Study on the Mechanism of Opuntia Dillenii Polysaccharide against Cadmium-Induced Liver Injury Based on Network Pharmacology and Oxidative Stress Pathway
- 0. Both the authors contributed equally
- 1. Department of Key Laboratory for Information System of Mountainous Areas and Protection of Ecological Environment, Guizhou Normal University, China
- 2. Department of Guizhou Engineering Laboratory for Quality Control Evaluation Technology of Medicine, Guizhou Normal University, China
- 3. Department of Innovation Laboratory, The Third Experiment Middle School of Guiyang, China
Abstract
This study aims to investigate the mechanism of action of Opuntia dillenii polysaccharides (ODPs) in protecting against cadmium-induced liver injury from the perspectives of network pharmacology and oxidative stress pathway. Using network pharmacology methods, we analyzed the interactions between ODPs and target molecules, and predicted potential mechanisms of action. Additionally, we focused on the oxidative stress pathway, which plays a crucial role in cadmium-induced liver injury. The results showed that the active components of ODPs were glucuronic acid, galacturonic acid, rhamnose, fucose, glucose, galactose, mannose, fructose, xylose and arabinose in order. According to the analysis of PPI network and the “compound - liver injury target-pathway” network, 69 ODPs anti-cadmium-induced liver injury targets were selected, including AKT1, CASP3, MAPK8, JAK2, MAPK14, IL2, MMP9, IGF1R, NOS2, etc. Enrichment analysis showed that the main signaling pathways involved were VEGF signaling pathway, cancer signaling pathway, PI3K-AKT signaling pathway, Rap1 signaling pathway, Ras signaling pathway, chemokine signaling pathway, sphingolipid signaling pathway, toll-like receptor signaling pathway, tumor necrosis factor signaling pathway, and MAPK signaling pathway. Network pharmacological analysis showed that cactus polysaccharide could significantly reduce the level of Caspase-3 and MMP-9 in liver, increase the level of IL-2, and effectively inhibit the occurrence of liver cell apoptosis and inflammation caused by cadmium. Studies on the mechanism of action based on traditional oxidative stress indexes show that ODPs has good antioxidant capacity, can significantly inhibit the activity of SOD and GSH-PX caused by cadmium, reduce the content of lipid peroxidation product MDA, and restore the content of antioxidant enzymes in liver to normal level. This study can provide a scientific basis for the in-depth development and application of ODPs, and also provide a potential scientific and technological basis for the development of drugs or health food for the treatment of cadmium-induced liver injury.
Keywords
• Opuntia Dillenii Polysaccharide
• Cadmium-Induced Liver Injury
• Network Pharmacology
• Oxidative Stress
• Action Mechanism
CITATION
Zhou B, Liu T, Wu Q, Deng Q, Chen H (2023) Study on the Mechanism of Opuntia Dillenii Polysaccharide against Cadmium-Induced Liver Injury Based on Network Pharmacology and Oxidative Stress Pathway. J Liver Clin Res 8(1): 1055.
INTRODUCTION
Opuntia dillenii (Ker-Gaw) Haw., also known as a type of succulent plant [1], typically grows in dry and semi-arid climates such as deserts and grasslands [2]. They are widely distributed in Americas, Africa, and Australia, among other regions [3]. O. dillenii come in various shapes, with some being spherical, flat, or spiny [4]. They have adapted to survive in arid and hot conditions by storing water [5]. In folk medicine, O. dillenii is extensively used for medicinal [6] and cosmetic purposes [7]. It contains a rich variety of active compounds such as alkaloids, flavonoids,and polysaccharides [8]. O. dillenii is believed to have multiple benefits, including heat-clearing and detoxification, moisturizing the lungs and relieving cough, lowering blood sugar, and possessing anti-inflammatory and analgesic properties [9]. It is also used for skin hydration, whitening, and treating skin issues in the field of [10].
Polysaccharides are one of the main chemical constituents in O. dillenii and exhibit various pharmacological activities [11]. Firstly, O. dillenii polysaccharides (ODPs) can enhance immune function by regulating the activity of the immune system and improving the body’s disease resistance [12]. Secondly, they have protective effects on the liver, reducing liver damage [13] and inflammation, and promoting the repair and regeneration of liver cells [14]. Additionally, ODPs possess antioxidant and anti- inflammatory effects, reducing the production of free radicals and protecting cells from oxidative stress damage [15]. In our previous study [16], we conducted pharmacological studies on the efficacy of ODPs in protecting against cadmium-induced liver injury from the perspectives of time-effect (1, 2, 3, 4 and 5 weeks)and dose-effect (0, 100, 200, 400 and 600 mg·kg-1). This study provided a basic understanding of the process and behavior of their pharmacological effects. However, the mechanism of its action against cadmium-induced liver injury is still unclear, which seriously hinders the industrial development and application of ODPs.
Network pharmacology is a research method that integrates network databases [17], and pharmacological knowledge to reveal the interactions between drugs or active compounds and targets in the human body [18]. It utilizes large-scale molecular interaction data and employs computational and systems biology approaches to predict and explore drug mechanisms [19], efficacy, and drug-target interaction networks [20]. Oxidative stress pathway is a common biochemical process in cells [21], and plays a crucial role in the development of many diseases [22]. During oxidative stress, excessive reactive oxygen species are produced, leading to cellular oxidative damage and subsequent inflammation, apoptosis, and tissue injury [23]. The oxidative stress pathway includes antioxidant enzyme systems, redox reactions, and free radical scavenging mechanisms [24]. Modulating these pathways can improve oxidative stress status.
When studying the mechanism of ODPs in protecting against cadmium-induced liver injury, we choose to approach it from the perspectives of network pharmacology and oxidative stress pathway for the following reasons: firstly, network pharmacology helps us comprehensively understand and predict the interactions between polysaccharides and other molecules in the human body. By employing network pharmacology methods, we can uncover the associations between polysaccharides and relevant targets and signaling pathways, thus revealing the mechanisms of action of polysaccharides in protecting against cadmium- induced liver injury. Secondly, the oxidative stress pathway is an important mechanism by which cadmium causes liver damage, making it meaningful to investigate the protective effects of ODPs. By analyzing the regulatory effects of polysaccharides on oxidative stress-related molecules and pathways, we can better understand the mechanisms of action of polysaccharides in protecting against cadmium-induced liver injury. Furthermore, combining network pharmacology with the oxidative stress pathway research approach allows us to comprehensively and deeply elucidate the protective effects of ODPs against cadmium- induced liver injury, providing a more reliable theoretical basis for the clinical application of polysaccharides. Therefore, this study aims to investigate the mechanism of action of ODPs in protecting against cadmium-induced liver injury from the perspectives of network pharmacology and oxidative stress. This will provide scientific support for the in-depth development and utilization of ODPs.
MATERIALS AND METHODS
Materials
ODPs (purity > 95%) were prepared by our research group. Cadmium chloride (CdCl2) was purchased from Tianjin Zhiyuan Chemical Reagent Co., Ltd. Mouse sandwich enzyme-linked immunosorbent assay (ELISA) kits for SOD, GSH-PX, MDA were purchased from Shanghai Guduo Biotechnology Co., Ltd., while mouse ELISA kits for Caspase-3, IL-2, MMP-9 were purchased from Nanjing Jiancheng Bioengineering Institute. Anhydrous ethanol, saline solution, bitter acid, xylene, n-butanol, neutral gum, paraffin, formaldehyde solution, hematoxylin, eosin, and other chemical reagents were purchased from Sinopharm Group Chemical Reagent Co., LTD. This study involved equipment and instruments such as a microbalance, pure water machine, micropipette, benchtop low-speed centrifuge, vortex oscillator, constant temperature and humidity chamber, freezer, microplate reader, automatic dehydrator, automatic embedding machine, slicer, slide stainer, constant temperature slide oven, slide sealer, inverted fluorescence microscope, and automated blood analyzer.
