Treatment of Muscle Tears with a Protein-Rich-Platelet-Rich Plasma Matrix
- 1. Department of Podesta Orthopedic Sports Medicine Institute, USA
- 2. Podesta Orthopedic & Sports Medicine Institute, USA
- 3. Medical School, Orlando College of Osteopathic Medicine, USA
- 4. Medical School (GBCS), The University of Queensland, Australia
- 5. Center for Collaborative Research, Nova Southeastern University, USA
- 6. Medical School, Max Planck University Center (UniMAX), Brazil
Abstract
Muscle tears are a common injury with limited treatment options that effectively accelerate healing. This case study explores the use of a Protein-Rich Platelet-Rich Plasma Matrix (PR-PRP), as a multipurpose biologic platform for treating acute muscle tears in two athletes of significantly different ages: a 19-year-old D1 intercollegiate female soccer player with a quadriceps - rectus femoris tear, and a 73-year-old recreational male tennis player with a hamstring - biceps femoris tear. Both individuals received a single injection of Protein-Rich PRP Matrix, delivered to the individual injury sites to enhance tissue regeneration through the sustained release of growth factors, chemokines, leukocytes, and provide a scaffold that supports cell migration, followed by a structured rehabilitation exercise program. Post treatment assessments and musculoskeletal ultrasound imaging demonstrated accelerated recovery, reduced pain, and enhanced tissue regeneration in both patients. These findings highlight the potential of Protein-Rich PRP matrices in acute muscle injuries regardless of the patient’s age.
Keywords
• Muscle tears
• Platelet-Rich Plasma (PRP)
• Protein Rich-Platelet Rich Plasma (PR-PRP)
• Muscle healing
Citation
Podesta L, Lack K, Everts P (2025) Treatment of Muscle Tears with a Protein-Rich-Platelet-Rich Plasma Matrix. J Muscle Health 7(1): 1016
ABBREVIATIONS
PRP: Platelet-Rich Plasm; PR-PRP: Protein-Rich Platelet-Rich Plasma Matrix
INTRODUCTION
Platelet-Rich Plasma (PRP) therapy has gained significant interest in recent years as a potential treatment for muscle injuries. PRP is derived from the patient’s blood, concentrated by centrifugation and then administered into the injured tissue, releasing growth factors believed to aid in tissue regeneration and healing [1]. Several studies have reported encouraging results regarding the effectiveness of PRP in acute muscle injuries. Halpern et al. [2], discussed how PRP can enhance muscle healing by stimulating angiogenesis, cell proliferation, and cell migration. Similarly, Patil et al. [3], demonstrated that PRP treatments enhanced recovery times and improved functional outcomes in athletes with acute muscle injuries.
A recent study by Kruse et al. [3], determined the Platelet-poor plasma resulted in faster return to sport than platelet-rich plasma for acute thigh muscle injuries with a noninferior injury recurrence rate. While PRP holds large potential, further research is necessary to develop standardized preparation protocols and delivery techniques to optimize treatment outcomes [2,4].
Other plasma derived treatments, such as Platelet-Rich Matrix (PRM) [5], has emerged as a promising treatment option for muscle and tendon injuries. These formulations, which contain a higher concentration of platelets and leukocytes and invading residence cells, compared to standard plasma, are believed to promote tissue regeneration and repair through biological mechanisms, including the actions of non-platelet based growth factors like, insulin-like growth factor-1 (IGF-1) and hepatocyte growth factor (HGF) are mainly present in the PPP fraction [5]. Platelet-rich matrix (PRM) and Platelet-rich fibrin (PRF) have emerged as effective treatments for muscle injuries, leveraging the body’s intrinsic healing mechanisms [4,6- 8]. These products, derived from the patient’s own blood, contain a higher concentration of platelets and leukocytes compared to standard plasma, thereby promoting tissue regeneration and repair through various mechanisms.
