Convergence of Acute Ischemic Stroke and Frailty: Exploring Therapeutic Synergies
- 1. Department of Neurology, Yancheng Third People’s Hospital, China
Abstract
One of the leading causes of death worldwide and the primary cause of long-term disability in affluent nations is frailty and stroke. According to earlier research, there is a strong correlation between stroke patients death and frailty. The aims of this review to investigate the association of acute ischemic stroke and frailty patients. The search was performed using the keywords acute ischemic and stroke frailty. The review systematically analyzed existing literature to provide a comprehensive overview of associations, different factors and inflammatory biomarkers. This review addresses the factors associated with these two diseases, emphasizing the role of ageing, factors (diet, physical activity, smoking), genetic background, and the presence of subclinical illnesses in influencing the risk of both frailty and acute ischemic stroke. The association between frailty and ischemic stroke is underscored, with shared risk factors and potential bidirectional influences. Moreover, the review highlights the intricate links between inflammatory markers, frailty, and acute ischemic stroke. Interleukin-6 (IL-6), Tumor necrosis factor-alpha (TNF-α), Lymphocytes and C-reactive protein (CRP) play a dual role in inflammation and are associated with age-related morbidity, frailty and stroke. The conclusion is that frailty and acute ischemic stroke are closely linked, sharing risk factors and having bidirectional influences, highlighting the need for targeted interventions and improved healthcare strategies for affected individuals.
Keywords
- Acute Ischemic Stroke
- Frailty
- Associations
- Inflammatory biomarkers
Citation
Liu J, Wang S, Shen Y, Shi H, Han L (2024) Convergence of Acute Ischemic Stroke and Frailty: Exploring Therapeutic Synergies. J Neurol Disord Stroke 11(3): 1222.
INTRODUCTION
One of the most prevalent illnesses in the world, stroke now ranks third in terms of disability and is the second biggest cause of mortality [1]. According to http:// world-stroke.org, one in six people will experience a stroke at some point in their lifetime, and more than 13.7 million individuals do so annually, with 5.8 million people dying as a result. Over 80 million people have survived strokes worldwide. Out of all stroke incidents, 9.5 million are ischemic strokes, with the remaining 70% being either subarachnoid or intracerebral hemorrhage. It is predicted that the US has a greater percentage of ischemic strokes (85- 87%) [2]. An abrupt decrease in blood flow to a section of the brain can cause an acute ischemic stroke, which leaves the affected area neurologicly impaired (AIS). It is brought on by an embolism or thrombosis that blocks a cerebral artery that supplies a particular part of the brain [3]. There is a core region of vascular occlusion where the brain sustains irreversible damage and an area of penumbra where the brain has lost function but is not injured due to a diminished blood supply. When given to suitable patients, evidence-based therapies for acute ischemic stroke (AIS), such as intravenous thrombolysis and endovascular clot extraction, have been demonstrated to enhance outcomes by removing the blockage and restoring blood flow to the afflicted brain regions. These treatments have advanced significantly in recent years [4-6]. The prevalence of stroke will pose a significant challenge to the healthcare and social service systems as societies get older. Furthermore, an earlier study found that elder stroke survivors had a greater death rate [1]. Consequently, it’s critical to recognize and manage the mortality risk in elderly stroke patients. Numerous clinical variables are linked to an increased risk of death, including age, the severity of the stroke, ischemic heart disease, and comorbidities [7].
A prevalent geriatric illness known as frailty is characterized by a drop in reserve and functional ability brought on by a cumulative multisystem deterioration, which increases the likelihood of negative outcomes when stressful situations arise [8,9]. Frailty was linked to a lower survival rate in a 2017 study involving 717 people who had an episode ischemic stroke, according to the investigators [10]. It has been documented that frailty increases an individual’s risk of falls, fractures, incapacity, and even death in older persons living in the community or in nursing homes [11,12]. Given that stroke is a highly stressful event, it seems sense to investigate the relationship between stroke survivors’ mortality and frailty. One risk factor for a shorter survival that stood alone was frailty, according to Winovich et al.’s analysis of the risk factors for ischemic stroke survival [8]. The relationship between stroke patients’ mortality and frailty has been the subject of numerous investigations recently [10,13]. A different American study [14], on 240 patients with intracerebral haemorrhage discovered that the frailty score did not correlate with a high risk of death (P = 0.11). In order to investigate the association between frailty and mortality, long-term follow-up research is essential, as previous studies sometimes had very short follow-up periods and produced conflicting findings [15]. By identifying the association between frailty and acute ischemic stroke patients, the review highlights shared risk factors such as aging, lifestyle choices, and genetics. Additionally, the review seeks to investigate common inflammatory biomarkers and different factors that may be pertinent to both ischemic stroke and frailty.
Frailty Risk and its Impact on Stroke Incidence
After a stroke, frailty is common. According to a recent metaanalysis of 18 studies involving 48,009 participants, 49% and 22%, respectively, of stroke patients are pre-frailty and frailty [16]. Frail individuals with stroke are typically older and more likely to be female [17]. It is crucial to understand how stroke affects frailty, even if the influence of frailty on stroke has received a lot of attention in the discussion of the links between frailty and stroke [Figure 1].
Figure 1: The impact of stroke on frailty and the various factors influencing the onset of frailty are interconnected. The figure illustrates that the risk factors of stroke may be associated with frailty in every stroke patient. These factors, identified as contributors to frailty according to [19, 20].
It has been shown that a prior stroke significantly influences both the decline of a frailty trajectory and the transition from robust to frail. The neurological abnormalities that follow a stroke are likely to worsen the phenotypic markers of frailty [18][Figure 1].
To determine who is eligible for stroke clinical trials, the pre-stroke modified Rankin Scale (mRS), which evaluates impairment, is frequently utilized. But it’s important to realize that frailty assessment cannot be replaced by mRS alone. Prestroke mRS demonstrates reasonable agreement with a frailty index, even though only one-third of the participants displayed evidence of frailty. Over half of the people were classified as dependent on pre-stroke mRS, and there was a group with fragility but minimal disability [21]. Pre-stroke mRS and phenotypical frailty assessments agree very little, although other research has found a significant level of agreement between pre-stroke mRS and a frailty index [22]. However, other studies have reported no statistically significant correlation between CFS and mRS [13,23,24]. The study found that stroke was only associated with a transition from prefrail to frail [25]. Stroke was associated with a worsening of frailty status in women, both in terms of the transition from robust to prefrail/frail and from prefrail to frail, whereas it was associated with an improvement in males with baseline prefrailty or frailty in multivariate models. However, these correlations differed according to gender [16]. Medicare individuals who are frail before to an acute ischemic stroke had a reduced chance of obtaining inpatient rehabilitation after their stroke compared to nonfrail patients with identical stroke severity. Following adjustments for comorbidities, stroke severity, and a prestroke functional ability test, the study examines discharge destination patterns in poststroke patients. According to this, fragility could be a good stand-in for prestroke functional ability and be used to examine variations in rehabilitation utilization [17].
The study conducted by Renedo et. [26], is higher hospital frailty risk score (HFRS) was associated with an increased risk for any stroke as well as ischemic and hemorrhagic stroke subtypes. The HFRS remained significant even after accounting for vascular risk factors, suggesting that the score had an independent effect. A substantial association indicating a causal relationship between genetically determined frailty and the likelihood of all ischemic stroke, all ischemic stroke, and all ICH was shown by Mendelian Randomization analysis. The study showed that the Frailty Index approach is valid for use in stroke and may be utilized for assessment of almost all stroke patients, despite its low concordance with other measures of frailty [27].
Association of Frailty and the risk stroke
Even after controlling for age, sex, and number of medications, there is still a marginally significant connection between the premorbid frailty index and the onset of post-stroke delirium [22]. Even after controlling for age, delirium, prestroke cognitive impairment, and stroke severity, pre-stroke frailty continues to show an independent correlation with improved post-stroke cognition [28]. Pre-stroke frailty traits, like a sluggish gait and weak hands, have been independently linked to post-stroke cognitive deterioration and a diminished capacity for performing activities of daily life. These relationships could have an impact on the reduced effectiveness of rehabilitation for those with post-stroke cognitive impairment linked with frailty. They also point to a critical area that has to be explored in order to determine whether frailty affects how well post-stroke cognitive rehabilitation works [29].
Following a stroke, frail people self-reported a lower quality of life than the non-frail group, which is correlated with a significant deterioration in quality of life. Even after controlling for age, sex, and NIHSS score, the main cause of this disparity is the considerable decline in the mobility and self-care categories [30]. After a stroke, frailty seems to affect how well a person responds to psychosocial therapies. Following such therapies, the non-frail cohort demonstrates notable gains in activities of daily living, while the frail cohort either shows no significant change at all or a trend towards worsening outcomes. The impact of frailty on therapy modification is especially noticeable in psychological therapies aimed at mortality and physical performance [31].
Decreased functional capacity and limited reserve are hallmarks of frailty, a common geriatric disease brought on by cumulative multisystem decline. When faced with pressures, this state makes negative consequences more likely [8, 9]. Studies show that elderly people living in the community as well as those residing in nursing homes have higher risks of falls, fractures, incapacity, and death when they are fragile [11,12]. It seems sense to look into the relationship between mortality and frailty in stroke survivors as stroke is a major stressor. After analysing the risk factors linked to ischemic stroke survival, Winovich et al., found that frailty was a separate risk factor for a lower chance of surviving [29]. Numerous researches have examined the relationship between stroke patients’ mortality and frailty in recent times [10,14]. The Canadian Study of Health and Ageing (CSHA) has developed a frailty index (CSHA-FI) based on a cumulative deficit model as one of the many instruments for frailty assessment [9]. According to this paradigm, a person’s overall weaknesses in terms of clinical indicators, symptoms, illness states, and limitations add up to their frailty. When compared to depending only on chronological age, it provides a more accurate assessment of ageing [11].