Animals
SPF-grade male KM mice (weighing 18-22 g) were purchased from Changsha Tiantong Biotechnology Co., Ltd., license number: SCXK (Xiang) 2019-0014. The experimental temperature was maintained at 22 ± 0.5 ?, humidity at 55 ± 5%, and a light cycle of 12 hours. All animal experiments in this study were conducted in accordance with the guidelines of the Eighth Edition of Experimental Animal Care (2011) and approved by the Animal Care and Use Committee of Guizhou Normal University.
Experimental methods
Active ingredient information and target prediction of ODPs: The monosaccharide composition information of ODPs was obtained by consulting the literature, and the Chemical Abstracts Service (CAS) number of each monosaccharide composition was obtained by https://www.chemsrc.com/ casindex/). The CAS number was input into the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP) to obtain the active ingredient information of ODP. Based on the online website PubChem (https://pubchem. ncbi.nlm.nih.gov/), the sdf structure corresponding to the ODP component was obtained, and the sdf structure was uploaded to the Pharm Mapper ( http://www.lilab-ecust.cn/pharmmapper/) platform for related target prediction [25].
Target screening related to cadmium-induced liver injury: Based on the human gene database Gene Cards and the online human Mendelian genetics database OMIM, diseases related to liver injury such as Cadmium-derived hepatitis, cholestasis, hepatocyte apoptosis, hepatocyte, and liver abscess were used as keywords to screen for high-scoring targets and remove duplicate target genes, obtaining information on liver injury-related targets [26].
Construction of protein-protein interaction network: Using the jvenn online website (http://www.bioinformatics. com.cn/static/others/jvenn/example.html), the target genes of polysaccharides and diseases were input into jvenn to obtain the intersection group, resulting in a Venny diagram. The intersection genes were imported into the STRING 11.0 database (https:// string-db.org/), selecting “homo sapiens” as the species and setting the minimum interaction threshold to “highest confidence (> 0.7)”, with default settings for the rest. A protein-protein interaction (PPI) network model was constructed, and further analysis of the PPI network was performed using Cytoscape 3.8.2 software [27].
GO enrichment and KEGG pathway analysis: Key targets with degrees higher than the average degree value in the PPI network were imported into the DAVID database for GO (Gene Ontology) and KEGG (Kyoto Encyclopedia of Genes and Genomes) enrichment analysis. Genes ranking in the top 10 were selected based on a screening condition of P < 0.05, and GO functional annotation was performed in terms of biological processes (BP), cellular components (CC), and molecular functions (MF) modules. The top 20 pathways were selected for KEGG enrichment analysis, and pathway bubble plots were created using the online website omicX (http://www.bioinformatics.com.cn) [28].
Analysis of drug-disease-target network: Using Cytoscape
3.8.2 software, a “compound-target-pathway” network diagram was constructed, and built-in tools were used to analyze the network topological parameters of effective compounds and targets, including degree, betweenness centrality, and closeness centrality. The core targets for liver injury and the main active components responsible for the therapeutic effects of ODPs were determined based on network topology parameters [29].
Animal grouping and administration: Based on network pharmacology and the results of our previous studies [16], key targets predicted by network pharmacology were validated in vivo. Forty healthy male KM mice were fed with A-grade feed twice a day in a well-ventilated environment with a temperature of 22 ± 0.5°C and a relative humidity of 55 ± 5%. They had unlimited access to drinking water. After a 7-day adaptation period, the initial body weight of the mice was measured. Then, the 40 mice were randomly divided into the following groups: blank control group (NC), model control group (MC), positive control group (YC), and ODPs administration group (ODP), with 10 mice in each group.
After a 7-day adaptation period, the blank control group received a daily intraperitoneal injection of physiological saline, with a dose of 0.20 mL. The model control group, positive control group, and ODP administration group received a daily intraperitoneal injection of cadmium chloride solution, with a dose of 2.0 mg·kg-1. The injections were administered every 3 days for all groups. Concurrently, the blank group and model group received a daily gavage of physiological saline, with a dose of 0.20 mL. The positive group received a daily gavage of glutathione solution, with a dose of 180 mg·kg-1. The ODP administration group received a daily gavage of ODP solution, with a dose of 200 mg·kg-1. The gavage was administered 3 hours after the injection, and the treatment lasted for 28 days. After sacrifice by cervical dislocation, liver tissues were collected for the determination of relevant oxidative stress markers using an ELISA enzyme immunoassay kit.
Statistical analysis
SPSS 26.0 was used to perform one-way ANOVA on the data, which was expressed as mean standard deviation ( X± SD), and the data had significant differences (P< 0.05), had a very significant difference (P< 0.01). Graphing the data using GraphPad Prism 8.0.2.
RESULTS
Monosaccharide composition information and target prediction of ODPs
According to the literature review [30,31], the monosaccharide composition of ODPs includes arabinose, xylose, fructose, glucose, glucuronic acid rhamnose, mannose, glucuronic acid, and fucose. The monosaccharide composition chemical information of ODPs was obtained from the TCMSP database, as shown in [Table 1].
Table 1: Monosaccharide composition information of ODPs.
|
Compound code |
Compound name |
Oral availability OA % |
Drug-likeness DL |
|
MOL007173 |
L-Arabinose |
52.64 |
0.03 |
|
MOL000731 |
Xylose |
58.74 |
0.03 |
|
MOL000053 |
Fructose |
1.68 |
0.03 |
|
MOL000734 |
Glucose |
24.40 |
0.03 |
|
MOL000814 |
Galactose |
47.94 |
0.04 |
|
MOL000424 |
Rhamnose |
50.50 |
0.04 |
|
MOL000051 |
Mannose |
1.76 |
0.03 |
|
MOL000386 |
Fucose |
42.51 |
0.03 |
|
MOL000384 |
Glucuronic acid |
3.35 |
0.04 |
|
MOL011643 |
Galacturonic acid |
40.39 |
0.06 |
Furthermore, 295 targets were predicted for the action of monosaccharide composition of ODPs using the Pharm Mapper platform.
Prediction of potential disease targets
Based on GeneCards and OMIM, targets with high scores were screened and duplicate target genes were removed. 116 targets related to Cadmium-derived hepatitis, 256 targets related to cholestasis, 186 targets related to hepatocyte apoptosis were obtained. In addition, there were 362 targets related to hepatocyte injury and 324 targets related to liver abscess, a total of 1073 liver injury targets were obtained by combining and deleting duplicate values.