CASE STUDY
Case 1
A 19-year-old female collegiate soccer player was evaluated for right thigh pain. While playing in a collegiate soccer summer league game, the patient sustained an acute injury to her right anterior thigh while kicking a soccer ball. She was unable to continue playing due to her pain. At presentation, the patient stated she has been playing soccer from a young age and had no history of prior injury to her right anterior thigh or lower extremity. She had previously consulted two Physical Therapists: one diagnosed her with a micro tear of the quadriceps tendon, while the other performed dry needling and cupping therapy for the right anterior thigh. The patient had not undergone a formal medical evaluation prior to presentation. On examination, the patient exhibited 4/5 weakness of the right thigh and tenderness in the right rectus femoris muscle. Nero vascular exam is found to be normal in both lower extremities and no other physical findings were observed. A diagnostic musculoskeletal ultrasound examination of the right complete thigh was performed the day of her initial visit to help determine the etiology of the patient’s pain and muscle. The diagnostic ultrasound showed an abnormal examination of the right thigh, consistent with a partial tear of the rectus femoris muscle belly (Figure 1)
Based on the history, mechanism of injury, clinical examination and ultrasound findings, a diagnosis of a partial tear of the right rectus femoris muscle was made. Since she had a minimal response from conventional conservative therapy and time requirements to return to play, a decision was made to treat the muscle injury with a cell-based therapy using autologous PRM under ultrasound guidance (Tables 1 and 2).
Table 1: Hematology Analysis Report (Beckman Coulter Hemoanalyzer) of each patient’s whole blood and NR-PRP sample.
|
Case 1: NR-PRP |
Case 2: NR-PRP |
||||||||
|
Whole Blood Analysis |
PRP Analysis |
Whole Blood Analysis |
PRP Analysis |
||||||
|
Total WB Volume (mls) |
60 ml |
Total LR-PRP Volume (mls) |
3 ml |
Total WB Volume (mls) |
60 ml |
Total LR-PRP Volume (mls) |
3.5 ml |
||
|
WBC |
6.36 x103/µL |
WBC |
67.83 x103/µL |
WBC |
3.46 x103/µL |
WBC |
28.04 x103/µL |
||
|
Lymph |
1.57 x103/µL |
Lymph |
22.69 x103/µL |
Lymph |
1.15 x103/µL |
Lymph |
18.72 x103/µL |
||
|
Mono |
0.35 x103/µL |
Mono |
6.57 x103/µL |
Mono |
0.26 x103/µL |
Mono |
4.77 x103/µL |
||
|
Neu |
4.17 x103/µL |
Neu |
37.50 x103/µL |
Neu |
1.96 x103/µL |
Neu |
4.38 x103/µL |
||
|
RBC |
3.66 x106/µL |
RBC |
2.97 x106/µL |
RBC |
3.93 x106/µL |
RBC |
1.92 x106/µL |
||
|
HCT |
33.0 % |
HCT |
28.2 % |
HCT |
39.2 % |
HCT |
19.8 % |
||
|
MPV |
7.16 fL |
MPV |
7.85 fL |
MPV |
9.19 fL |
MPV |
9.20 fL |
||
|
Total # Plts WB (x103/µL) |
222.2 x103/µL |
Total # Plts PRP (x103/µL) |
3318.9 x103/L |
Total # Plts WB (x103/µL) |
145.3 x103/µL |
Total # Plts PRP (x103/µL) |
2379.2 x103/µL |
||
|
|
|
Total Platelets Captured (%) |
74.68 % |
|
|
Total Platelets Captured (%) |
95.52 % |
||
|
|
|
Platelet Concentration / baseline |
14.94 X |
|
|
Platelet Concentration / baseline |
16.37 X |
||
|
|
|
Total Deliverable / Available PLTs (x106) |
9,957x106 |
|
|
Total Deliverable / Available PLTs (x106) |
8,327 x106 |
||
|
|
|
Total # PLTs/ml (x103 / ml) |
3320 x103 / ml |
|
|
Total # PLTs/ml (x103 / ml) |
2379 x103 / ml |
||
|
Case 1: Protein Fibrin Matrix |
Case 2: Protein Fibrin Matrix |
||||||||
|
Autologous PPP Volume |
30 ml |
Autologous PPP Volume |
30 ml |
||||||
|
Protein Fibrin Matrix Volume |
3.0 mls |
Protein Fibrin Matrix Volume |
3.0 mls |
||||||
Table 2: Individual patient procedural characteristics (Location, Bioformulation, Total Platelet Dose, Protein Matrix Volume, Total Injection Volume/site, and Activation status).