Clinical fragility was found to be an independent risk factor for 28-day death in patients with ischemic stroke in 2020 research [20]. In the Clinical Frailty Scale, the odds ratio was 1.03 (95% CI: 1.01–1.05, P < 0.01) for every one-point rise. On the other hand, a different study [32], that was carried out in the United States and involved 240 patients who had experienced intracerebral haemorrhage revealed that there was no significant correlation between frailty and an increased risk of death (P = 0.12), according to the frailty index.
Patients who had previously been diagnosed with frailty, as determined by the FRAIL scale, had a higher one-year mortality risk than those who were not frail. Crucially, this increased risk persisted in spite of confounding factors such inadequate handgrip strength, polypharmacy, instrumental activities of daily living (IADL), and activities of daily living (ADL) [33]. The study’s conclusions highlight frailty as a unique and independent risk factor for stroke patients’ one-year death. This implies that early frailty screening by physicians or nurses is crucial, as is the implementation of appropriate therapies. These interventions could include customised tactics, such physical activity campaigns and suitable dietary plans, to lessen the effect of frailty on stroke patients’ one-year mortality risk [34,35].
Prevalence of frailty and stroke
In contrast to Evans et al.’s earlier study [36], which discovered that the prevalence of frailty among ischemic stroke patients was 54.04%, the prevalence of frailty among stroke patients was determined to be 22.5%. Given that the average age of 72.73 years was lower than the median age of 87 years in the previous study for frail patients and 83 years for nonfrail patients, age may be the main reason for this discrepancy. As patients age, there is an increased risk of frailty. In the 60–69 age range, the prevalence of frailty was reported to be 12% (95% CI: 11%–14%), while in the 80–89 age group, it was 31% (95% CI: 29%–34%) [37]. Apart from age, the intensity of the ailment and the tools utilised to measure frailty are other factors that could influence prevalence reports [38].
Numerous research works have examined the relationship between frailty and death. Consistent with our results, the authors of a 2017 study comprising 717 patients who experienced an ischemic stroke noted a correlation between fragility and a shorter survival time [39]. Comparing stroke patients with and without frailty, our investigation revealed that frailty was an independent risk factor for 28-day death [36]. Although the outcomes of our study were comparable, there were some noticeable variations. While the studies that were cited only looked at ischemic stroke, our analysis included haemorrhagic stroke as well.
A recent study indicated that the prevalence of haemorrhagic stroke was higher than that of ischemic stroke [40]. On the other hand, Kim et al., found that patients who experienced spontaneous intracerebral haemorrhage and were fragile, as determined by the modified Frailty Index, did not have a higher mortality risk than those who were not frail [41]. These differences highlight the complex link, impacted by variables like the kind of stroke and length of follow-up, between frailty and mortality. The authors postulated that doctors may have a propensity to choose patients who are more severely disabled for evaluation of their frailty, thereby creating a potential bias towards certain patients. Imaoka et al.’s investigation of 156 patients who experienced spontaneous intracerebral haemorrhage, however, allayed this worry. According to the study’s findings, multivariate analytic models showed a roughly two-fold increase in the risk of death (OR = 1.97, 95% CI: 1.34–2.90) for every point increase in the modified Frailty Index [42,43].
Gender base prevalence: The results of a previous study [32], which indicated that female stroke patients had a higher prevalence of frailty than male patients, were validated by gender stratification. The gender of stroke patients was linked to a higher incidence of frailty, per a pooled analysis of 15 studies (OR=1.76, 95%CI:1.63–1.91). First, because women live longer on average and have a higher risk of stroke as they age, women have a larger overall prevalence of stroke [44]. Because of their worse prognosis, higher disability, and lower quality of life, women are more likely to have fragility following a stroke [45]. This is another often disregarded variation in stroke risk by gender. A high rate of vitamin D insufficiency impacted the ability of postmenopausal women over 60 who participated in these trials to contract and use their muscles [46]. Age was observed to positively correlate with frailty in seven studies that used age as a continuous variable (MD = 6.732, 95%CI: 3.55–9.91, P < 0.001). However, in elderly stroke patients, the effects of the illness have led to a drop in the physiological reserve and a partial decline in the functions of various organs and systems. This raises the possibility of fragility and lessens the body’s capacity to endure damaging external stimuli. The frequency of frailty varies from 7% to 12% in those over 65 to as high as 30% in those over 80, per similar data [20]. Because a stratified analysis of age could not be performed by the included study, we may look into the fragility of stroke patients in different age groups more. Even if women and age are unmodifiable risk factors, medical personnel should promptly and precisely analyse the patient’s condition, pay attention to their nutritional state, and provide sufficient intake of critical protein, amino acids, and vitamin D.
Diabetes, hyperlipidemia, and atrial fibrillation (AF) were identified in the meta-analysis as additional risk factors for frailty among stroke sufferers. According to a prior study [47], people with type 2 diabetes had much lower levels of frailty than those without the illness. Atherosclerosis, the pathological aetiology of stroke, can result from diabetes-related damage to the arterial endothelium [48]. Due to the negative relationship between IR and muscle content, individuals with diabetes are more likely to experience IR-related muscle mass loss and eventually become weak [49]. Hyperlipidaemia was associated with frailty in stroke patients (OR = 1.46, 95%CI: 1.04–2.04, P = 0.030) [50]. It might have been because elevated blood lipid levels in older persons were associated with an increased risk of arteriosclerosis. Debilitating symptoms are the consequence of tissue, organ, and system damage caused by chronic arteriosclerosis. Through cell cycle arrest and oxidative stress, it also hastens cellular senescence [47]. The exact method might not be obvious because different researches produced different results. Frailty was found to be correlated with low total cholesterol (TC) and high HDL-C by Bastos-Barbosa [51]. The studies suggested that AF may exacerbate physical function in stroke patients, perhaps contributing to frailty. Individuals with AF were more likely to fall and had a lower functional capacity than those without AF [52,53].
Specific-Female Risk Factors of stroke
There are risk factors specific to women that need to be considered. These could include variables that can be changed directly as well as variables that can’t be changed directly but may still be important for risk prediction, such as reproductive life span. A recent meta-analysis discovered that, in comparison to a life span of 36 to 38 years, a reproductive life span of less than 30 years was associated with a 75% increase in risk. An increased risk of stroke is associated with these factors. The time from menarche to menopause is known as lifetime oestrogen exposure [54]. Premature (40 years old) or early (40–44 years old) menopause appears to be the primary factor causing this association, while both early and late menarche also likely to increase the risk of stroke. These findings hold true for other recent prospective investigations [55-57]. Parity and breastfeeding are two more factors related to reproductive health that are linked to stroke risk. Parity data indicate that women who have had five or more live births are more likely to have a stroke than women who have had one or two live births; however, in some research, this association was abolished when confounding variables, such as body mass index, were taken into account [55,58]. According to recent data on breastfeeding from the Women’s Health Initiative, women who reported ever breastfed had a 23% decreased risk of incident stroke; additionally, the reduction appeared to increase with longer reported breastfeeding periods [59]. Unfavourable pregnancy outcomes such as preterm delivery, gestational hypertension, preeclampsia, and foetal growth limitation have been frequently associated with an increased long-term risk of cardiovascular disease, including stroke, in the mother [60]. Atherosclerotic cardiovascular disease, which includes stroke, has been consistently linked to a history of unfavourable pregnancy outcomes, according to recent data from the Women’s Health Initiative [61]. According to certain research, patients with a history of preeclampsia have a threefold increased risk of stroke in the future (peripartum stroke excluded) [62]. Preterm delivery (65% higher risk), prenatal hypertension (80% increased risk), and foetal growth restriction (30% increased risk) have all been linked to an increased risk of stroke in the future [63]. Additionally, some research shows a link between a history of pregnancy-related hypertension illnesses and potential vascular cognitive impairment [64]. Future cerebrovascular disease as well as unfavourable pregnancy outcomes may be predisposed by a high-risk maternal vascular phenotype. On the other hand, unfavourable pregnancy outcomes could cause immunologic intolerance and an elevated inflammatory state, which might affect the mother’s vasculature in the long run. These are some of the putative mechanisms of these associations [65].
Female Stroke Pathophysiology: It is quite uncommon for atherosclerotic disease to cause maternal stroke. Subarachnoid haemorrhage, or ICH, is the cause of up to 50% of strokes in mothers, as opposed to 87% of strokes in the overall population. Cardio embolism, dissection, infarction, or haemorrhage; cerebral venous sinus or cortical vein thrombosis; reversible cerebral vasoconstriction syndrome leading to vasospasm- related ischemia or convexity Subarachnoid haemorrhage, hypertensive ICH, and rupture of vascular anomalies such as cerebral aneurysms, arteriovenous malformations, or moyamoya vasculopathy are common stroke causes observed in pregnant and postpartum patients [66,67]. All of these acute cerebrovascular disorders are more likely to occur due to the special and complicated physiology of pregnancy and its aftereffects, especially hypertensive disorders of pregnancy (Figure 2).
Figure 2: The physiology of stroke in mothers (A) Pathological mechanisms involving the cerebrovascular tree and cervical artery dissection may cause stroke during pregnancy or puberty (B) Pregnancy-related physiological changes that impact the immunological, hematologic, and cardiovascular systems may all raise the risk of stroke. Pregnancy-related pathological problems include cardiac remodelling, pulmonary imbalance, and hypertensive heart disease.