PPI network construction of core targets
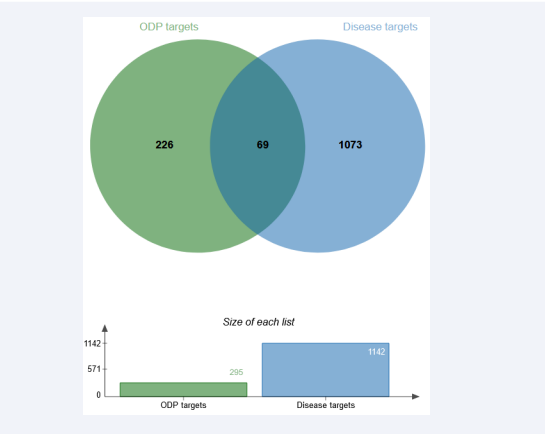
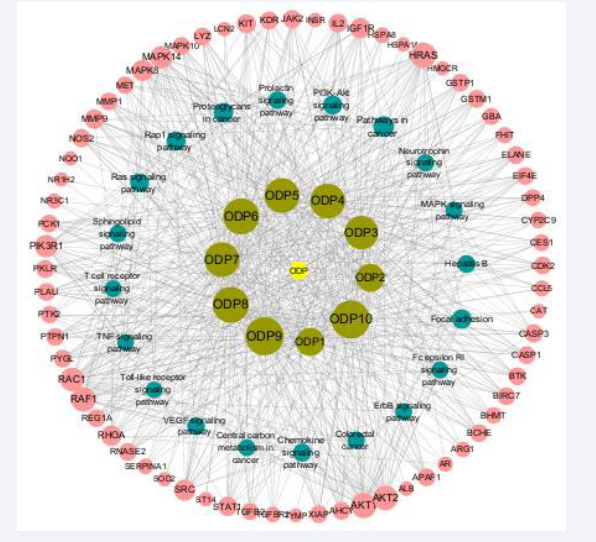
The intersection of ODPs active ingredient information and cadmium-induced liver injury related targets was obtained by using jvenn online website (Figure 1).
Figure 1: Venn diagram of ODPs and cd-induced liver injury related targets. Note: The green circle in the figure represents the collection of targets related to the active chemical components of ODPs, and the blue circle represents the collection of targets related to cadmium-derived liver injury.
69 potential targets of ODP associated with cadmium-induced liver injury were screened, accounting for about 5% of the total. The 69 potential targets are AHCY, AKT1, APAF1, BCHE, BIRC7, BTK, CASP1, CDK2, CES1, EIF4E, ELANE, F2, GBA, GST, M1, GSTP1, HRAS, IGF1R, IL2, LYZ, MAPK10, MAPK14, MMP1, MMP9, NOS2, PCK1, PKLR, PLAU, PTPN1, PYGL, RAC1, RAF1, REG1A, RNASE2, SRC, AKT2, ARG1, CASP3, CCL5, DPP4, etc.
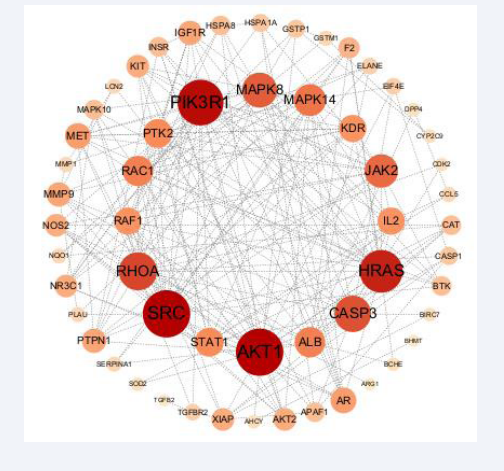
The intersection gene was imported into the STRING database to obtain the PPI network diagram of common targets. The downloaded file was imported into cytoscape 3.8.2 to obtain the PPI network diagram (Figure 2).
Figure 2: Protein interaction network of potential targets. Note: The size of the circle and the depth of the color in the figure indicate the size of the corresponding target degree value. The larger the circle and the darker the color, the larger the degree value; The thickness of the edge and the depth of the color indicate the correlation between the target, and the thicker the edge and the darker the color, the better the correlation.
Degree value was used as the evaluation criterion, and degree > average value (8.04) was selected as the key gene. As can be seen from Figure 2, core targets with high connectivity include AKT1, SRC, PIK3R1, HRAS, RHOA, CASP3, MAPK8, JAK2, MAPK14, ALB, RAC1, STAT1, PTK2, IL2, RAF1, KDR, AR, AKT1, SRC, PIK3R1, HRAS, Rhoa, CasP3, MapK8, JAK2, MAPK14, ALB, RAC1, Stat1, MET, PTPN1,MMP9, IGF1R. The topological parameters of potential targets are shown in Table 2.
Table 2: Topological analysis of potential targets of ODPs on cadmium-induced liver injury.
|
Shared name |
Betweenness Centrality |
Closeness Centrality |
Degree |
AverageShortest PathLength |
Clustering Coefficient |
|
AKT1 |
0.15 |
0.57 |
24.00 |
1.75 |
0.27 |
|
SRC |
0.10 |
0.58 |
24.00 |
1.73 |
0.36 |
|
PIK3R1 |
0.07 |
0.55 |
23.00 |
1.82 |
0.38 |
|
HRAS |
0.06 |
0.56 |
21.00 |
1.80 |
0.40 |
|
RHOA |
0.08 |
0.54 |
18.00 |
1.84 |
0.38 |
|
CASP3 |
0.11 |
0.55 |
17.00 |
1.82 |
0.26 |
|
MAPK8 |
0.17 |
0.53 |
16.00 |
1.89 |
0.38 |
|
JAK2 |
0.02 |
0.47 |
15.00 |
2.15 |
0.42 |
|
MAPK14 |
0.02 |
0.49 |
14.00 |
2.04 |
0.44 |
|
ALB |
0.13 |
0.50 |
13.00 |
2.00 |
0.24 |
|
RAC1 |
0.01 |
0.49 |
13.00 |
2.05 |
0.54 |
|
STAT1 |
0.03 |
0.48 |
12.00 |
2.09 |
0.45 |
|
PTK2 |
0.01 |
0.46 |
12.00 |
2.16 |
0.62 |
|
IL2 |
0.03 |
0.50 |
11.00 |
1.98 |
0.51 |
|
RAF1 |
0.02 |
0.47 |
11.00 |
2.13 |
0.44 |
|
KDR |
0.01 |
0.45 |
11.00 |
2.22 |
0.64 |
|
AR |
0.01 |
0.47 |
10.00 |
2.15 |
0.53 |
|
MET |
0.00 |
0.47 |
10.00 |
2.11 |
0.71 |
|
PTPN1 |
0.00 |
0.45 |
9.00 |
2.22 |
0.64 |
|
MMP9 |
0.06 |
0.46 |
9.00 |
2.16 |
0.28 |
|
IGF1R |
0.00 |
0.43 |
9.00 |
2.35 |
0.64 |
|
KIT |
0.01 |
0.47 |
8.00 |
2.15 |
0.61 |
|
NOS2 |
0.04 |
0.45 |
8.00 |
2.20 |
0.46 |
|
XIAP |
0.02 |
0.45 |
8.00 |
2.24 |
0.29 |
|
AKT2 |
0.00 |
0.44 |
8.00 |
2.27 |
0.61 |
|
NR3C1 |
0.01 |
0.42 |
8.00 |
2.38 |
0.43 |
|
F2 |
0.02 |
0.43 |
7.00 |
2.33 |
0.33 |
|
CAT |
0.05 |
0.46 |
6.00 |
2.18 |
0.33 |
|
INSR |
0.00 |
0.43 |
6.00 |
2.35 |
0.67 |
|
HSPA8 |
0.01 |
0.43 |
6.00 |
2.31 |
0.47 |
|
APAF1 |
0.01 |
0.42 |
6.00 |
2.36 |
0.40 |
|
BTK |
0.00 |
0.44 |
6.00 |
2.29 |
0.87 |
|
MAPK10 |
0.00 |
0.44 |
6.00 |
2.25 |
0.73 |
|
GSTP1 |
0.13 |
0.38 |
5.00 |
2.65 |
0.20 |
|
HSPA1A |
0.00 |
0.37 |
5.00 |
2.67 |
0.30 |
|
CASP1 |
0.00 |
0.40 |
5.00 |
2.51 |
0.50 |
|
EIF4E |
0.00 |
0.41 |
4.00 |
2.45 |
0.67 |
|
ELANE |
0.00 |
0.35 |
4.00 |
2.85 |
0.50 |
|
TGFBR2 |
0.04 |
0.40 |
4.00 |
2.47 |
0.17 |
|
LCN2 |
0.00 |
0.35 |
3.00 |
2.87 |
0.67 |
|
SERPINA1 |
0.00 |
0.35 |