|
Case 1: Rectus femoris muscle PR-PRP Bioformulation |
||||
|
NR-PRP ml |
TPD |
PFM ml |
TVI ml |
Activation |
|
1.0 |
3.3 billion |
2.0 |
3.0 |
yes |
|
Case 2: Biceps femoris muscle PR-PRP Bioformulation |
||||
|
NR-PRP ml |
TPD |
PFM ml |
TVI ml |
Activation |
|
1.0 |
3.3 billion |
2.0 |
3.0 |
yes |
Post treatment the patient was instructed to apply heat to the treatment site 4-5 times per day to optimize the physiologic environment and stimulate blood flow. Recommendations were made to maintain knee extension for two weeks to minimize load and forces across the injured muscle that would occur with knee flexion. The patient presented for a reevaluation 1 week post procedure with minimal treatment site discomfort VAS 1-2/10, but substantially better prior to treatment. She continued to maintain knee extension to minimize stress and load across the rectus femoris muscle treatment site. At the beginning of the third week post treatment, the patient was started on a formal goal-based muscle specific rehabilitation exercise program .
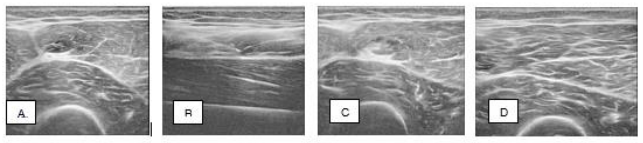
At 6 weeks post procedure, the patient was pain free and completed her exercise program and returned to play soccer. She returned for an in-person reevaluation at 19 weeks post procedure. She remained pain free, exhibited normal strength during her physical exam, and a diagnostic musculoskeletal ultrasound of the right quadriceps was performed with evidence of a healed rectus femoris muscle (Figure 1).
Figure 1 Case 1: Ultrasound images of the right rectus femoris muscle partial tear injury, A. Pretreatment; B. and C. Time of treatment; D. 24 weeks post-treatment.
Case 2
A 73-year-old male tennis player presented with an acute pain in the left posterior thigh while playing tennis. He stated he had a heavy total body exercise training program the day prior and after an extensive dynamic warmup prior to playing tennis the day of injury. While planting the left foot and eccentrically loading his hamstring muscles to make a shot, he experienced a sudden sharp pain in the posterior mid-thigh and was unable to continue playing. He had difficulty walking and extending his knee. He reported no prior injuries to the left lower extremity in the past. Physical exam revealed tenderness over the mid biceps femoris muscle belly with a small painful palpable defect in the muscle. Diagnostic ultrasound evaluation revealed a hematoma at the side of injury within the muscle belly and disruption of the normal muscle morphology (Figure 1). A decision was made to treat the muscle injury with a cell-based therapy using autologous PRM under ultrasound guidance (Tables 1 and 2). The same post procedure instructions were recommended to this patient as with case 1. The patient presented for a reevaluation 1 week post procedure with no post-procedural pain VAS 0/10. He continued to limit knee flexion and extension to minimize stress and load across the biceps femoris muscle treatment site. At the beginning of the third week post treatment, the patient was started on a formal goal-based muscle specific rehabilitation exercise program (Table 3). At 4 weeks post procedure, he was reevaluated and at his own discretion, decided to go snow skiing. He reported to be able to ski without any pain or restrictions and exhibited normal strength during his physical exam. At 8 weeks post procedure, he was reevaluated having no pain in his left hamstring muscle. He began a progressive return to play tennis. A diagnostic musculoskeletal ultrasound Figure 1 Case 1: Ultrasound images of the right rectus femoris muscle partial tear injury, A. Pretreatment; B. and C. Time of treatment; D. 24 weeks post-treatment. of the left hamstring was performed with evidence of a healed biceps femoris muscle (Figure 2).
Figure 2 Case 2: Ultrasound images of the right biceps femoris muscle partial tear injury, A. and B. Pretreatment; C. 24 weeks post-treatment.