Potential mechanism for the association of frailty and stroke
It is unknown how stroke patients’ mortality and fragility are related. These relationships could be explained by a number of factors. First, a large body of research across a wide range of groups and environments has demonstrated that frailty, a state marked by poor functional capacity and cumulative deficiencies of several systems, increases the risk of mortality [12,68]. Stroke incidents occurred in older persons, and either an ischemic or hemorrhagic stroke was a major stressor that exacerbated the deleterious effects of frailty on patients and ultimately probably raised their chance of death. Second, stroke patients who are frail may not be able to withstand intrusive medical interventions like surgery, artificial ventilation, and medication during the acute disease stage, increasing their risk of death. This is because frail patients have a lower physiological reserve. Third, stroke patients typically have incapacity [69], and dysphagia [70], during the chronic and rehabilitative phases. This leads to prolonged inactivity and an increased risk of malnutrition [71], hence aggravating the frailty situation. Frailty in stroke patients may lead to a vicious cycle of malnourishment and inactivity that ultimately worsens the patient’s chances of death and severe disability [15].
The association between pre-stroke frailty state and the prognosis for acute cerebral infarction was investigated in this cohort study. It was discovered that pre-stroke frailty independently influenced 28-day mortality but not 1-year mortality. Furthermore, co-infection, NIHSS score, and advanced age were all independently linked to 1-year death. Prior to the stroke, frailty was linked to severe disability at 28 days and 1 year, which may have a detrimental impact on a person’s capacity to function. Prior research has established a correlation between pre-stroke frailty state and unfavourable outcomes, including stroke severity [72], mortality [13], short-term functional outcome [73], reduced capacity for daily activities [29], discharge location [17], and cognitive impairment following a stroke [28]. The results of this investigation demonstrated that, whereas pre-stroke frailty status did not independently affect long- term mortality, it did increase the chance of short-term death. Diseases other than stroke account for the majority of long-term mortality outcomes following a stroke [74]. According to the study, cerebral infarction and its associated complications such as significant seizures and cerebral hernias were the primary causes of death in patients who died young, while heart failure and post-stroke pneumonia develop after the acute stage. A deterioration in the physiological regulating mechanisms is the pathophysiology of frailty. As a result, there is a dynamic imbalance, reduced resilience, and heightened susceptibility to stressors. Clinical signs of frailty with higher death and disability arise when a specific level of dysregulation takes place [75]. Pre-frail or frail people’s functional ability rapidly deteriorates when stress events occur (e.g., acute cerebral infarction) [76], increasing the likelihood of acute disease severity and short- term death). Following a stroke, patients are twice as likely to experience fragility as those who do not experience a stroke [16]. Frailty affects about one in four acute stroke patients, and an additional four patients experience frailty if their pre-stroke state is pre-frail. According to these investigations, pre-stroke frailty did not independently influence 1-year mortality. Older persons over 80 years of age showed a considerably reduced connection between pre-stroke frailty and long-term survival following stroke, which may be related to heterogeneity, according to a study with a mean follow-up period of 1.6 years. Following an acute cerebral infarction, older persons typically have greater NIHSS and infection rates, which exacerbates the decline of their pre-existing frailty condition [22]. Pre-stroke fragility may not have as strong of a prognostic influence on post-stroke long-term mortality as other research have demonstrated. These factors include advanced age, co-infection, and a higher NIHSS score. Consequently, long-term mortality can be effectively decreased by focusing on the prevention and treatment of infection during the acute phase [77,78]. Pre-stroke frailty deterioration was substantially correlated with decreased functional improvement. Since all patients at our centre received rehabilitation counselling and instruction while they were in the hospital, post-stroke rehabilitation exercises were not included in this study. Additionally, there is a vicious loop since
pre-stroke frailty was found to be substantially correlated with the severity of strokes and post-stroke neurologic impairment made frailty worse [27]. Patients with acute ischemic stroke treated with endovascular stroke therapy had a lower chance of favourable 3-month outcomes when their frailty risk was high [79]. Concurrently, participants experiencing mild stroke had lower health-related quality-of-life, and this decline persisted for patients experiencing in-hospital frailty between three and eighteen months after the stroke [30]. Frailty is quite common and is linked to poor outcomes and higher medical expenses. Pre-frailty, defined as those with adverse risk factors who are not yet experiencing significant physical and physiological changes associated with frailty, is a dynamic process that transitions from quantitative to qualitative change between robust and frail persons [80]. It’s unclear when pre-frailty in this study gives way to frailty. It is possible that vascular alterations and frailty predate the commencement of an overt cerebrovascular event and that the patient’s frailty was made worse by the acute shock. Notably, research indicates that extended periods of sedentary behaviour and anorexia raise the risk of frailty in older adults [30,75]. Primary care interventions that support healthy eating and physical activity have the potential to stop the progression from pre-frailty to frailty [81].
Risk Factors Considerations
Epidemiological data linking putative predisposing factors to the eventual development of disease has led to the evolution of the phrase “risk factor.” Strong, dose-related risk variables that are predictive over a range of samples, pathogenically plausible, and backed by additional research are considered important [82]. Data from long-term research have demonstrated that lowering high blood pressure, quitting smoking, engaging in regular physical activity, and maintaining a healthy weight and diet are some of the most effective lifestyle changes to minimise the risk of stroke [83,84]. It has been shown that changing lifestyle risk factors, even little, is feasible and significantly reduces risk. The most accurate assessment of a person’s future risk of cardiovascular disease comes from their genetic background, knowledge on risk factors and behaviors, and the existence of subclinical illnesses [85].
Ageing is a complex multifactorial process that differs widely between individuals and within a person’s tissue. Among the biological markers of ageing are telomere attrition, loss of proteostasis, stem cell depletion, dysregulated nutrition sensing, mitochondrial dysfunction, cellular senescence, altered intercellular communication, and genomic instability [86]. Age- related loss of homeostasis and a compromised stress response make a person more susceptible to a variety of diseases and disabilities. As people age, their risk of developing cardiovascular disease, dementia, stroke, diabetes, and cancer increases. The idea that ageing is the primary risk factor for the majority of these complicated diseases and that preventing ageing itself is essential to preventing these age-related disorders forms the foundation of the rapidly developing area of “geroscience [87]. Regional subgroup studies showed that North America had the lowest prevalence of frailty among stroke patients, while Asia had the highest frequency. Moreover, studies [88], found that access to high-quality healthcare and affluence lower the risk of frailty, which may assist to explain why frailty prevalence differs across LMICs and HICs. The Frail Scale [89], Fried Frailty Phenotype, and Hospital Frailty Risk Score [90], were utilised by the majority of the included research to determine the prevalence of frailty.
According to this meta-analysis, the prevalence of frailty was 12.0% when evaluation was restricted to the 11-item Modified Frailty Index and greatest (32.6%) when assessment was restricted to the FRAIL scale [9]. Fluctuations in the sensitivity and specificity of these measurements may account for variations in the prevalence of frailty. Future research should focus on developing a standard frailty assessment for stroke victims. According to a pooled analysis of the included studies, ischemic stroke was associated with a higher frequency of frailty than hemorrhagic stroke. Acute ischemic stroke may have occurred in certain study participants in addition to multi-system underlying diseases and cerebrovascular risk factors. Furthermore, a few researchers had severely compromised limb function and were going through the acute phase or the aftermath of a stroke [75,81].
GWAS studies of frailty and stroke
Genome-wide association studies on parental lifespan conducted with the UK Biobank participants have identified relationships with several loci, including rs1051730 (CHRNA3), rs1317286 (CHRNA3/5), rs429358 (APOE), rs55730499 (LPA), rs1556516 (CDKN2BAS, CDKN2A/2B), rs28926173 (MC2R),
and rs11065979 (ATXN2). Frailty, stroke, cancer, cardiovascular disorders, and Alzheimer’s disease have all been associated with these genes [91,92]. The gene locus for the cholinergic receptor, nicotinic alpha (CHRNA5-CHRNA3-CHRNB4), which codes for ligand-gated ion channels involved in neurotransmission, is located on chromosome 15q25. Other disorders associated with this locus include the onset of smoking and lung cancer. LPA 61 encodes lipoprotein(a), which is involved in the transportation of cholesterol and triglycerides. The three main diseases type 2 diabetes, coronary heart disease, and stroke that are linked to genetic variations in this LPA region (6q26) [93]. The 9p21 gene is a GWAS hotspot associated with multiple complex illnesses, including as dementia, diabetes, cancer, and cardiovascular disease. It comprises CDKN2BAS, also known as ANRIL, and cyclin-dependent kinase inhibitors 2A/2B (CDKN2A/2B) [94]. Tumor suppressor genes, CDKN2A and CDKN2B, control the cell cycle, apoptosis, senescence, and ageing processes. Adrenocorticotropic hormone (ACTH) receptor MC2R is involved in immune system and blood sugar regulation [95]. The adrenal glands produce glucocorticoid hormones (cortisol, corticosteroid) when ACTH binds to its receptors.
The pleiotropic gene APOE, which codes for Apolipoprotein E, is implicated in a variety of physiological processes, including CNS physiology, inflammation, and lipoprotein metabolism. The most researched genotypes in relation to multiple negative outcomes, including Alzheimer’s disease, stroke, hypertension, and cardiovascular disorders, are the ε4, ε3, and ε2 alleles of Apolipoprotein E (APOE). While the ε3 allele is thought to be neutral and the ε2 allele has been demonstrated to have a cognitive protective effect, the ε4 allele functions as a risk allele for Alzheimer’s disease [96,97].