3.00 |
2.87 |
0.67 |
|
NQO1 |
0.02 |
0.34 |
3.00 |
2.91 |
0.33 |
|
CCL5 |
0.00 |
0.36 |
3.00 |
2.78 |
0.33 |
|
GSTM1 |
0.00 |
0.29 |
3.00 |
3.40 |
0.67 |
|
MMP1 |
0.00 |
0.32 |
3.00 |
3.09 |
0.67 |
|
PLAU |
0.00 |
0.40 |
3.00 |
2.51 |
0.67 |
|
AHCY |
0.04 |
0.28 |
2.00 |
3.60 |
0.00 |
|
SOD2 |
0.00 |
0.38 |
2.00 |
2.60 |
1.00 |
|
BCHE |
0.00 |
0.34 |
2.00 |
2.91 |
1.00 |
|
DPP4 |
0.00 |
0.34 |
2.00 |
2.91 |
0.00 |
|
BIRC7 |
0.00 |
0.37 |
2.00 |
2.71 |
1.00 |
|
CDK2 |
0.00 |
0.38 |
2.00 |
2.60 |
1.00 |
|
CYP2C9 |
0.00 |
0.28 |
2.00 |
3.62 |
1.00 |
|
BHMT |
0.00 |
0.22 |
1.00 |
4.58 |
0.00 |
|
ARG1 |
0.00 |
0.31 |
1.00 |
3.18 |
0.00 |
|
TGFB2 |
0.00 |
0.29 |
1.00 |
3.45 |
0.00 |
It can be seen from the data in the table that the average betweennesss centrality is 0.03 and the average closenessss centrality is 0.42. The Average Shortest PathLength was 2.46 and the average Clustering Coefficient was 0.48.
GO enrichment and KEGG pathway analysis
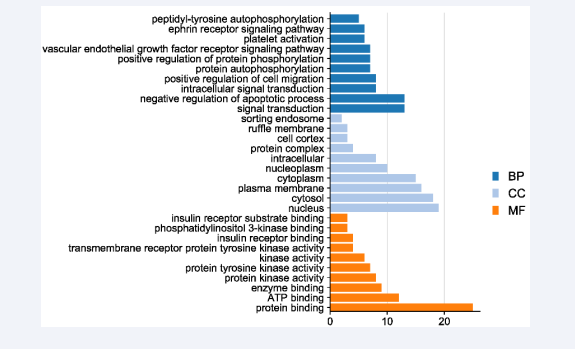
The gene ontological biological function enrichment and KEGG signaling pathway analysis of ODPs against cadmium- induced liver injury were carried out through DAVID database, and visualization processing was performed. As can be seen from Figure 3,
Figure 3: GO analysis bar chart.
ODPs are mainly involved in biological processes including negative regulation of apoptosis, intracellular signal transduction, positive regulation of cell migration, positive regulation of protein phosphorylation, vascular endothelial growth factor receptor signaling pathway, and platelet activation. In terms of cell composition, it mainly acts on the nucleus, cytoplasm, plasma membrane, nucleoplasma and protein complex. In terms of molecular function, it is mainly related to the activities of protein binding, ATP binding, protein kinase activity, protein tyrosine kinase activity, transmembrane receptor protein tyrosine kinase activity, insulin receptor binding and so on.
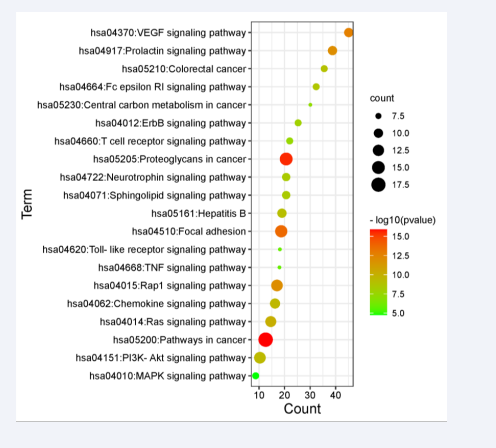
Through the enrichment analysis of KEGG pathways in DAVID database, 89 pathways with significant correlation were found (P<0.05), the first 20 related core pathways were screened out according to the P-value (Figure 4),
Figure 4: Enrichment analysis of KEGG pathway.
mainly involving VEGF signaling pathway, cancer signaling pathway, PI3K-AKT signaling pathway, hepatitis B, Rap1 signaling pathway, Ras signaling pathway, chemokine signaling pathway, sphingolipid signaling pathway, and toll-like receptor signaling pathway. Tumor necrosis factor signaling pathway, MAPK signaling pathway, etc. In addition, apoptosis, Jak-STAT signaling pathway, HIF-1 signaling pathway also play an important role in ODPs anti- cadmium-induced liver injury.
Drug-disease-target network diagram
The main targets and pathways of cadmium-induced liver injury alleviated by ODPs were analyzed by cytosacpe 3.8.2, and a “drug-disease-target network diagram” was constructed (Figure 5),
Figure 5: ODPs alleviates cadmium-derived liver injury related target-core pathway network.
which shows that ODPs has the characteristics of multi-target and multi-pathway action, and can better alleviate cadmium- induced liver injury. Among them, the monosaccharides in ODPs were glucuronic acid, galacturonic acid, rhamnose, fucose, glucose, galactose, mannose, fructose, xylose, and arabinose in descending order [Table 3].
Table 3: Characteristic parameters of network nodes related to monosaccharide composition of ODPs
|
Compound code |
Compound name |
Betweenness centrality |
Closeness centrality |
Degree |
|
ODP1 |
L-Arabinose |
0.02 |
0.50 |
34.00 |
|
ODP2 |
Xylose |
0.02 |
0.50 |
34.00 |
|
ODP3 |
Fructose |
0.07 |
0.57 |
47.00 |
|
ODP4 |
Glucose |
0.06 |
0.59 |
50.00 |
|
ODP5 |
Galactose |
0.06 |
0.59 |
50.00 |
|
ODP6 |
Rhamnose |
0.13 |
0.62 |
53.00 |
|
ODP7 |
Mannose |
0.06 |
0.59 |
50.00 |
|
ODP8 |
Fucose |
0.13 |
0.62 |
53.00 |
|
ODP9 |
Glucuronic acid |
0.11 |
0.66 |
58.00 |
|
ODP10 |
Galacturonic acid |
0.11 |
0.66 |
58.00 |
The main targets of polysaccharide in alleviating liver injury were AKT1, HRAS, RAF1, AKT2, RAC1, MAPK14, PIK3R1, SRC,MAPK8, IGF1R, RHOA, and MMP9 [Table 4].