DISCUSSION
Muscle injuries occur at all levels of sport and can result in significant loss of practice and playing time. These muscle injuries are also associated with significant risk of recurrence. Autologous point of care blood products, such as PRP and PPP have been studied to treat acute muscle injuries with the goal to expedite healing, return to play and minimize recurrence. Muscle injuries account for 29% of injuries in sports [6]. Specific muscle injury prevention programs have been studied and implemented such as Nordic hamstring curls, and Copenhagen abductor exercises in addition to the FIFA 11+ program [9-12]. PRP has been studied extensively and has been shown to modulate anti-inflammatory proregenerative M2 macrophage response to muscle injury. It is believed to have a direct and indirect impact on satellite cell activity [13].
The proliferation and differentiation of myogenic precursors by satellite cells have the ability to rescue muscle cells from apoptosis [6,13]. PRP impacts surrounding cells through concentrated growth factors released from platelets following degranulation (Table 4) [14]. An important part of the mitogenic response of PRP is platelet-derived growth factor (PDGF) working synergistically with vascular endothelial growth factor (VEGF), transforming growth factor b1 (TGF-b1), insulinlike growth factor-1 (IGF-1), and hepatocyte growth factor (HGF) [6,14].
Table 4: Protein rich platelet concentrate/matrix content.
|
cPRP component |
Structure |
Key content |
Main Functions |
|
Platelets |
α-granules |
Growth factors: PDGF (AA-BB-CC), VGEF, TGF (α,β), FGH (α,β), CTGF |
Growth factor-based regulation of tissue repair via cell proliferation, differentiation, mitosis, chemotaxis, epithelial repair. |
|
|
|
Adhesive proteins: Fibronectin, Vitrofibrinogen, vWF, P-selectin, Integrins α-IIbβ, Phosphatidylserline |
Platelet aggregation, platelet-endothelial, cell interaction, thrombus formation. |
|
|
Coagulation factors: Factors IV, XI, XIII, plasminogen, Plasmin, Antithrombin, Tissue factor |
Hemostasis, thrombus formation. |
|
|
|
Angiogenic regulators: IL8, Thrombospondin, Angiostatin, PF- 4, TIMP-1,4, MMO-1,2,9, Angiopoetin, Endostatin, SDF-1, PMP |
Angiogenic cascades and reestablishing vascular |
|
|
|
Cytokines: IL1, IL4, IL6, TNFα, SDF-1 |
Inflammation, antimicrobial, bacteria, side activity. |
|
|
|
Compliment proteins: C3, C4 |
Phagocytosis, chemotaxis, platelet activation. |
|
|
|
Exosomes: mRNA, miRNA, CXCL4, CXCL7 |
Cell adhesion, paracrine communication, regulation of cell fate, modulation of inflammatory response. |
|
|
Dense granules |
ADP, ADT, TNFα, calcium, serotonin, epinephrine, pyrophosphates |
Platelet activation, vasoconstriction. |
|
|
Lysosomes |
Collagenase, Elastase, Cathepsin, α-arabinoside, β-galactosidase |
Matrix degradation, antimicrobial activity. |
|
|
Multivesicular bodies |
Exosomes, Extracellular vesicles |
Cell proliferation, PGF, transportation, platelet-cell communication |
|
|
|
|
|
|
|
PPP |
Plasma proteins (>300) |
Albumin, fibrinogen, alpha-2-macroglobulin |
Blood clotting, maintain blood pressure, carrier functions, immunity, pH regulation |
|
|
Coagulation factors |
Tissue factor, Factor I, II, IV, V, VII, vWF |
Intrinsic and extrinsic coagulation pathways, clot formation |
|
Growth factors |
IGF-1, HGF |
Bone growth, glucose transport in fat and muscle, muscle production, mitogenesis, cell growth, cell proliferation |
Additional questions have risen due to the inconsistent effects of PRP questioning its optimal application and the appropriate balance of growth factors to treat muscle injuries. Studies comparing unmodified PRP to modified nonneutrophil containing PRP and PPP have determined that the modified PRP and PPP formulations preferentiallyinduce myoblast cells into the muscle cell differentiation pathway. However, unmodified PRP leads to increased myoblast proliferation [6,15]. In addition, PRP has the potential to induce reperfusion of blood in muscle lesions, based on angiogenesis pathways initiated by LRPRP [16]. While the key response to initial injury is the proliferation of myoblasts, the differentiation pathway is necessary for induction of cells into muscle tissue [15]. Increased muscle fibrosis has been identified clinically after PRP treatment in contrast to observed increased muscle repair and healing with native muscle architecture with PPP treatment [6]. Clinical results can vary significantly due to variations of PRP preparation, platelet capture rate, bioformulations, cellular dosing, and application techniques. The debate continues to exist regarding the potential benefits of PPP versus PRP for the treatment of muscle injuries. PPP and modified PPP has been found to be beneficial over PRP in directing skeletal myoblasts to differentiate into mature muscle tissue [6,15]. The clinical benefits of PPP can include increased coordination of muscle recovery and improve tissue quality resulting in accelerated recovery while decreasing recurrence rates. The PPP fraction, which is typically discarded due to its historically perceived lack of therapeutic benefit, contains its own molecular and cellular components. These components are preserved for further concentration of plasma-specific growth factors, such as insulin-like growth factor-1 (IGF-1) and hepatocyte growth factor (HGF) [7].
The PPP fractions also contain exosomes and other extravesicular structures that are essential for cell-to-cell communication and cell signaling [17]. Technological advancements have facilitated the development of new biological preparation concepts, such as concentrating PPP through modified ultrafiltration to elevate plasma levels of proteins like fibrinogen, alpha 2 macroglobulin (A2M), IGF-1, HGF, cytokines, and other biomolecules. Modern low-volume ultrafiltration devices, featuring small membrane pore sizes, produce a protein-rich product that can be used independently or combined with high-definition PRP formulations to create a consolidated multicellular protein-rich platelet concentrate (PR-PRP) preparation. Compared to PRP alone, PR-PRP contains a greater variety of growth factors and other significant non-platelet derivatives. Several studies have reported synergistic effects between platelet-derived and nonplatelet-derived growth factors. Once activated, PR-PRP functions as a biological matrix, retaining and facilitating interactions between its cellular and molecular constituents. Moreover, the versatility of PR-PRP technology enables the creation of patient-specific formulations for both nonsurgical and surgical applications. This adaptability allows for tailored treatments that address the unique needs of individual patients, enhancing the efficacy and precision of therapeutic interventions. The post procedural, rehabilitation and recovery process is extremely important and often overlooked. The appropriate post treatment recovery and exercise progressions are extremely important in achieving positive outcomes.
Each patient and injured tissue are unique and require specific intervention and tissue specific rehabilitation. Early protection and tissue-specific progressive loading are critical components to successful outcomes following orthobiologic intervention. Each tissue heals and responds differently. Muscle, tendon, ligament, and articular cartilage each have unique healing properties that require tissue-specific loading. Using a criteria-based loading and exercise progression, guided by dynamic imaging when appropriate is helpful to further advance the goal-oriented rehabilitation program [18]. Lastly, from this case study we observed no negative effects of age as a predetermining factor on positive outcome. Therefore, we disagree with earlier publications that age influences patient outcomes following PRP treatments [19].
REFERENCES
- Everts P, Onishi K, Jayaram P, Lana JF, Mautner K. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. Int J Mol Sci. 2020; 21:7794.
- Halpern BC, Chaudhury S, Rodeo SA. The role of platelet-rich plasma in inducing musculoskeletal tissue healing. HSS J. 2012; 8: 137-145.
- Prasad P, Jadhav M, Suvvari TV, Thomas V. Therapeutic uses of platelet-rich plasma (PRP) in sport injuries-A narrative review. J Orthop Rep. 2024; 3: 100287.
- Ryan KC, Elena V. Platelet-Poor Plasma for the Treatment of Acute Hamstring Muscle Injuries in Collegiate Football Athletes: A Cohort Study. Clin J Sport Med. 2024.