More extensive methods like to the GWAS in the UK biobank are required to offer a more comprehensive view of the underlying genetics of frailty. It’s possible that more pathways come together to create frailty than previously thought [37]. In addition to disease signs and symptoms, the individual components of the cumulative frailty index include complicated disorders such as diabetes, cancer, heart disease, stroke, and chronic lung disease. It is possible for several biochemical pathways to become active in frailty, resulting in a mosaic phenotype that is challenging to identify at the population level. Several main factors can lead to a state of frailty. Diabetes, heart disease, neurological disorders, or other conditions could all contribute to the same cumulative frailty score in different people. Pleiotropic effects of SNPs and genes show that each of these traits has a certain amount of shared genetic components in addition to its own distinct genetic components [98]. Remarkably, the biggest GWAS in the UK biobank also revealed associations between regions that had previously been linked to a variety of variables. Since GWAS only look at common variations, these phenotypic complexities combined with lacking heritability may make it difficult to unravel the underlying biology of frailty. Both rare mutations and epigenetic modifications may be significant contributors to frailty [12,99]. Geographical location and various ethnic groups also have an impact on life expectancy [100]. This study suggested that age-related features like frailty in various contexts may have distinct genetic and epigenetic markers.
Biomarkers in ischemic stroke and frailty
While endogenous pyrogenic compounds from proteins have been documented since the 1940s and 1950s, the first interleukin was discovered in 1979 and is called IL-1 [101]. Two crucial roles for IL-1 in the innate immune system response and inflammation management are fever induction and acute phase response. Eleven components make up IL-1 at this time: seven agonists (IL-1α, IL-1β, IL-18, IL-33, IL-36α, IL-36β, and IL-36 γ),
one anti-inflammatory cytokine (IL-37), and three antagonists (IL-1Ra, IL-36Ra, and IL-38). There has been research on the possibility of using circulating IL-33 levels as markers of sickness severity or death [102]. Remarkably, decreased circulating levels of IL-33 were associated with either a greater infarction volume and a more severe stroke [53] or a higher risk of death in patients with acute ischemic stroke (AIS) [103]. Although elevated levels of IL-33 have been linked to several immune-regulated illnesses, including rheumatoid arthritis, asthma, multiple sclerosis, atopic dermatitis, and Sjögren’s syndrome, IL-33 appears to be helpful in atherosclerosis. In addition to heart failure and other cardiovascular diseases, several other age-related acute and chronic illnesses have shown the importance of sST2 in predicting outcomes. These include acute distress, stroke, systemic sclerosis, neurodegenerative diseases, type 2 diabetes (T2D), metabolic syndrome, cancers, chronic kidney disease (CKD), hypertension, chronic obstructive pulmonary disease and critically ill patients [103,104]. When cognitive impairment occurs, blood NfL remains elevated for three to six months after an acute neuronal event, such as an ischemic or hemorrhagic stroke [105,106]. This surge occurs in the early days after the neuronal event. Blood NfL levels were linked to the lesion burden [107].
Common inflammatory biomarkers of frailty
The current study has yielded frailty biomarkers that primarily focus on inflammation, oxidative stress, nutrition, and other related factors. According to the findings of numerous studies on inflammatory biomarkers, TNF-α and IL-6 levels are significantly higher in older, frail individuals [108-110]. These are common biomarkers of inflammation linked to frailty (Figure 3). To promote, strengthen, and safeguard older individuals’ capacity to age healthily, the WHO developed the ICOPE (Integrated Care for Older Persons) recommendations [111]. This suggests that the use of instruments and/or biomarkers is crucial in the diagnosis and prevention of frailty. Because the internal phenotype of frailty is ignored in favour of the external phenotype, which includes decreased muscle mass, activity, slower pace, and other physical manifestations, effective early diagnosis and treatments are currently impossible to obtain. The inherent phenotype of physiological illnesses in the body are known as biomarkers [112][Figure 3].
Figure 3: Displays the common inflammatory biomarkers associated with frailty and stroke. Previous studies have concluded that these biomarkers are directly linked to stroke during frailty, as evidenced by [34, 45].
Interleukin 6: According to [113], IL-6 is sometimes referred to as the “geriatric factor” because of its dual pro- and anti- inflammatory properties. According to [114], IL-6 has the ability to both stimulate and inhibit the synthesis of acute reactants, including fibrinogen and CRP, as well as the inflammatory markers TNF-α and IL1β. According to earlier research, older individuals exhibit greater levels of the inflammatory biomarkers CRP and IL-6 than do younger individuals [115]. When it comes to older folks, those who are frailty-affected have a much greater IL-6 level than those who are not. Additionally, it has been discovered that IL-6 is linked to adverse health outcomes and age-related morbidity in older persons, including slow speed, decreased independence, and all-cause mortality [116]. It is clear from cross-sectional research that inflammatory indicators and frailty are related [117]. According to a recent meta-analysis, therapies aimed at promoting frailty, such exercise and dietary assistance, may also lower concentrations of IL-6 and CRP [118]. According to the research mentioned above, IL-6 is a potential biomarker for frailty. IL-6 levels can be used to measure the impact of frailty therapies in older adults. However, there are unavoidably drawbacks to significant variability between research because of the various frailty evaluation measures utilised in the aforementioned investigations. We suggest that future research look into the possibility of employing more than two frailty assessment instruments for the study subjects when assessing frailty in order to further verify the dependability of the conclusion that IL-6 is strongly associated to frailty. This will enable us to ascertain if the relationship between IL-6 and frailty varies depending on the frailty evaluation instrument used.
C-reactive protein CRP: The first protein to be identified during the acute phase of inflammation was CRP (c-reactive protein), which belongs to the “Pentraxin” protein family. Hepatocytes are stimulated to produce CRP by proinflammatory stimuli such as IL-6, IL-1, and IL-17 [119]. By enlisting complement systems and phagocytes, CRP facilitates the removal of infections and injured cells by identifying them and the host harm cells they cause. Numerous age-related illnesses, including type 2 diabetes mellitus and cardiovascular disease [120] are influenced by CRP [121]. Research has revealed greater CRP in the elderly, a positive correlation between CRP concentration and the degree of frailty [122], and a correlation between high middle-aged CRP and the likelihood of frailty in old age [123]. The concentration of CRP can be lowered by frailty measures interventions. The aforementioned findings imply that frailty and CRP are related. A small number of studies, including some big ones, such as a cross- sectional study of 1723 older people aged 70 to 84, contradict this conclusion, though, and the findings imply that CRP is not linked to frailty [124]. Furthermore, CRP does not appear to be
linked to the risk of frailty, according to findings from a nested case-control study of 1900 postopausal women [125]. There are gender variations in the correlation between CRP and frailty as well: women exhibit a stronger correlation between CRP and the risk of frailty [126]. Referring to previous secondary research, CRP can be regarded as an effective marker of frailty, even though the results of these studies differ [127,128].
Tumor necrosis factor (TNF-α): The peptide cytokine TNF-α is mostly generated by T cells, monocytes, and macrophage subsets. Through the activation of numerous pathways, TNF-α can boost the synthesis of IL-6 and mediate the inflammatory response under pathological situations as well as immune surveillance under physiological conditions. While there is a slight correlation between TNF-α and frailty, it is still present compared to CRP and IL-6. One the one hand, research indicates that older adults with frailty have higher levels of TNF-α and mRNA than those without [129]. TNF-α concentrations were also found to be greater in the frailty group than in the nonfrailty group, and TNF-α concentrations were linked to frailty, according to a recently published meta-analysis of 21 cross-sectional studies [127,130]. However, as Diego Marcos-Pérez et al., pointed out, a number of investigations revealed no conclusive link between TNF-α and frailty. Furthermore, a 2023 study by Anna Picca et al., revealed that while frailty therapies had no discernible impact on TNF-α concentration, they did appear to lower CRP and IL-6 concentrations in older persons. Even though the aforementioned results differ, TNF-α remains a plausible biomarker of frailty. To further evaluate the relationship between TNF-α and frailty, future research must investigate the mechanism by which TNF-α contributes to frailty [131].
Lymphocytes: Possible explanations for the association between lymphocytes and frailty include immunosenescence, which is characterized by changes in lymphocyte function and number, and inflammation, which is exacerbated by low lymphocyte counts [132]. Moreover, studies have demonstrated a negative correlation between lymphocyte count and the degree of frailty, with lower lymphocyte counts in older frail persons [92,133]. It is important to note that a low lymphocyte count indicates frailty. Nonetheless, certain study findings run counter to the conclusions made above. Guilherme Eustáquio Furtado’s cross-sectional study looked at the connection between frailty and inflammatory markers in the saliva and blood of 358 elderly women 75 years of age and older. The results showed that although the lymphocyte counts of the three categories did not differ significantly, the lymphocyte counts of robust older people were greater than those of prefrailty or frailty.
The blood levels of inflammatory markers following a stroke have been the subject of numerous research examining their relationship to various outcomes, including long-term disability cognitive impairment [15], musculoskeletal involvement [134,135], depression [136], and future stroke risk [137]. According to studies [138], cognitive impairment in stroke patients has been associated with interferons [139,140], and gamma, C-reactive protein, tumor necrosis factor, and other common inflammatory indicators. Less research has linked the inflammatory markers high sensitive C reactive protein (HsCRP), soluble CD40 Ligand (CD40-L), serum amyloid A (SAA), tumour necrosis factor receptor-1 (TNFR1), and monocyte chemoattractant protein-1 (MCP-1) to an increased risk of lacunar stroke [141]. In the meanwhile, negative outcomes are thought to be predicted by inflammatory indicators. These markers include baseline serum amyloid protein (SAP), alpha-2 macroglobulin (A2M), and metalloproteinase 9 pre-post tissue-plasminogen activator (tPA) variations (?) [142]. Wijeratne and Wijeratne used publicly available, universal serial white cell counts in the context of COVID-19 and PCNS (Long COVID) to illustrate the clinical application of serial systemic immune-inflammatory indicators (SSIIi). This could be investigated to clarify the connection between low-grade inflammation and cognitive fragility in the context of frailty and stroke. As mentioned before, ischemia and/or inflammatory brain damage can cause frailty in a number of ways, either directly or indirectly.