Table 4: Characteristic parameters of cadmium-derived liver injury related network nodes.
|
Shared name |
Betweenness Centrality |
Closeness Centrality |
Degree |
|
AKT1 |
0.05 |
0.58 |
28.00 |
|
HRAS |
0.04 |
0.58 |
28.00 |
|
RAF1 |
0.03 |
0.52 |
26.00 |
|
AKT2 |
0.03 |
0.52 |
26.00 |
|
RAC1 |
0.03 |
0.55 |
24.00 |
|
MAPK14 |
0.03 |
0.53 |
21.00 |
|
PIK3R1 |
0.02 |
0.48 |
21.00 |
|
SRC |
0.02 |
0.52 |
18.00 |
|
MAPK8 |
0.02 |
0.48 |
18.00 |
|
IGF1R |
0.01 |
0.51 |
16.00 |
|
RHOA |
0.01 |
0.47 |
16.00 |
|
MMP9 |
0.01 |
0.49 |
14.00 |
|
KIT |
0.01 |
0.49 |
13.00 |
|
STAT1 |
0.01 |
0.49 |
13.00 |
|
IL2 |
0.01 |
0.49 |
12.00 |
|
NOS2 |
0.00 |
0.48 |
11.00 |
|
CASP3 |
0.01 |
0.48 |
11.00 |
|
JAK2 |
0.01 |
0.48 |
11.00 |
|
XIAP |
0.00 |
0.48 |
10.00 |
|
KDR |
0.01 |
0.47 |
10.00 |
|
AHCY |
0.00 |
0.48 |
10.00 |
|
APAF1 |
0.00 |
0.48 |
10.00 |
|
BCHE |
0.00 |
0.48 |
10.00 |
|
BIRC7 |
0.00 |
0.48 |
10.00 |
|
BTK |
0.00 |
0.48 |
10.00 |
|
CASP1 |
0.00 |
0.48 |
10.00 |
|
CDK2 |
0.00 |
0.48 |
10.00 |
|
EIF4E |
0.00 |
0.48 |
10.00 |
|
ELANE |
0.00 |
0.48 |
10.00 |
|
GSTM1 |
0.00 |
0.48 |
10.00 |
|
GSTP1 |
0.00 |
0.48 |
10.00 |
|
LYZ |
0.00 |
0.48 |
10.00 |
|
MAPK10 |
0.00 |
0.48 |
10.00 |
|
MMP1 |
0.00 |
0.48 |
10.00 |
|
PCK1 |
0.00 |
0.48 |
10.00 |
|
PKLR |
0.00 |
0.48 |
10.00 |
|
PLAU |
0.00 |
0.48 |
10.00 |
|
PTPN1 |
0.00 |
0.48 |
10.00 |
|
PYGL |
0.00 |
0.48 |
10.00 |
|
REG1A |
0.00 |
0.48 |
10.00 |
|
RNASE2 |
0.00 |
0.48 |
10.00 |
The main pathways involved are VEGF signaling pathway, cancer pathway, PI3K AKT signaling pathway, Rap1 signaling pathway, Ras signaling pathway, and chemokine signaling pathway [Table 5].
Table 5: Characteristic parameters of network nodes related to core paths.
|
Shared name |
Betweenness Centrality |
Closeness Centrality |
Degree |
|
Pathways in cancer |
0.01 |
0.42 |
17.00 |
|
Focal adhesion |
0.01 |
0.41 |
14.00 |
|
Proteoglycans in cancer |
0.01 |
0.41 |
14.00 |
|
Rap1 signaling pathway |
0.00 |
0.41 |
13.00 |
|
PI3K-Akt signaling pathway |
0.00 |
0.41 |
13.00 |
|
Ras signaling pathway |
0.00 |
0.41 |
12.00 |
|
Chemokine signaling pathway |
0.00 |
0.40 |
11.00 |
|
VEGF signaling pathway |
0.00 |
0.40 |
10.00 |
|
Prolactin signaling pathway |
0.00 |
0.40 |
10.00 |
|
Hepatitis B |
0.00 |
0.40 |
10.00 |
|
Colorectal cancer |
0.00 |
0.40 |
9.00 |
|
Neurotrophin signaling pathway |
0.00 |
0.40 |
9.00 |
|
Sphingolipid signaling pathway |
0.00 |
0.40 |
9.00 |
|
Fc epsilon RI signaling pathway |
0.00 |
0.39 |
8.00 |
|
ErbB signaling pathway |
0.00 |
0.39 |
8.00 |
|
T cell receptor signaling pathway |
0.00 |
0.39 |
8.00 |
|
Central carbon metabolism in cancer |
0.00 |
0.39 |
8.00 |
|
MAPK signaling pathway |
0.00 |
0.39 |
8.00 |
Effects of ODPs on oxidative stress in cadmium- induced liver injury
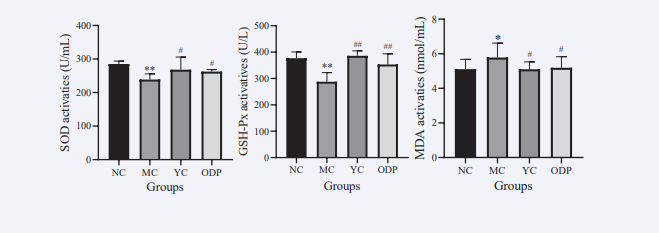
As shown in Figure 6,
Figure 6: Effects of ODPs on SOD, GSH-Px and MDA in mice.
compared with NC group, SOD and GSH- Px contents of mice in MC group were significantly decreased by 16.21% and 23.67%, respectively, while MDA contents were significantly increased by 13.18% (P < 0.05). Compared with MC group, SOD and GSH-PX contents were significantly decreased by 16.21% and 23.67%, respectively. In YC group, SOD and GSH-Px contents were significantly increased by 12.25% and 34.22%, and MDA content was significantly decreased by 11.76%; in ODP group, SOD and GSH-PX contents were significantly increased by 9.85% and 22.72%, and MDA contents were significantly decreased by 11.54%, with significant differences (P < 0.05)..
Effect of ODPs on the related targets of cadmium- induced liver injury
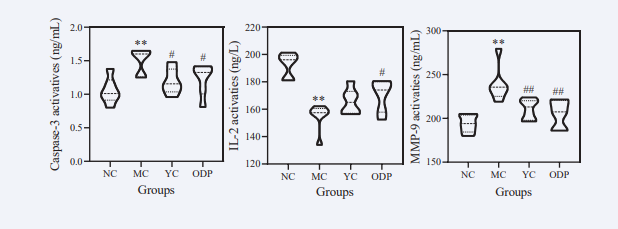
As shown in Figure 7,
Figure 7: Effects of ODPs on caspase-3, IL-2 and MMP-9 in mice. Note : Data are expressed as mean ± standard deviation ( ± S.D ). Compared with the blank group, * * P < 0.01, with a very significant difference. Compared with the model group, # P < 0.05, with significant difference, # # P < 0.01, with extremely significant difference.
compared with the NC group, the content of Caspase-3 and MMP-9 in the MC group increased by 43.39 % and 23.31 %, respectively, and the content of IL-2 decreased by 19.65 %, with significant difference (P < 0.01). Compared with MC group, the contents of Caspase-3 and MMP-9 in YC group were significantly decreased by 20.71 % and 11.85%, and the content of IL-2 was increased by 7.22 %. The levels of Caspase-3 and MMP-9 in the ODP group increased by 18.36 % and 14.29 %, and the content of IL-2 increased by 10.56 %, with significant difference (P < 0.05).