- Everts PA, Lana JF, Alexander RW, Dallo I, Kon E, Ambach MA, et al. Profound Properties of Protein-Rich, Platelet-Rich Plasma Matrices as Novel, Multi-Purpose Biological Platforms in Tissue Repair, Regeneration, and Wound Healing. Int J Mol Sci. 2024; 25:7914.
- Raum G, Kenyon C, Bowers R. Platelet-Poor versus Platelet-Rich Plasma for the Treatment of Muscle Injuries. Curr Sports Med Rep. 2024; 23: 222-228.
- Miroshnychenko O, Chang WT, Dragoo JL. The Use of Platelet-Rich and Platelet-Poor Plasma to Enhance Differentiation of Skeletal Myoblasts: Implications for the Use of Autologous Blood Products for Muscle Regeneration. Am J Sports Med. 2017; 45: 945-953.
- Hamilton B, Tol JL, Almusa E, Boukarroum S, Eirale C, Farooq A, et al. Platelet-rich plasma does not enhance return to play in hamstring injuries: a randomized controlled trial. Br J Sports Med. 2015; 49: 943-950.
- Van Dyk N, Behan FP, Whiteley R. Including the Nordic hamstring exercise in injury prevention programs halves the rate of hamstring injuries: a systematic review and meta-analysis of 8459 athletes. Br J Sports Med. 2019; 53: 1362-1370.
- Ishøi L, Krommes K, Husted RS, Juhl CB, Thorborg K. Diagnosis, prevention and treatment of common lower extremity muscle injuries in sport- grading the evidence: a statement paper commissioned by the Danish Society of Sports Physical Therapy (DSSF). Br J Sports Med. 2020; 54: 528-537.
- Thorborg K, Krommes KK, Esteve E, Clausen MB, Bartels EM, Rathleff MS. Effect of specific exercise-based foot-ball injury prevention programs on the overall injury rate in football: a systematic review and meta-analysis of the FIFA 11 and 11+ programs. Br J Sports Med. 2017; 51: 562-571.
- Harøy J, Clarsen B, Wiger EG, Øyen MG, Serner A, Thorborg K, et al. The adductor strengthening program prevents groin problems among male football players: a cluster-randomized controlled trial. Br J Sports Med. 2019; 53: 150-157.
- Chellini F, Tani A, Zecchi-Orlandini S, Sassoli C. Influence of platelet- rich and platelet-poor plasma on endogenous mechanisms of skeletal muscle repair/ regeneration. Int J Mol Sci. 2019; 20: 683.
- Borrione P, Gianfrancesco AD, Pereira MT, Pigozzi F. Platelet-rich plasma in muscle healing. Am J Phys Med Rehabil. 2010; 89: 854-861.
- Miroshnychenko O, Chang WT, Dragoo JL. The use of platelet-rich and platelet-poor plasma to enhance differentiation of skeletal myoblasts: implications for the use of autologous blood products for muscle regeneration. Am J Sports Med. 2017; 45: 945-953.
- Everts PA, Lana JF, Onishi K, Buford D, Peng J, Mahmood A, et al. Angiogenesis and Tissue Repair Depend on Platelet Dosing and Bioformulation Strategies Following Orthobiological Platelet-Rich Plasma Procedures: A Narrative Review. Biomed. 2023; 11: 1922.
- Li Y, Zhu Z, Li S, Xie X, Qin L, Zhang Q, et al. Exosomes: compositions, biogenesis, and mechanisms in diabetic wound healing. J Nanobiotechnology. 2024; 22: 398.
- Podesta L, Mattfeld R, Khadavi M, Honbo E. Rationale and Clinical Guidelines for Post-Orthobiologic Rehabilitation, In Manchikanti L, Navani A, Atluri S, Sanapati M. (eds) Manchkanti’s Essentials of
-
Regenerative Medicine in Interventional Pain Management 2nd Edition. Springer, Cham. 2024.
- Chowdhary K, Sahu A, Iijima H, Shinde S, Borg-Stein J, Ambrosio F. Aging Affects the Efficacy of Platelet-Rich Plasma Treatment for Osteoarthritis. Am J Phys Med Rehabil. 2023; 102: 597-604.