Nonetheless, the correlations discovered between inflammatory markers and cognitive decline, musculoskeletal atrophy, and frailty represent only a portion of the overall picture of the relationship between the brain and body. Regular use of more accessible biomarkers, such SSIIi, and total blood protein profile investigations may assist identify stronger signals or factors that contribute to the development of diseases like stroke, enabling more focused research and treatment [43,143].
2. There were 259 patients in total for the experiment; their mean age was 69±12 years, and 46 percent of them were female. Higher odds of a great outcome were linked to an increased brain parenchymal percentage (odds ratio per percent increase, 1.078 [95% CI, 1.008–1.153]). An independent relationship between a one-point increase in CFS and 28-day mortality was found via multivariable analysis (OR 1.03 (1.01–1.05)). The median NIHSS considerably decreased in 63 thrombolysed adults; it did not fall in frail individuals (15 (IQR 11.5) to 16 (IQR 16.5), P =0.23), but it did fall in non-frail individuals (12.5 (interquartile range (IQR) 9.25) to 5 (IQR 10.5), P 0.01). An independent connection (coefficient 1.07, P = 0.03) was found in the multivariable analysis between a one-point rise in CFS and a one-point drop in post-thrombolysis NIHSS improvement. Clinical fragility appears to be linked to a slower rate of improvement in NIHSS following stroke thrombolysis and is independently associated with 28-day mortality following an ischemic stroke [13,15Discussion
The intricate relationship between inflammatory markers, frailty, and acute ischemic stroke (AIS) underscores the complexity of these conditions and their interplay in shaping health outcomes. C-reactive protein (CRP), tumor necrosis factor-alpha (TNF-α), and lymphocyte counts emerge as key biomarkers reflecting the inflammatory processes underlying both frailty and stroke. CRP, a prominent acute-phase reactant, has been associated with various age-related diseases, including cardiovascular disease and type 2 diabetes [120]. Studies have revealed a positive correlation between CRP levels and frailty severity [122], indicating its potential as a marker for frailty.
However, contradictory findings exist [124], necessitating further research to elucidate the precise role of CRP in frailty. Moreover, interventions targeting frailty have shown promising results in lowering CRP levels [118], emphasizing the dynamic nature of this biomarker. Similarly, TNF-α, primarily produced by immune cells, exhibits a complex relationship with frailty. Further investigation into the mechanisms underlying TNF- α’s contribution to frailty is warranted to clarify its role as a biomarker. Lymphocyte counts, reflective of immunosenescence and inflammation, have been negatively correlated with frailty severity [133]. However, conflicting findings highlight the need for a nuanced understanding of lymphocyte dynamics in frail individuals, considering factors such as age, gender, and health status. In the context of ischemic stroke, inflammatory markers play a crucial role in predicting outcomes such as cognitive impairment, musculoskeletal involvement, and mortality risk [15]. The relationship between inflammatory markers and cognitive decline underscores the broader impact of inflammation on neurological health, extending beyond stroke outcomes. The bidirectional influences between frailty, inflammatory markers, and ischemic stroke suggest a reciprocal relationship, where inflammation contributes to both frailty development and stroke severity. This intricate interplay opens avenues for therapeutic synergies, emphasizing the importance of targeted interventions and personalized healthcare approaches. It is unclear how frailty affects the course of a stroke, especially in terms of mortality. A number of conditions that impact cerebrovascular health, such as diabetes, ischemic heart disease, hypertension, atrial fibrillation, and a decrease in the prescription of anticoagulants for atrial fibrillation, are linked to frailty [37]. Our findings, however, show that clinical frailty was unrelated to traditional vascular risk factors, indicating that the worse outcomes after an ischemia insult might be explained by a worldwide decrease in physiological reserve. It is uncertain to what degree a loss of “cerebrovascular reserve” to withstand an insult is a sign of frailty [144,145].
Frailty was found to have a small but cumulative effect. The relationship between frailty and stroke has only been briefly studied in a few research, most of which have focused on longer- term functional outcomes. At six months after a stroke, a lower ability to perform activities of daily living (a proxy for frailty) was linked to worse functional outcomes and an increased probability of institutionalisation [146]. Another factor that has been linked to post-stroke cognition as an independent moderator is pre- stroke fragility [28]. After a stroke, the 6-minute walk test can accurately predict death. Similarly, although stroke severity was not taken into account, decreased premorbid grip strength and walking speed were linked to both cognitive decline and stroke fatality [29]. However, in the context of an acute stroke, measures like the 6-minute walk test and grip strength are unfeasible and cannot be obtained in the past. On the other hand, in our “real- world” group, CFS assessment of premorbid fragility was possible in the acute setting [147].
CONCLUSION
This review unveils the intricate connection between acute Ischemic Stroke and frailty, highlighting shared risk factors like aging, lifestyle choices, and genetics. The bidirectional influences suggest a reciprocal relationship, emphasizing the need for nuanced comprehension. The interplay of frailty, inflammatory markers (IL-6, TNF-α, CRP), and Ischemic Stroke opens avenues for therapeutic synergies. This understanding sets the stage for targeted interventions and improved healthcare approaches for individuals facing both frailty and acute Ischemic Stroke.
AUTHOR CONTRIBUTIONS
JL searched data and wrote the manuscript; SW and YS modified the manuscript; HS reviewed and edited the manuscript; LH conceived and supervised the study, and article drafting. All authors contributed to the report, read and approved the final manuscript.
FUNDING
This work was supported by the Special Funds for Science Development of the Clinical Teaching Hospitals of Jiangsu Vocational College of Medicine (No. 20229107) and Clinical Medicine Project of Nantong University (No. 2023JZ026).
REFERENCES
- Global, Regional, and Country-specific Lifetime Risks of Stroke, 1990 and 2016. N Engl J Med. 2018; 379: 2429-2437.
- Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics - 2019 update: a report from the American Heart Association. Circulation. 2019; 139: e56-e528.
- Phipps MS, Cronin CA. Management of acute ischemic stroke. BMJ. 2020; 368: 16983.
- Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega- Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018; 378: 708-718.
- Eastin TM, Dye JA, Pillai P, Lopez-Gonzalez MA, Huang L, Boling WW, et al. Delayed revascularization in acute ischemic stroke patients. Front Pharmacol. 2023; 14: 1124263.
- Goyal M, Menon BK, Van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016; 387: 1723-1731.
- Rønning OM, Stavem K. Predictors of mortality following acute stroke: a cohort study with 12 years of follow-up. J Stroke Cerebrovasc Dis. 2012; 21: 369-372.
- Avers D. The older adult who is frail. Guccione’s Geriatric Physical Therapy E-Book. 2019. 283.
- Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013; 381: 752-762.
- Imaoka Y, Kawano T, Hashiguchi A, Fujimoto K, Yamamoto K, Nishi T, et al. Modified frailty index predicts postoperative outcomes of spontaneous intracerebral hemorrhage. Clin Neurol Neurosurg. 2018; 175: 137-143.
- De Vries O, Peeters G, Lips P, Deeg D. Does frailty predict increased risk of falls and fractures? A prospective population-based study. Osteoporos Int. 2013; 24: 2397-2403.
- Wang Q, Wang Y, Lehto K, Pedersen NL, Williams DM, Hägg S. Genetically-predicted life-long lowering of low-density lipoprotein cholesterol is associated with decreased frailty: a Mendelian randomization study in UK biobank. EBioMedicine. 2019; 45: 487- 494.
- Evans NR, Wall J, To B, Wallis SJ, Romero-Ortuno R, Warburton EA. Clinical frailty independently predicts early mortality after ischaemic stroke. Age Ageing. 2020; 49: 588-591.
- Kim MG, Gandhi C, Azizkhanian I, Epstein B, Mittal A, Lee N, et al. Frailty and spontaneous intracerebral hemorrhage: does the modified frailty index predict mortality? Clin Neurol Neurosurg. 2020; 194: 105816.
- Zhang XM, Jiao J, Xu T, Wu XJ. The association between frailty of older stroke patients during hospitalization and one-year all-cause mortality: A multicenter survey in China. Int J Nurs Sci. 2022; 9: 162- 168.
- Palmer K, Vetrano DL, Padua L, Romano V, Rivoiro C, Scelfo B, et al. Frailty syndromes in persons with cerebrovascular disease: A systematic review and meta-analysis. Front Neurol. 2019; 10: 1255.
- Seamon BA, Simpson KN. The effect of frailty on discharge location for medicare beneficiaries after acute stroke. Arch Phys Med Rehabi. 2019; 100: 1317-1323.
- Lee Y, Kim J, Han ES, Ryu M, Cho Y, Chae S. Frailty and body mass index as predictors of 3-year mortality in older adults living in the community. Gerontology. 2014; 60: 475-482.
- Oliver-Williams C, Vladutiu CJ, Loehr LR, Rosamond WD, Stuebe AM. The association between parity and subsequent cardiovascular disease in women: the atherosclerosis risk in communities study. J Womens Health. 2019; 28: 721-727.
- Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012; 60: 1487-1492.
- Fearon P, McArthur KS, Garrity K, Graham LJ, McGroarty G, Vincent S, et al. Prestroke modified rankin stroke scale has moderate interobserver reliability and validity in an acute stroke setting. Stroke. 2012; 43: 3184-3188.
