%) showing lambda light chain. Patients showing multiple osteolytic lesions were 48/50 (96%) and Patients with fibrosis in bone marrow biopsy were 33/50 (66%) with grade I fibrosis: 15/33 (30%), grade II fibrosis: 11/33(22%), grade III fibrosis: 6/33 (12%) and grade IV fibrosis: 1/33 (2%).
Cyclin D1 results on BMB
Cyclin D1 positive patients were 19/50 (38%). While patients with cyclin D1 negative were 31/50 (62%). Positive reaction was described as grade1 were 13/50 (26%), grade 2 were 2/50 (4%), and grade 3 were 4/50 (8%) shown in Figure 1.
Correlation of Cyclin D1 with demographic data and initial laboratory investigations
Correlation of cyclin D1 expression with our patients' demographic characteristic and initial laboratory investigations, shows statistically significant correlation between cyclin D1 positivity and elevated total protein level with P value = 0.041 , with bone marrow fibrosis with P value = 0. 033 and with initial serum B2 microglobulin level with P value =0.05 (Table 1).
Correlation of Cyclin D1 with response of treatment & clinical outcome
Patients were divided according to their response to treatment after 1 year into two groups: responded cases; those are Patients who responded to the treatment: 28/50(56%) and refractory or relapsed cases were 22/50 (44%). Among the patients who shows refractoriness to treatment 13/22 (59%) shows cyclin D1 positivity, and there was a statistically significant correlation between cyclin D1 positive expression and response of the patients with P. value = 0.006 (Table 2).
Table 2: Correlating of cyclin D1 expression with response to treatment and outcome of the patients:
|
|
Cyclin D1 –ve (%) |
Cyclin D1 +ve (%) |
P value |
|
Response |
|
0.006 |
|
|
Respond (n=28) |
22 (78.6%) |
6 (21.4%) |
|
|
Relapse/Refractory (n=22) |
9 (40.9%) |
13 (59.1%) |
|
|
Outcome |
|
0.006 |
|
|
Alive (n=35) |
26 (74.3%) |
9 (25.7%) |
|
|
Died (n=15) |
5 (33.3%) |
10 (66.6%) |
|
*Significant P value < 0.05
Patient who survived were 35/50(70%) and those who died were 15/50 (30%). Among the non survivors 10/15 (66.6%) were cyclin D1 positive, with statistically significant correlation between cyclin D1 positivity and poor outcome of the patients with P value = 0.006 (Table 2).
Correlation of bone marrow fibrosis with response of treatment
In our study bone marrow fibrosis was associated with poor treatment response, as among MM patients with fibrosis (33 patients), 14/33 (42.4%) responded and 19/33(57.6%) showed relapse/refractoriness. statistically significant correlation was found between poor response of the patients and the fibrosis of bone marrow (P value = 0.007). (Table 3).
Table 3: Correlating bone marrow fibrosis with the treatment response
|
Bone marrow fibrosis |
Respond (%) |
Relapse/ Refractory (%) |
P value |
|
No fibrosis (no=17) |
14 (82.4%) |
3 (17.6%) |
0.007* |
|
Fibrosis (no=33) |
14 (42.4%) |
19 (57.6%) |
|
*Significant P value < 0.05
Association between cyclin D1 expression and patients' follow up laboratory investigations
There was no statistically significant association between cyclin D1 expression and patients' follow up laboratory investigations, including hemoglobin level, TLC, platelet count, calcium level, LDH, albumin, total protein, and percentage of plasma cell in BMA, urea, creatinine, and ß2 microglobulin (Table 4).