DISCUSSION
The accumulation of cadmium in the liver can cause severe damage. Studies have shown that cadmium toxicity is associated with oxidative stress [32], and lipid peroxidation in liver cells [33]. Reactive oxygen species (ROS) such as superoxide anion, hydrogen peroxide, and hydroxyl radical promote the generation of ROS [34], thus disrupting the balance of the oxidative/ antioxidant system. Effective removal of free radicals and inhibition of oxidative substances require antioxidant barriers, including enzymatic and non-enzymatic systems [35]. The most important enzymatic antioxidants are superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase (CAT). SOD catalyzes the dismutation of superoxide radicals into hydrogen peroxide (H2O2) and molecular oxygen (O2) [36], thus providing an important defense mechanism against the toxicity of superoxide radicals. Matrix metalloproteinase-9 (MMP-9) is a protease that degrades the extracellular matrix and plays a major role in basement membrane degradation [37]. In clinical diagnosis, the analysis of SOD and MMP-9 in combination is often used as an auxiliary indicator for diagnosis [38]. Malondialdehyde (MDA) is the final product of cell membrane lipid peroxidation [39]. MDA affects the activity of mitochondrial respiratory chain complexes and key enzymes in the mitochondria [40], and its production can further exacerbate membrane damage [41]. Therefore, measuring MDA levels can indirectly reflect the degree of lipid peroxidation and cell damage. The results of this study showed that the content of SOD, MMP-9, and GSH-Px in the liver of mice in the cadmium model group was significantly decreased, while the MDA content was significantly increased, indicating an excess production of superoxide anion radicals in the mice, lipid peroxidation of cell membranes, and disruption of the redox balance. The polysaccharide group and positive control group had significantly higher levels of SOD, MMP-9, and GSH-Px, and significantly lower levels of MDA compared to the model group, suggesting that ODPs can eliminate excessive superoxide anion radicals in the body, inhibit lipid peroxidation of biomembranes,and repair structural damage to cell membranes, thereby restoring liver cell function.
It has been proven that cadmium-induced hepatotoxicity can induce cell apoptosis [42]. Studies have shown that cadmium can decrease the expression of the cell apoptosis-related factor B-cell lymphoma 2 (Bcl-2) [43], increase the levels of Bcl-2-associatedX (Bax) and activated caspase-3 (Caspase-3) [44], indicating the activation of the mitochondria-mediated intrinsic apoptotic pathway and the induction of liver cell apoptosis. Activated Caspase-9 has been demonstrated to be a major factor in tissue hypoxia-induced apoptosis [45], and after activation, Caspase-9 can cleave and activate downstream Caspase-3 [46], which is an important final execution molecule in various apoptotic pathways and a significant marker of cell apoptosis [47]. Once activated, Caspase-3 can cause cell apoptosis in multiple ways [48]. Activated Caspase-3 can cleave many intracellular proteins and inactivate nucleases, leading to the destruction of the cell nucleus and morphological changes characteristic of cell apoptosis [49]. The results of this study showed that the level of Caspase-3 was significantly increased in the liver of mice in the model group, while the content of Caspase-3 in the polysaccharide group and positive control group was significantly lower than in the cadmium-treated group, indicating that ODPs can inhibit liver cell apoptosis through the mitochondria-mediated caspase- dependent pathway.
In cadmium-induced hepatotoxicity, cadmium-activated liver cells release a large amount of inflammatory factors [50]. Common pro-inflammatory factors [51] include tumor necrosis factor-alpha (TNF-α), interleukins (IL-1, IL-6, IL-8), etc., while anti-inflammatory factors include IL-2, IL-4, IL-10, which mainly inhibit the release of pro-inflammatory mediators and prevent excessive inflammatory responses [52]. These inflammatory factors further activate the expression of adhesion molecules [53], including E-selectin, ICAM-1, VCAM-1, P-selectin, and the β2 integrin family member Mac-1. The abundant expression of adhesion molecules in liver cells triggers a series of cellular and humoral responses, ultimately leading to inflammation and secondary liver damage [54]. In this study, the IL-2 content in the liver of mice in the model group was significantly decreased, while the IL-2 content in the polysaccharide group and positive control group was significantly higher than in the model group. Combined with the KEGG pathway enrichment analysis, ODPs can inhibit the excessive activation of immune cells, increase the release of anti-inflammatory mediators, and maintain the relative balance between the body’s inflammatory response and compensatory anti-inflammatory response, thereby avoiding liver cell damage caused by excessive inflammatory response.
CONCLUSION
Based on the previous research work, this project combines network pharmacology to explore the mechanism of ODPs in alleviating cadmium-induced liver damage. The results were validated and analyzed through in vivo experiments. The main conclusions are as follows: (1) The results of this study demonstrate that ODPs have the characteristics of multi-target and multi-pathway actions, effectively alleviating cadmium- induced liver damage. (2) Based on the analysis of the PPI network and the “compound-liver injury target-pathway” network, 69 target genes associated with ODPs in the treatment of cadmium-induced liver injury were identified, including AKT1, CASP3, MAPK8, JAK2, MAPK14, IL2, MMP9, IGF1R, NOS2, etc.Enrichment analysis revealed that the main signaling pathways involved are the VEGF signaling pathway, cancer pathway, PI3K- AKT signaling pathway, etc. (3) Based on traditional oxidative stress indicators, ODPs exhibited good antioxidant capacity, significantly inhibiting the decrease in SOD and GSH-Px activities induced by cadmium, reducing the level of lipid peroxidation product MDA, and restoring the level of antioxidant enzyme content in the liver to a normal range. Validation analysis based on network pharmacology showed that ODPs significantly reduced the levels of Caspase-3 and MMP-9 in the liver, increased the level of IL-2, effectively inhibiting cadmium-induced liver cell apoptosis and inflammatory reactions.
CRediT authorship contribution statement
Biyun Zhou: Data curation, and analysis, Writing – original draft, Writing – review & editing, Modify. Ting Liu: Visualization, Investigation, Data curation, and analysis. Ruhai Chen: Visualization, Investigation, Data curation, and analysis. Qing Wu: Visualization, Investigation, Funding acquisition. Qingfang Deng: Visualization, Investigation, Data curation, and analysis. Huaguo Chen: Conceptualization, Methodology, Visualization, Investigation, Funding acquisition, Modify.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
ACKNOWLEDGMENTS
This study was supported by the Collaborative Innovation Center of Biology and Information Technology in Karst Area of Guizhou Province (qian Jiaoji [2022] 010) and research on the key technology of the integration of Guizhou green tea and digital technology (Construction contract [2022]Chiu CS, Cheng YT, Chan YJ, Lu WC, Yang KM, Li, PH, et al. Mechanism and inhibitory effects of cactus (Opuntia dillenii) extract on melanocytes and its potential application for whitening cosmetics. Sci Rep. 2023; 13: 501.