- Taylor-Rowan M, Cuthbertson G, Keir R, Shaw R, Drozdowska B, Elliott E, et al. The prevalence of frailty among acute stroke patients, and evaluation of method of assessment. Clin Rehabil. 2019; 33: 1688-1696.
- Palmer K, Vetrano DL, Padua L, Romano V, Scelfo B, Marengoni A, et al. Frailty syndromes in persons with cerebrovascular disease: a systematic review and meta-analysis. Front Neurol. 2019; 10: 1255.
- Trevisan C, Veronese N, Maggi S, Baggio G, Toffanello ED, Zambon S, et al. Factors influencing transitions between frailty states in elderly adults: The Progetto Veneto Anziani Longitudinal Study. J Am Geriatr Soc. 2017; 65: 179-184.
- Lee JS, Auyeung TW, Leung J, Kwok T, Woo J. Transitions in frailty states among community-living older adults and their associated factors. J Am Med Dir Assoc. 2014; 15: 281-286.
- Renedo D, Acosta JN, Koo AB, Rivier C, Sujijantarat N, de Havenon A, et al. Higher Hospital Frailty Risk Score Is Associated With Increased Risk of Stroke: Observational and Genetic Analyses. Stroke. 2023; 54: 1538-1547.
- Evans NR, Todd OM, Minhas JS, Fearon P, Harston GW, Mant J, et al. Frailty and cerebrovascular disease: Concepts and clinical implications for stroke medicine. Int J Stroke. 2022; 17: 251-259.
- Taylor-Rowan M, Keir R, Cuthbertson G, Shaw R, Drozdowska B, Elliott E, et al. Pre-stroke frailty is independently associated with post-stroke cognition: a cross-sectional study. J Int Neuropsychol Soc. 2019; 25: 501-506.
- Winovich DT, Longstreth Jr WT, Arnold AM, Varadhan R, Zeki Al Hazzouri A, Cushman M, et al. Factors associated with ischemic stroke survival and recovery in older adults. Stroke. 2017; 48: 1818- 1826.
- Wæhler IS, Saltvedt I, Lydersen S, Fure B, Askim T, Einstad MS, et al. Association between in-hospital frailty and health-related quality of life after stroke: the Nor-COAST study. BMC Neurol. 2021; 21: 100.
- Ertel K, Glymour M, Glass T, Berkman L. Frailty modifies effectiveness of psychosocial intervention in recovery from stroke. Clin Rehabil. 2007; 21: 511-522.
- Huang Y, Yan F, Wang X, Chen X, Chong H, Su W, et al. Prevalence and Risk Factors of Frailty in Stroke Patients: A Meta-Analysis and Systematic Review. J Nutr Health Aging. 2023; 27: 96-102.
- Cano-Escalera G, Graña M, Irazusta J, Labayen I, Gonzalez-Pinto A, Besga A. Mortality Risks after Two Years in Frail and Pre-Frail Older Adults Admitted to Hospital. J Clin Med. 2023; 12: 3103.
- Xue QL, Bandeen-Roche K, Tian J, Kasper JD, Fried LP. Progression of Physical Frailty and the Risk of All-Cause Mortality: Is There a Point of No Return? J Am Geriatr Soc. 2021; 69: 908-915.
- Muscedere J, Waters B, Varambally A, Bagshaw SM, Boyd JG, Maslove D, et al. The impact of frailty on intensive care unit outcomes: a systematic review and meta-analysis. Intensive Care Med. 2017; 43: 1105-1122.
- Whiteley W, Jackson C, Lewis S, Lowe G, Rumley A, Sandercock P, et al. Inflammatory markers and poor outcome after stroke: a prospective cohort study and systematic review of interleukin-6. PLoS Med. 2009; 6: e1000145.
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001; 56: M146-M156.
- Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007; 62: 722-727.
- Middaugh SJ, Whitehead WE, Burgio KL, Engel BT. Biofeedback in treatment of urinary incontinence in stroke patients. Biofeedback Self Regul. 1989; 14: 3-19.
- Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci. 2007; 62: 738-743.
- Gelber DA, Good DC, Laven LJ, Verhulst SJ. Causes of urinary incontinence after acute hemispheric stroke. Stroke. 1993; 24: 378- 382.
- Greenham M, Gordon AL, Cooper A, Ditchfield M, Coleman L, Hunt RW. Social functioning following pediatric stroke: contribution of neurobehavioral impairment. Dev Neuropsychol. 2018; 43: 312-328.
- Hassan EB, Phu S, Warburton E, Humaith N, Wijeratne T. Frailty in Stroke - A Narrated Review. Life. 2021; 11: 891.
- Appelros P, Nydevik I, Viitanen M. Poor outcome after first-ever stroke: predictors for death, dependency, and recurrent stroke within the first year. Stroke. 2003; 34: 122-126.
- Bushnell CD, Chaturvedi S, Gage KR, Herson PS, Hurn PD, Jimenez MC, et al. Sex differences in stroke: challenges and opportunities. J Cereb Blood Flow Metab. 2018; 38: 2179-2191.
- Navaneethan PR, Kekre A, Jacob KS, Varghese L. Vitamin D deficiency in postmenopausal women with pelvic floor disorders. J Midlife Health. 2015; 6: 66-69.
- Li G, Prior JC, Leslie WD, Thabane L, Papaioannou A, Josse RG, et al. Frailty and risk of fractures in patients with type 2 diabetes. Diabetes Care. 2019; 42: 507-513.
- Lau LH, Lew J, Borschmann K, Thijs V, Ekinci EI. Prevalence of diabetes and its effects on stroke outcomes: A meta-analysis and literature review. J Diabetes Investig. 2019; 10: 780-792.
- El Assar M, Laosa O, Mañas LR. Diabetes and frailty. Curr Opin Clin Nutr Metab Care. 2019; 22: 52-57.
- Wong TY, Massa MS, O’Halloran AM, Kenny RA, Clarke R. Cardiovascular risk factors and frailty in a cross-sectional study of older people: implications for prevention. Age Ageing. 2018; 47: 714-720.
- Bastos-Barbosa RG, Ferriolli E, Coelho EB, Moriguti JC, Nobre F, et al. Association of frailty syndrome in the elderly with higher blood pressure and other cardiovascular risk factors. Am J Hypertens. 2012; 25: 1156-1161.
- Hung C-Y, Wu T-J, Wang K-Y, Huang J-L, Loh E-W, Chen Y-M, et al. Falls and atrial fibrillation in elderly patients. Acta Cardiol Sin. 2013; 29: 436-443.
- Magnani JW, Wang N, Benjamin EJ, Garcia ME, Bauer DC, Butler J, et al. Atrial fibrillation and declining physical performance in older adults: the health, aging, and body Composition study. Circ Arrhythm Electrophysiol. 2016; 9: e003525.
- Mishra SR, Chung HF, Waller M, Dobson AJ, Greenwood DC, Cade JE, et al. Association between reproductive life span and incident nonfatal cardiovascular disease: a pooled analysis of individual patient data from 12 studies. JAMA Cardiol. 2020; 5: 1410-1418.
- Mishra SR, Chung HF, Waller M, Mishra GD. Duration of estrogen exposure during reproductive years, age at menarche and age at Menopause, and risk of cardiovascular disease events, All-Cause and cardiovascular mortality: a systematic review and Meta-Analysis. BJOG. 2021; 128: 809-821.
- Okoth K, Chandan JS, Marshall T, Thangaratinam S, Thomas GN, Nirantharakumar K, et al. Association between the reproductive health of young women and cardiovascular disease in later life: umbrella review. BMJ. 2020; 371: m3502.
- Welten SJ, Onland-Moret NC, Boer JM, Verschuren WM, van der Schouw YT. Age at menopause and risk of ischemic and hemorrhagic stroke. Stroke. 2021; 52: 2583-2591.
- Vladutiu CJ, Meyer ML, Malek AM, Stuebe AM, Mosher A, Aggarwal S, et al. Racial Differences in the association between parity and incident stroke: Results from the reasons for geographic and racial differences in stroke study. J Stroke Cerebrovascul Dis. 2017; 26: 749-755.
- Jacobson LT, Hade EM, Collins TC, Margolis KL, Waring ME, Van Horn LV, et al. Breastfeeding history and risk of stroke among parous postmenopausal women in the Women’s Health Initiative. J Am Heart Assoc. 2018; 7: e008739.
- Parikh NI, Gonzalez JM, Anderson CA, Judd SE, Rexrode KM, Hlatky MA, et al. Adverse pregnancy outcomes and cardiovascular disease risk: unique opportunities for cardiovascular disease prevention in women: a scientific statement from the American Heart Association. Circulation. 2021; 143: e902-e916.
- Søndergaard MM, Hlatky MA, Stefanick ML, Vittinghoff E, Nah G, Allison M, et al. Association of adverse pregnancy outcomes with risk of atherosclerotic cardiovascular disease in postmenopausal women. JAMA Cardiol. 2020; 5: 1390-1398.
- Grandi SM, Filion KB, Yoon S, Ayele HT, Doyle CM, Hutcheon JA, et al. Cardiovascular disease-related morbidity and mortality in women with a history of pregnancy complications: systematic review and meta-analysis. Circulation. 2019; 139: 1069-1079.
- Wu P, Gulati M, Kwok CS, Wong CW, Narain A, O’Brien S, Chew- Graham CA, et al. Preterm delivery and future risk of maternal cardiovascular disease: a systematic review and meta-analysis. J Am Heart Assoc. 2018; 7: e007809.
- Adank MC, Hussainali RF, Oosterveer LC, Ikram MA, Steegers EA, Miller EC, et al. Hypertensive disorders of pregnancy and cognitive impairment: a prospective cohort study. Neurology. 2021; 96: e709-e718.