Table 4: Association between cyclin D1 expression and patients' follow up laboratory investigations
|
Parameter |
Cyclin D1 negative (n= 26) |
Cyclin D1 positive (n= 9) |
P value |
||
|
No = 35 |
Range |
Mean ± SD |
Range |
Mean ± SD |
|
|
Hb (g/dl) |
5.0 - 13.0 |
10.4 ± 2 |
6.0 - 12 |
9.4 ± 2.1 |
0.239 |
|
TLC (×103/cm3) |
3.0 - 11.0 |
6.6 ± 2.1 |
3.0 - 8.0 |
5.3 ±1.4 |
0.073 |
|
Platelets (×103/cm3) |
55 - 500 |
243.7 ± 108 |
150 - 300 |
212.2 ± 51.9 |
0.362 |
|
Calcium (mg/dl) |
7.9 - 12 |
9.2 ± 0.9 |
8.1 - 13.2 |
9.8 ± 1.4 |
0.093 |
|
LDH (u/l) |
75 - 420 |
194.2 ± 78.3 |
120 - 550 |
229.4 ± 134.5 |
0.565 |
|
Albumin (g/dl) |
2.6 - 4.3 |
3.5 ± 0.4 |
2.1 - 4.1 |
3.1 ± 0.6 |
0.056 |
|
Total protein (g/dl) |
5.1 - 11.7 |
7.8 ± 1.4 |
6.1 - 12 |
8.5 ± 1.9 |
0.342 |
|
Parameter |
Range |
Median |
Range |
Median |
P value |
|
BMA (% of plasma cells) |
1.0 - 86 |
3 |
3.0 - 35 |
4 |
0.11 |
|
Urea (mg/dl) |
17 - 114 |
35 |
25 - 312 |
28 |
0.697 |
|
B2 micro (µg/ml) |
2.1 - 15 |
3.6 |
2.6 - 35 |
3.8 |
0.271 |
|
Creatinine (mg/dl) |
0.56 - 14 |
0.88 |
0.5 – 9.3 |
0.9 |
0.868 |
*Significant P value < 0.05
DISCUSSION
Cyclin D1 plays an important regulatory role in the normal cell cycle, dysregulations of cyclin D1 were found to be associated with early oncogenic effects in MM [18]. Our results demonstrated a significant correlation between bone marrow fibrosis at the time of diagnosis with both cyclin D1 positivity and response of the patients with P value = 0.033 and 0.007 respectively. Bone marrow fibrosis at the time of diagnosis and in the follow up of the patients of MM has not been studied in detail by many authors, however, Subramanian et al., 2007 who performed a study on 54 MM patients found that increased marrow fibrosis correlated significantly with poorly differentiated plasma cell morphology and mitotic figures which by themselves are considered poor prognostic markers in MM patients [19].
Serum B2 microglobulin is considered a reliable marker for the tumor burden in MM cases, it has been used in the initial stratification of the patients and in the follow up of their response. Our study showed a significant correlation between cyclin D1 positivity and elevated serum B2 microglobulin with P value (= 0.005).and this result was in agreement with Hoechtlen-Vollmar et al.,2002 who stated a significant correlation between cyclin D1 positive MM patients and elevated serum B2 microglobulin with P value =0.002 [20].
In our study; we found no statistically significant correlation between cyclin D1 expression and both age and gender of the patients (P value = 0.707 and 0.771), this was approved by Padhi et al.,2013 who stated that both groups cyclin D1 whether positive and negative showed no statistical significance difference as regards age and gender, in their study using the same IHC technique in examining cyclin D1 with a rabbit monoclonal antibody [16] and by Cook et al .,2006 who also found non- significant difference between cyclin D1 positive and negative MM patients in respect to age and gender [21].
Our study showed a non-significant correlation between hemoglobin concentration and cyclin D1 expression (P value = 0.183). While Padhi et al., 2013 stated that cyclin D1 positive group had significantly lower hemoglobin level (P value = 0.03) [16]. Also, our study demonstrated lack of significant correlation between cyclin D1 expression and any of; serum calcium level, creatinine level, type of monoclonal protein and light chain phenotype (P value = 0.075, 0.879, 0.61and 0.336 respectively). In consistent to our findings, Cook et al., 2006 [21] and Markovic et al., 2004 found that there was no statistically significant difference between cyclin D1 expression with any of the above parameters [22].
In this study , we found that cyclin D1 positive patients are considered to be associated with worse prognosis, it showed significant correlation with both response and outcome (P value=0.006 for both), concurring this result with Sewify et al.,2014 who stated that there is an association between cyclin D1 gene amplification, disease severity and lower overall survival, this may be due to the presence of higher percentage of plasma cells in the BM and more aggressive osteolytic lesions in cyclin D1 positive patients. They stated that cyclin D1 amplification also had an adverse prognostic value in MM patients [18]. Nazarovs et al.,2021 found that cyclin D1 expression was correlated with higher calcium level and more common osteolytic lesions in their study which involved 122 multiple myeloma patients and considered its expression as a poor prognostic marker in those patients [23].