REFERENCES
- Ahmed MS, El Tanbouly ND, Islam WT, Sleem AA, El Senosy AS. Antiinflammatory flavonoids from Opuntia dillenii (Ker-Gawl) Haw. flowers growing in Egypt. Phytother Res. 2005; 19: 807-809.
- Ben Lataief S, Mohamed-Nizar Z, Rahmani R, Najjaa H, Gharsallah N, Zourgui L, et al. Chemical composition, antioxidant, antimicrobial and cytotoxic activities of bioactive compounds extracted from Opuntia dilleniicladodes. J Food Meas Charact. 2021; 15: 782-794.
- Boehm H, “Opuntia dillenii” - An Interesting and Promising Cactaceae Taxon. J Prof Assoc Cactus Dev. 2008; 10: 148-170.
- Bouhrim M, Ouassou H, Boutahiri S, Daoudi NE, Mechchate H, Gressier B, et al. Opuntia dillenii (Ker Gawl.) Haw., Seeds Oil Antidiabetic Potential Using In Vivo, In Vitro, In Situ, and Ex Vivo Approaches to Reveal Its Underlying Mechanism of Action. Molecules. 2021; 26: 1677.
- Contino M, Leonardi C, Genovese C, Scalisi EM, Pecoraro R, Ignoto S, et al. Antioxidant activity of two Opuntia Mill. species fruit extracts on human sperm quality after a freeze-thaw cycle. Nat Prod Res. 2023; 37: 2725-2731.
- Ejaz S, Khaleeq A, Robina T, Muhammad. A novel link between angiogenesis and natural products: Anti-angiogenic effects of Opuntia dillenii. Cent Eur J Biol. 2014; 9: 298-308.
- Al-Naqeb G, Cafarella C, Aprea E, Ferrentino G, Gasparini A, Buzzanca C, et al. Supercritical Fluid Extraction of Oils from Cactus Opuntia ficus-indica L. and Opuntia dillenii Seeds. Foods. 2023; 12: 618.
- Gomez-Lopez I, MP Portillo, MP Cano, Characterization, bioaccessibility and biological activities of betalains and phenolic compounds from opuntia stricta var. Dillenii fruits. Ann Nutr Metab. 2022; 78: 21-21.
- Loukili EH, Bouchal B, Bouhrim M, Abrigach F, Genva M, Zidi K, et al. Chemical Composition, Antibacterial, Antifungal and Antidiabetic Activities of Ethanolic Extracts of Opuntia dillenii Fruits Collected from Morocco. J Food Qual. 2022; 2022: 11-20.
- Wu S, Yu Zhang, Shengfu Li, Changhua Li. Effects of cactus Opuntia dillenii polysaccharide-based coatings loaded with glutathione on the preservation of freshly cut Chinese water chestnut. Food Chem. 2023; 401: 134187.
- Long-Yan Zhao, Song-Lian Zhang, Qing-Xia Yuan, Jie Cheng, Fu-Hua Zeng. Immunomodulatory effects of Opuntia dillenii polysaccharides on specific immune function of mice. Zhong yao cai. 2012. 35: 98-102.
- Kalegowda P, AS Chauhan, SMN Urs, Opuntia dillenii (Ker-Gawl) Haw cladode mucilage: Physico-chemical, rheological and functional behavior. Carbohydr Polym, 2017; 157: 1057-1064.
- Lu WC, Chien-Shan Chiu, Yung-Jia Chan, Amanda Tresiliana Mulio, Po-Hsien Li. Recent Research on Different Parts and Extracts of Opuntia dillenii and Its Bioactive Components, Functional Properties, and Applications. Nutrients. 2023; 15: 2962.
- Yang Q, Huaguo Chen, Xin Zhou, Junzeng Zhang. Optimum extraction of polysaccharides from Opuntia dillenii and evaluation of its antioxidant activities. Carbohydr Polym. 2013; 97: 736-742.
- Liu T, Bianli Li, Xin Zhou, Huaguo Chen. A Study on the Time-Effect and Dose-Effect Relationships of Polysaccharide from Opuntia dillenii against Cadmium-Induced Liver Injury in Mice. Foods. 2022; 11: 1340.
- Hassan Shokri Garjan, Yadollah Omidi, Mehdi Poursheikhali Asghari, Reza Ferdousi. In-silico computational approaches to study microbiota impacts on diseases and pharmacotherapy. Gut Pathog. 2023. 15: 10.
- Hou Y, Guoyu Wang , Shuo Han, Huaman Liu, Xinhua Jia. Network pharmacology and molecular docking to explore the pharmacological mechanism of Yifei Tongluo granules in treating idiopathic pulmonary fibrosis: A review. Medicine. 2023. 102: e33729.
- Wei, WX, YH Jiang. To Investigate the Clinical Efficacy and Potential Mechanism of Tongxinluo Capsules in Preventing Coronary Restenosis Based on Meta-Analysis and Network Pharmacology Analysis. Evid Based Complement Alternat Med: 2023; 2023: 7985459.
- Zhao L, Zhang H, Li N, Chen J, Xu H, Wang Y, et al. Network pharmacology, a promising approach to reveal the pharmacology mechanism of Chinese medicine formula. J Ethnopharmacol. 2023; 309: 116306.
- Kanwugu ON, TV Glukhareva. Activation of Nrf2 pathway as a protective mechanism against oxidative stress-induced diseases: Potential of astaxanthin. Arch Biochem Biophys. 2023; 741: 109601.
- Lin Q, Li K, Chen Y, Xie J, Wu C, Cui C, et al. Oxidative Stress in Diabetic Peripheral Neuropathy: Pathway and Mechanism-Based Treatment. Mol Neurobiol. 2023; 60: 4574-4594.
- Liu S, Jia Y, Meng S, Luo Y, Yang Q, Pan Z, et al. Mechanisms of and Potential Medications for Oxidative Stress in Ovarian Granulosa Cells: A Review. Int J Mol Sci. 2023; 24: 9205.
- Liu Y, Shi Y, Han R, Liu C, Qin X, Li P, et al. Signaling pathways of oxidative stress response: the potential therapeutic targets in gastric cancer. Front Immunol. 2023; 14: 1139589.
- Ge X, Zhang Y, Fang R, Zhao J, Huang J. Exploring the inhibition mechanism of interleukin-1-beta in gouty arthritis by polygonum cuspidatum using network pharmacology and molecular docking: A review. Medicine. 2023; 102: ee34396.
- Liu X., YingYan Kuang, CaiRu Bian, ShaoWen Hu, YuanFang Xie, BeiBei Zhao, et al. Exploring the mechanism of action of herbal compounding in the treatment of myasthenia gravis based on network pharmacology. Biotechnol Genet Eng Rev. 2023: 23: 11-16.
- Noor F, Asif U, Ashfaq UA, Qasim M, Qamar MTU. Machine learning for synergistic network pharmacology: a comprehensive overview. Briefings in Bioinformatics. 2023; 24: 10-16.
- Simeonov M, Kostova B, Vassileva E. Interpenetrating Polymer Networks of Polyacrylamide with Polyacrylic and Polymethacrylic Acids and Their Application for Modified Drug Delivery - a Flash Review. Pharm Nanotechnol. 2023; 11: 25-33.