- Rexrode KM, Madsen TE, Yu AY, Carcel C, Lichtman JH, Miller EC. The impact of sex and gender on stroke. Circ Res. 2022; 130: 512-528.
- Lee S, Kim Y, Navi BB, Abdelkhaleq R, Salazar-Marioni S, Blackburn SL, et al. Risk of intracranial hemorrhage associated with pregnancy in women with cerebral arteriovenous malformations. J NeuroInterv Surg. 2021; 13: 707-710.
- Swartz RH, Cayley ML, Foley N, Ladhani NNN, Leffert L, Bushnell C, et al. The incidence of pregnancy-related stroke: a systematic review and meta-analysis. Int J Stroke. 2017; 12: 687-697.
- Kaddour H, Tranquille M, Okeoma CM. The past, the present, and the future of the size exclusion chromatography in extracellular vesicles separation. Viruses. 2021; 13: 2272.
- Yang Y, Shi Y-Z, Zhang N, Wang S, Ungvari GS, Ng CH, et al. The disability rate of 5-year post-stroke and its correlation factors: a national survey in China. PLoS One. 2016; 11: e0165341.
- Ikeda T, Morotomi N, Kamono A, Ishimoto S, Miyazawa R, Kometani S, et al. The effects of timing of a leucine-enriched amino acid supplement on body composition and physical function in stroke patients: a randomized controlled trial. Nutrients. 2020; 12: 1928.
- Bouziana SD, Tziomalos K. Malnutrition in patients with acute stroke. J Nutr Metab. 2011; 2011: 167898.
- Kanai M, Noguchi M, Kubo H, Nozoe M, Kitano T, Izawa KP, et al. Pre-stroke frailty and stroke severity in elderly patients with acute stroke. J Stroke Cerebrovasc Dis. 2020; 29: 105346.
- Noguchi M, Kubo H, Kanai M, Nozoe M, Shimada S. Relationship between pre-stroke frailty status and short-term functional outcome in older patients with acute stroke–a mediation analysis. Arch Gerontol Geriatr. 2021; 94: 104370.
- Singh R-J, Chen S, Ganesh A, Hill MD. Long-term neurological, vascular, and mortality outcomes after stroke. Int J Stroke. 2018; 13: 787-796.
- Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. 2019; 394: 1365-1375.
- Dent E, Martin FC, Bergman H, Woo J, Romero-Ortuno R, Walston JD. Management of frailty: opportunities, challenges, and future directions. Lancet. 2019; 394: 1376-1386.
- Finlayson O, Kapral M, Hall R, Asllani E, Selchen D, Saposnik G. Risk factors, inpatient care, and outcomes of pneumonia after ischemic stroke. Neurology. 2011; 77: 1338-1345.
- Zhang S, He W-B, Chen N-H. Causes of death among persons who survive an acute ischemic stroke. Curr Neurol Neurosci Rep. 2014; 14: 1-11.
- Pinho J, Küppers C, Nikoubashman O, Wiesmann M, Schulz JB, Reich A, et al. Frailty is an outcome predictor in patients with acute ischemic stroke receiving endovascular treatment. Age Ageing. 2021; 50: 1785-1791.
- Romero-Ortuno R, Wallis S, Biram R, Keevil V. Clinical frailty adds to acute illness severity in predicting mortality in hospitalized older adults: an observational study. Eur J Intern Med. 2016; 35: 24-34.
- Serra-Prat M, Sist X, Domenich R, Jurado L, Saiz A, Roces A, et al. Effectiveness of an intervention to prevent frailty in pre-frail community-dwelling older people consulting in primary care: a randomised controlled trial. Age Ageing. 2017; 46: 401-407.
- Carandang R, Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Kannel WB, et al. Trends in incidence, lifetime risk, severity, and 30-day mortality of stroke over the past 50 years. JAMA. 2006; 296: 2939- 2946.
- D’Agostino Sr RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008; 117: 743-753.
- Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991; 22: 312-318.
- Kelly-Hayes M. Influence of age and health behaviors on stroke risk: lessons from longitudinal studies. J Am Geriatr Soc. 2010; 58: S325-S328.
- Cruz-Jentoft AJ, Kiesswetter E, Drey M, Sieber CC. Nutrition, frailty, and sarcopenia. Aging Clin Exp Res. 2017; 29: 43-48.
- Angulo J, El Assar M, Álvarez-Bustos A, Rodríguez-Mañas L. Physical activity and exercise: Strategies to manage frailty. Redox Biol. 2020; 35: 101513.
- Dhamoon MS, Longstreth W, Bartz TM, Kaplan RC, Elkind MS. Disability trajectories before and after stroke and myocardial infarction: the cardiovascular health study. JAMA Neurol. 2017; 74: 1439-1445.
- Wondergem R, Pisters MF, Wouters EJ, Olthof N, de Bie RA, Visser- Meily JM, et al. The course of activities in daily living: who is at risk for decline after first ever stroke? Cerebrovasc Dis. 2017; 43: 1-8.
- Cassidy JM, Cramer SC. Spontaneous and therapeutic-induced mechanisms of functional recovery after stroke. Transl Stroke Res. 2017; 8: 33-46.
- Joshi PK, Pirastu N, Kentistou KA, Fischer K, Hofer E, Schrau KE, et al. Genome-wide meta-analysis associates HLA-DQA1/DRB1 and LPA and lifestyle factors with human longevity. Nat Commun. 2017; 8: 910.
- Pilling LC, Atkins JL, Bowman K, Jones SE, Tyrrell J, Beaumont RN, et al. Human longevity is influenced by many genetic variants: evidence from 75,000 UK Biobank participants. Aging (Albany NY). 2016; 8: 547-560.
- Mora S, Kamstrup PR, Rifai N, Nordestgaard BG, Buring JE, Ridker PM. Lipoprotein (a) and risk of type 2 diabetes. Clin Chem. 2010; 56: 1252-1260.
- Pasmant E, Sabbagh A, Vidaud M, Bièche I. ANRIL, a long, noncoding RNA, is an unexpected major hotspot in GWAS. FASEB J. 2011: 25: 444-448.
- Meimaridou E, Hughes C, Kowalczyk J, Chan L, Clark A, Metherell L. ACTH resistance: genes and mechanisms. Endocr Dev. 2013; 24: 57-66.
- Conejero-Goldberg C, Gomar J, Bobes-Bascaran T, Hyde T, Kleinman J, Herman M, et al. APOE2 enhances neuroprotection against Alzheimer’s disease through multiple molecular mechanisms. Mol Psychiatry. 2014; 19: 1243-1250.
- Raber J, Wong D, Yu G-Q, Buttini M, Mahley RW, Pitas RE, et al. Apolipoprotein E and cognitive performance. Nature. 2000; 404: 352-354.
- Sivakumaran S, Agakov F, Theodoratou E, Prendergast JG, Zgaga L, Manolio T, et al. Abundant pleiotropy in human complex diseases and traits. Am J Hum Genet. 2011; 89: 607-618.
- Livshits G, Malkin I, Bowyer RC, Verdi S, Bell JT, Menni C, et al. Multi- OMICS analyses of frailty and chronic widespread musculoskeletal pain suggest involvement of shared neurological pathways. Pain. 2018; 159: 2565-2572.
- Organization WH. Monitoring Health for the Sustainable Development Goals (SDGs). World Health Organization: 2016.
- Nomenclature SSR. Revised Nomenclature for Antigen-Nonspecific T Cell Proliferation and Helper Factors. J Immunol. 1979; 123: 2928- 2929.
- Mohanty SD, Lekan D, McCoy TP, Jenkins M, Manda P. Machine learning for predicting readmission risk among the frail: Explainable AI for healthcare. Patterns. 2022; 3: 100395.
- Krychtiuk KA, Stojkovic S, Lenz M, Brekalo M, Huber K, Wojta J, et al. Predictive value of low interleukin-33 in critically ill patients. Cytokine. 2018; 103: 109-113.
- Günther F, Straub R, Hartung W, Luchner A, Fleck M, Ehrenstein B. Increased serum levels of soluble ST2 as a predictor of disease progression in systemic sclerosis. Scand J Rheumatol. 2022; 51: 315-322.
- Gattringer T, Pinter D, Enzinger C, Seifert-Held T, Kneihsl M, Fandler S, et al. Serum neurofilament light is sensitive to active cerebral small vessel disease. Neurology. 2017; 89: 2108-2114.
- Tiedt S, Duering M, Barro C, Kaya AG, Boeck J, Bode FJ, et al. Serum neurofilament light: a biomarker of neuroaxonal injury after ischemic stroke. Neurology. 2018; 91: e1338-e1347.
- Duering M, Konieczny MJ, Tiedt S, Baykara E, Tuladhar AM, Van Leijsen E, et al. Serum neurofilament light chain levels are related to small vessel disease burden. J Stroke. 2018; 20: 228-238.
- Bruunsgaard H, Andersen-Ranberg K, Jeune B, Pedersen AN, Skinhøj P, Pedersen BK. A high plasma concentration of TNF-α is associated with dementia in centenarians. J Gerontol A Biol Sci Med Sci. 1999; 54: M357-M364.
- Capuron L, Schroecksnadel S, Féart C, Aubert A, Higueret D, Barberger-Gateau P, et al. Chronic low-grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: role in neuropsychiatric symptoms. Biol Psychiatr. 2011; 70: 175-182.
- Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, et al. The origins of age-related proinflammatory state. Blood. 2005; 105: 2294-2299.