Also, a recent meta-analysis confirmed that CCND1 overexpression can be a predictive biomarker for patients with MM treated with bortezomib, receiving ASCT, or in relapsed and refractory period [24]. On the same way Tasidou et al., 2012 who studied 130 newly diagnosed patients using IHC, concluded that positivity for cyclin-D1 expression enhanced disease activity and independently associated with inferior survival (P value = 0.001). This result supports the role of cyclin-D1 in the disease prognosis [25].
In consistent to our findings, Pruneri et al., 2008, used IHC and FISH in 48 MM patients, stated that their analysis for cyclin D1 expression was significantly associated with higher percentage of plasma cell in bone marrow and advanced clinical stage proving that cyclin D1 expression is more frequent in advanced stages than in early clinical stages (P value = 0.044) [26]. On the contrary, Markovic et al., 2004 whose results did not reveal prognostic significance of cyclin D1 overexpression (P value = 0.76) in MM cases [22].
LIMITATION
The main limitation of this study was we did not conduct any power analysis to calculate the sample size selected for this study. In addition, we only use IHC method and did not do other techniques like RT-PCR and FISH due to financial issues (no Fund).
CONCLUSION
There was significant correlation between cyclin D1 positivity in our MM patients and their response of treatment, they were more likely to have worse prognosis than cyclin D1 negative patients. Cyclin D1 detection by IHC in MM patient is considered feasible technique, readily available and cheaper than molecular techniques.
Ethics Statement
This study was approved by Ethical Committee of the National Cancer Institute, Cairo University. The research was carried out in conformity with the Declaration of Helsinki's ethical principles. To participate in this study, all subjects signed an informed consent form.
Informed consent
Informed written consent was obtained from all participants after the study objectives were explained and before blood sampling. Confidentiality of patient data was guaranteed.
REFERENCES
- Hussain M, Yellaprangada S, Al Hadidi S. Differential diagnosis and therapeutic advances in multiple myeloma. Blood lymphat Cancer. 2023; 13: 33-57.
- Moreau P, Miguel J, Sonneveld P, Mateos MV, Zamagni E, Avet- Loiseau H, et al. Multiple Myeloma: ESMO clinical practice guidelines for diagnosis, treatment and follow up clinical practice guidelines. ESMO- Ann Oncol. 2017; 28: 52-61.
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues (Revised 4th edtion).IARC: Lyon. 2017; 243-248.
- Ibrahim A, Khaled H, Mikhail N, Baraka H, Kamel H. Cancer incidence in Egypt: Results of National Population Based Cancer Registry Program. J Cancer Epidemiol. Hindawy Publishing Corporation. 2014; 437971.
- El Husseiny NM, Kasem N, El Azeeim HA, Mattar MW. Multiple myeloma: a descriptive study of 217 Egyptian patients. Ann Hematol. 2014; 93: 141-145.
- Dimopoulos MA, Terpos E, Boccadoro M, Delimpasi S, Beksac M, Katodritou E, et al. APOLLO Trial investigators. Daratumumab plus Pomalidomide and dexamethasone versus pomalidolide and dexamethasone alone in previously treated multiple myeloma (APOLLO): an open-label, randomized, phase 3 trial. Lancet Oncol. 2021; 22: 801-812.
- Hanbali A, Hussanein M, Rasheed W, Aljurf M, Al sharif F. The evolution of prognostic factors in multiple myeloma. Adv Hematol. 2017; 4812637.
- Hydbring P, Malumbres M, Sicinski P. Non-canonical functions of cell cycle cyclins and cyclin-dependent kinases. Nat Rev Mol Cell Biol. 2016; 17: 280-292.
- Jiang Y, Zhang C, Lu L, Wang X, Liu H, Hong L et al. The prognostic role of cyclin D1 in Multiple myeloma: A systematic review and meta- analysis. Technol Cancer Res Treat. 2022; 21: 1-16.