- Wu H, Sha Tu, Zewei Zhuo, Rui Jiang, Ruijie Zeng, Qi Yang, et al. Investigating the Mechanisms of Bisdemethoxycurcumin in Ulcerative Colitis: Network Pharmacology and Experimental Verification. Molecules. 2022; 28: 68.
- Wu S. Effect of Opuntia dillenii polysaccharide on gelling properties of Trichiurus lepturus myobrilar protein. Int J Biol Macromol. 2019; 130: 636-639.
- Zhao LY, QJ Lan, ZC Huang, LJ Ouyang, FH Zeng. Antidiabetic effect of a newly identified component of Opuntia dillenii polysaccharides. Phytomedicine. 2011. 18: 661-668.
- Agha F, Zehra Batool, Tuba Sharf Batool, Rida Nisar, Fizza Naqvi, Sadia Saleem, et al. Tree nuts supplementation instigates the oxidative status and improves brain performance in male rats. Pak J Pharm Sci. 2020; 33: 2785-2791.
- Ahmadian R, Mahmoud Reza Heidari, Bibi Marjan Razavi, Hossein Hosseinzadeh. Alpha-mangostin Protects PC12 Cells against Neurotoxicity Induced by Cadmium and Arsenic. Biol Trace Elem Res. 2023; 201: 4008-4021.
- Aja PM, Friday I, Izekwe 1, Ademola C Famurewa, Ezebuilo U Ekpono, Felix E Nwite, Ikechuku O Igwenyi, et al. Hesperidin protects against cadmium-induced pancreatitis by modulating insulin secretion, redox imbalance and iNOS/NF-kappa B signaling in rats. Life Sci. 2020; 259: 118268.
- Bovio F, Pasquale Melchioretto, Matilde Forcella, Paola Fusi, Chiara Urani. Cadmium promotes glycolysis upregulation and glutamine dependency in human neuronal cells. Neurochem Int. 2021; 149: 105144.
- Dabrowski A, Barbara M Onopiuk, Halina Car, Pawe? Onopiuk, Zofia N D?browska, Joanna Rogalska, et al. Beneficial Impact of an Extract from the Berries of Aronia melanocarpa L. on the Oxidative-Reductive Status of the Submandibular Gland of Rats Exposed to Cadmium. Antioxidants. 2020; 9: 185.
- Cammisotto PG, L Campeau. In vivo Crispr-Cas9 targeting matrix metalloproteinase-9 improves bladder voiding in prediabetic female Tally Ho mice. European Urology. 2023; 83: S964-S964.
- Chen X, Shuaixing Wang, Wenli Xu, Mingming Zhao, Youyi Zhang, Han Xiao. Metformin Directly Binds to MMP-9 to Improve Plaque Stability. J Cardiovasc Dev Dis. 2023. 10: 54.
- Carneiro Santiago LT, Natália Alves de Freitas 1, José Donizeti de Meira Junior, José Eduardo Corrente, Verônyca Gonçalves Paula, Debora Cristina Damasceno, et al. Oxidative status in colostrum and mature breast milk related to gestational age and fetal growth. J Matern Fetal Neonatal Med. 2023; 36: 2183763.
- Cordiano R, Di Gioacchino M, Mangifesta R, Panzera C, Gangemi S, Minciullo PL, et al. Malondialdehyde as a Potential Oxidative Stress Marker for Allergy-Oriented Diseases: An Update. Molecules. 2023; 28: 25-29.
- Merino de Paz N, García González M, Gómez Bernal F, uevedo Abeledo JCQ, de Vera González A, López Mejias R, et al. Relationship between Malondialdehyde Serum Levels and Disease Features in a Full Characterized Series of 284 Patients with Systemic Lupus Erythematosus. Antioxidants. 2023; 12: 11-15.
- Al-Azemi M, Omu FE, Kehinde EO, Anim JT, Anim JT, Omu AE, et al. Lithium protects against toxic effects of cadmium in the rat testes. J Assist Reprod Genet. 2010; 27: 469-476.
- Bao RK, Zheng SF, Wang XY. Selenium protects against cadmium- induced kidney apoptosis in chickens by activating the PI3K/AKT/ Bcl-2 signaling pathway. Environ Sci Pollut Res. 2017; 24: 20342- 20353.
- Kamel EO, Gad Elrab WM, Ahmed MA, Mohammedsaleh ZM, Hassanein EHM, Ali FEM, et al. Candesartan Protects Against Cadmium-Induced Hepatorenal Syndrome by Affecting Nrf2, NF-kappa B, Bax/Bcl-2/ Cyt-C, and Ang II/Ang 1-7 Signals. Biol Trace Elem Res. 2023; 201: 1846-1863.
- Huang R, Ding L, Ye Y, Wang K, Yu W, Yan B, et al. Protective effect of quercetin on cadmium-induced renal apoptosis through cyt-c/caspase-9/caspase-3 signaling pathway. Front in Pharmacol. 2022; 13:990993.
- Labib H, Galal A. Caffeine versus antioxidant combination (Antox) and their role in modifying cadmium-induced testicular injury in adult male albino rats. Andrologia. 2021; 53: e13948-e13948.
- Li X, Chen T, Wu X, Li Z, Zhang X, Jiang X, et al. Evolutionarily Ancient Caspase-9 Sensitizes Immune Effector Coelomocytes to Cadmium- Induced Cell Death in the Sea Cucumber, Holothuria leucospilota. Frontiers in Immunology. 2022; 13: 927880.
- Wei Z, Nie G, Yang F, Pi S, Wang C, Cao H, et al. Inhibition of ROS/ NLRP3/Caspase-1 mediated pyroptosis attenuates cadmium-induced apoptosis in duck renal tubular epithelial cells. Environ Pollut. 2021; 273: 115919.
- Yi L, Zhang L, Xiong C, Zhang Y, Chen L, Wang Y, et al. Effect of reactive oxygen species in cadmium chloride induced apoptosis of mouse Leydig cells. Wei sheng yan jiu. 2023; 52: 142-147.
- Liu W, Gong Z, Zhang K, Dong W, Zou H, Song R, et al. Paeonol protects renal tubular cells against cadmium-induced cytotoxicity via alleviating oxidative stress, inhibiting inflammatory responses and restoring autophagy. J Inorg Biochem. 2022; 230: 111733.
- Markiewicz-Gorka I, Chowaniec M, Martynowicz H, Wojakowska A, Jaremków A, Mazur G, et al. Cadmium Body Burden and Inflammatory Arthritis: A Pilot Study in Patients from Lower Silesia, Poland. International Journal of Environmental Research and Public Health. 2022; 19: 3099.
- Ramadan,MA, Saif Eldin AS. Effect of occupational cadmium exposure on the thyroid gland and associated inflammatory markers among workers of the electroplating industry. Toxicol Ind Health. 2022; 38: 210-220.
- Salama SA, Abd-Allah GM, Gad HS, Kabel AM, et al. Galangin attenuates cadmium-evoked nephrotoxicity: Targeting nucleotide-binding domain-like receptor pyrin domain containing 3 inflammasome, nuclear factor erythroid 2-related factor 2, and nuclear factor kappa B signaling. J Biochem Mol Toxicol. 2022; 36: e23059.
- Shahzadi A, Tariq N, Sonmez H, Waquar S, Zahid A, Javed MA, et al. Potential effect of luteolin, epiafzelechin, and albigenin on rats under cadmium-induced inflammatory insult: In silico and in vivo approach. Frontiers in Chemistry. 2023; 11:1036478. 3-11).