- Won CW, Ha E, Jeong E, Kim M, Park J, Baek JE, et al. World health organization integrated care for older people (ICOPE) and the integrated care of older patients with frailty in primary care (ICOOP_ frail) study in korea. Ann Geriatr Med Res. 2021; 25: 10-16.
- Wood AD, Strachan AA, Thies F, Aucott LS, Reid DM, Hardcastle AC, et al. Patterns of dietary intake and serum carotenoid and tocopherol status are associated with biomarkers of chronic low-grade systemic inflammation and cardiovascular risk. Brt J Nutr. 2014; 112: 1341- 1352.
- Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Ann Rev Med. 2000; 51: 245-270.
- Marcos-Pérez D, Sánchez-Flores M, Proietti S, Bonassi S, Costa S, Teixeira JP, et al. Association of inflammatory mediators with frailty status in older adults: results from a systematic review and meta- analysis. GeroScience. 2020; 42: 1451-1473.
- Collerton J, Martin-Ruiz C, Davies K, Hilkens CM, Isaacs J, Kolenda C, et al. Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: cross-sectional findings from the Newcastle 85+ Study. Mech Ageing Dev. 2012; 133: 456-466.
- Hsu B, Hirani V, Cumming RG, Naganathan V, Blyth FM, Wright FC, et al. Cross-sectional and longitudinal relationships between inflammatory biomarkers and frailty in community-dwelling older men: the concord health and ageing in men project. J Gerontol A Biol Sci Med Sci. 2019; 74: 835-841.
- Picca A, Coelho-Junior HJ, Calvani R, Marzetti E, Vetrano DL. Biomarkers shared by frailty and sarcopenia in older adults: A systematic review and meta-analysis. Ageing Res Rev. 2022; 73: 101530.
- Byrne T, Cooke J, Bambrick P, McNeela E, Harrison M. Circulating inflammatory biomarker responses in intervention trials in frail and sarcopenic older adults: A systematic review and meta-analysis. Exp Gerontol. 2023; 177: 112199.
- Eklund CM. Proinflammatory cytokines in CRP baseline regulation. Adv Clin Chem. 2009; 48: 111-136.
- Nurmohamed NS, Belo Pereira JP, Hoogeveen RM, Kroon J, Kraaijenhof JM, Waissi F, et al. Targeted proteomics improves cardiovascular risk prediction in secondary prevention. Eur Heart J. 2022; 43: 1569-1577.
- Yoldemir SA, Arman Y, Akarsu M, Altun O, Ozcan M, Tukek T. Correlation of glycemic regulation and endotrophin in patients with type 2 Diabetes; pilot study. Diabetol Metab Syndr. 2021; 13: 1-9.
- Ahmadi-Abhari S, Luben RN, Wareham NJ, Khaw KT. Distribution and determinants of C-reactive protein in the older adult population: European Prospective Investigation into Cancer-Norfolk study. Eur J Clin Invest. 2013; 43: 899-911.
- Soysal P, Stubbs B, Lucato P, Luchini C, Solmi M, Peluso R, et al. Inflammation and frailty in the elderly: a systematic review and meta-analysis. Ageing Res Rev. 2016; 31: 1-8.
- Liu P, Li Y, Li S, Zhang Y, Song Y, Ji T, Li Y, et al. Serum progranulin as a potential biomarker for frailty in Chinese older adults. Aging Clin Exp Res. 2023; 35: 399-406.
- Reiner AP, Aragaki AK, Gray SL, Wactawski-Wende J, Cauley JA, Cochrane BB, et al. Inflammation and thrombosis biomarkers and incident frailty in postmenopausal women. Am J Med. 2009; 122: 947-954.
- Gale CR, Baylis D, Cooper C, Sayer AA. Inflammatory markers and incident frailty in men and women: the English Longitudinal Study of Ageing. Age. 2013; 35: 2493-2501.
- Xu W, Liang Y, Lin Z. Association between Neutrophil–Lymphocyte Ratio and Frailty: The Chinese Longitudinal Healthy Longevity Survey. Front Med. 2022; 8: 783077.
- Marcos-Pérez D, Sánchez-Flores M, Maseda A, Lorenzo-López L, Millán-Calenti JC, Gostner JM, et al. Frailty in older adults is associated with plasma concentrations of inflammatory mediators but not with lymphocyte subpopulations. Front Immunol. 2018; 9: 1056.
- Greiwe JS, Cheng B, Rubin DC, Yarasheski KE, Semenkovich CF. Resistance exercise decreases skeletal muscle tumor necrosis factor α in frail elderly humans. FASEB J. 2001; 15: 475-482.
- Soysal P, Isik AT, Carvalho AF, Fernandes BS, Solmi M, Schofield P, et al. Oxidative stress and frailty: A systematic review and synthesis of the best evidence. Maturitas. 2017; 99: 66-72.
- Zhang L, Zeng X, He F, Huang X. Inflammatory biomarkers of frailty: A review. Exp Gerontol. 2023; 179: 112253.
- Núñez J, Sastre C, D’Ascoli G, Ruiz V, Bonanad C, Miñana G, et al. Relation of low lymphocyte count to frailty and its usefulness as a prognostic biomarker in patients> 65 years of age with acute coronary syndrome. Am J Cardiol. 2020; 125: 1033-1038.
- Navarro-Martínez R, Serrano-Carrascosa M, Buigues C, Fernández- Garrido J, Sánchez-Martínez V, Castelló-Domenech AB, et al. In Frailty syndrome is associated with changes in peripheral inflammatory markers in prostate cancer patients undergoing androgen deprivation therapy. Urol Oncol. 2019; 2019: 976-987.
- Dorsch S, Ada L, Canning CG. Lower limb strength is significantly impaired in all muscle groups in ambulatory people with chronic stroke: a cross-sectional study. Arch Phys Med Rehabil. 2016; 97: 522-527.
- Myint P, Poole K, Warburton E. Hip fractures after stroke and their prevention. QJM. 2007; 100: 539-545.
- Bensimon K, Herrmann N, Swardfager W, Yi H, Black SE, Gao F-Q, et al. Kynurenine and depressive symptoms in a poststroke population. Neuropsychiatr Dis Treat. 2014; 1827-1835.
- Wijeratne T, Gillard Crewther S, Sales C, Karimi L. COVID-19 pathophysiology predicts that ischemic stroke occurrence is an expectation, not an exception - a systematic review. Front Neurol. 2021; 11: 607221.
- Pacifici R, Rifas L, McCracken R, Avioli LV. The role of interleukin-1 in postmenopausal bone loss. Exp Gerontol. 1990; 25: 309-316.
- Moraga P, Collaborators G.C. o. D. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017; 390: 1151-1210.
- Katan M, Luft A. Global burden of stroke. Semin Neurol. 2018; 38: 208-211.
- Scherbakov N, Doehner W. Sarcopenia in stroke - facts and numbers on muscle loss accounting for disability after stroke. J Cachexia Sarcopenia Muscle. 2011; 2: 5-8.
- Gori AM, Giusti B, Piccardi B, Nencini P, Palumbo V, Nesi M, et al. Inflammatory and metalloproteinases profiles predict three-month poor outcomes in ischemic stroke treated with thrombolysis. J Cereb Blood Flow Metab. 2017; 37: 3253-3261.
- Benjamin LA, Allain TJ, Mzinganjira H, Connor MD, Smith C, Lucas S, et al. The role of human immunodeficiency virus–associated vasculopathy in the etiology of stroke. J Infect Dis. 2017; 216: 545-553.
- Polidoro A, Stefanelli F, Ciacciarelli M, Pacelli A, Di Sanzo D, Alessandri C. Frailty in patients affected by atrial fibrillation. Arch Geronto Geriatr. 2013; 57: 325-327.
- Induruwa I, Evans NR, Aziz A, Reddy S, Khadjooi K, Romero-Ortuno R. Clinical frailty is independently associated with non-prescription of anticoagulants in older patients with atrial fibrillation. Geriatr Gerontol Int. 2017; 17: 2178-2183.
- Colantonio A, Kasl SV, ostfeld am, Berkman LF. Prestroke physical function predicts stroke outcomes in the elderly. Arch Phys Med Rehabil. 1996; 77: 562-566.
- Longstreth Jr W, Bernick C, Fitzpatrick A, Cushman M, Knepper L, Lima J, et al. Frequency and predictors of stroke death in 5,888 participants in the Cardiovascular Health Study. Neurology. 2001; 56: 368-375.].
![The impact of stroke on frailty and the various factors influencing the onset of frailty are interconnected. The figure illustrates that the risk factors of stroke may be associated with frailty in every stroke patient. These factors, identified as contributors to frailty according to [19, 20].](https://www.jscimedcentral.com/public/assets/images/uploads/image-1724915107-1.PNG)
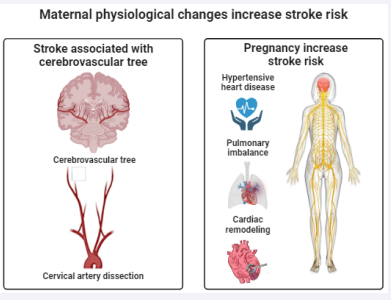
![Displays the common inflammatory biomarkers associated with frailty and stroke. Previous studies have concluded that these biomarkers are directly linked to stroke during frailty, as evidenced by [34, 45].](https://www.jscimedcentral.com/public/assets/images/uploads/image-1724915688-1.PNG)








































































































