- Li Z, Liu J, Zhang X, Fang L, Zhang C, Zhang Z, et al. Prognosis and significance of cyclin D1 expression in renal cell carcinoma: A systematic review and meta-analysis. Pathol Oncol Res. 2020; 26: 1401-1409.
- He Q, Wu J, Liu XL, Ma YH, Wu XT, Wang WY, et al. Clinicopathological and prognostic significance of cyclin D1 amplification in patients with breast cancer: A meta-analysis. J Buon. 2017; 22: 1209-1216.
- Kowalczyk MM, Baranska M, Fendler W, Borkowska EM, Kobos J, Borowiec M, et al. G870A polymorphic variants of CCND1 gene and cyclin D1 protein expression as prognostic markers in laryngeal lesions. Diagnostics. 2022; 12: 1059.
- Zlamalikova L, Moulis M, Salek D, Jarkovsky J, Smarda J and Smardova J. Expression of D-type cyclins in mantle cell lymphoma and diffuse large B-cell lymphomas. Oncol Rep. 2016; 35: 2673-2680.
- Shah V, Sherbone AL, Walker BA, Johnson DC, Boyle EM, Ellis S, et al. Prediction of outcome in newly diagnosed myeloma: a meta-analysis of the molecular profiles of 1905 trial patients. Leukemia. 2018; 32: 102-110.
- Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014; 15: e538-548.
- Padhi S, Varghese RGB, Ramdas A. Cyclin D1 expression in multiple myeloma by immunohistochemistry: Case series of 14 patients and literature review. Indian J Med Paediatr Oncol. 2013; 34: 283-291.
- Sonneveld P, Avet-Loiseau A, Lonial S, Usmani S, Siegel D, Anderson K, et al. Treatment of multiple myeloma with high-Risk cytogenetics: a consensus of the international myeloma working group. Blood. 2016; 127: 2955-2962.
- Sewify EM, Afifi OA, Mosad E, Zaki AH, El Gammal SA. Cyclin D1 amplification in multiple myeloma is associated with multidrug resistance expression. Clin Lymphoma Myeloma Leuk. 2014; 14: 215-222.
- Subramanian R, Basu D, Dutta TK. Significance of bone marrow fibrosis in multiple myeloma. Pathology. 2007; 39: 512-515.
- Hoechtlen-Vollmar W, Menzel G, Bartl R, Lamerz R, Wick M, Seidel D. Amplification of cyclin D1 gene in multiple myeloma: clinical and prognostic relevance. Br J Haematol. 2002; 109: 30-38.
- Cook JR, Hsi ED, Worley S, Tubbs RR, Hussein M. Immunohistochemical analysis identifies two cyclin D1+ subsets of plasma cell myeloma, each associated with favorable survival. Am J Clin Pathol. 2006; 125: 615-624.
- Markovic O, Marisavljevic D, Cemerikic V, Suvajdzic N, Milic N, ColovicM. Immunohistochemical analysis of cyclin D1 and p53 in multiple myeloma. Med Oncol. 2004; 21: 73-80.
- Nazarvos J, Breiksa A, Kleina R, Lejniece S, Voicehovska J, MomekovG. Prognostic value of plasmablastic morphology and p21, p53 and cyclin D1 expression in myeloma cells: retrospective study of 122 patients with first time newly diagnosed multiple myeloma. Biotechnol Biotechnol Equip. 2021; 35: 1941-1947.
- Jiang Y, Zhang C, Lu L, Wang X, Liu H, Jiang Y, et al. The Prognostic Role of Cyclin D1 in Multiple Myeloma: A Systematic Review and Meta- Analysis. Technol Cancer Res Treat. 2022; 21:15330338211065252.
- Tasidou A, Roussou M, Terpos E, Kastritis E, Gkotzamanidou M, Gavriatopoulou M, et al. Increased expression of cyclin D1 on trephine bone marrow biopsies independently predicts for shorter overall survival in patients with multiple myeloma treated with novel agents. Am J Hematol. 2012; 87: 734-736.
- Pruneri G, Alietti A, Angelli L, Morabito F, Laszlo D, Calabrese L, et al. Immunoreactivity for cyclin D1 is a reliable marker of gene aberration in plasma cell myeloma but does not specify patient’s prognosis. Leuk Res. 2008; 32: 1628-1632.