Eased BDNF Decline, Attenuated Cofilin1 Activation, and Decreased Dendrites Dendritic Spine Loss Are Involved in Antidepressant Effects of Resveratrol in Menopausal Mice
- 1. College of Life Science, Anhui Normal University, China
- 2. Anhui College of Traditional Chinese Medicine, China
- 3. Department of Anatomy, China
ABSTRACT
The decrease of estrogen is a common cause of menopausal depression, but there is still a lack of effective drugs with no side effects. Previous studies have shown that resveratrol, as a natural extract of polyphenols, has antidepressant effects in a variety of depression models, but the effect and mechanism of resveratrol on menopausal depression are unclear. In this study, B6.Cg-TgN (Thy-YFP-H)-2Jrs transgenic mice were ovariectomized combined with chronic restraint stress (OVX-CRS) to establish a model of menopausal depression. The antidepressant effect of resveratrol was evaluated by tail suspension test (TST), forced swimming test (FST), sucrose preference test (SPT) and novel inhibition feeding test (NSFT). Using the characteristic expression of yellow fluorescent protein (YFP) in excitatory neurons of transgenic mice, the effects of resveratrol on the density of dendrites and dendritic spines were evaluated by three-dimensional imaging technique. BDNF, cofilin1 and p-cofilin1 were quantitatively analyzed by qPCR or/and immunofluorescence quantification to explore the effects of resveratrol on synaptic plasticity in hippocampus and medial prefrontal cortex (mPFC) and its mechanism. The results revealed that CRS significantly increased the immobility time in TST, prolonged the feeding latency and reduced the food intake in NSFT, and decreased the sucrose consumption in SPT. Simultaneously, treatment with resveratrol significantly improved depression-like behaviors. In addition, resveratrol significantly increased the density of dendrites and dendritic spines in hippocampus and mPFC, augmented the density of filopodia-type spines and thin-type spines in hippocampal CA1, and upregulated the density of thin-type spines in mPFC. Consistent with these changes, resveratrol treatment significantly increased the density of p-cofilin1 immunoreactive dendritic spines and the mRNA level of BDNF in these brain regions. The results suggest that although there are some sex differences in the efficacy and antidepressant mechanism of resveratrol based on neuronal plasticity, resveratrol, the results of which are similar to those in the male model of depression, can improve the synaptic plasticity in the corresponding brain regions by upregulating BDNF levels, enhancing the phosphorylation of cofilin 1, increasing the density of dendrites and dendritic spines in the hippocampus and mPFC, and ultimately alleviating menopausal depression-like behaviors.
KEYWORDS
- Resveratrol
- Menopausal depression
- Dendrites and dendritic spines
- Cofilin1
- BDNF
CITATION
Xu H, Zhang ZQ, Chen G, Ge MJ, Yu ZH, et al. (2024) Eased BDNF Decline, Attenuated Cofilin1 Activation, and Decreased Dendrites/ Dendritic Spine Loss Are Involved in Antidepressant Effects of Resveratrol in Menopausal Mice. J Neurol Disord Stroke 11(3): 1223.
INTRODUCTION
Major depressive disorder (MDD) is one of the leading causes of disability and death worldwide, with its incidence varying widely across genders, with women often twice as high as men [1]. In addition, women are four times more likely to have MDD recurrence than men [2], and women exhibit higher symptom severity, greater dysfunction, less typical depressive symptoms, and higher rates of anxiety comorbidities [3,4]. Studies have shown that the high incidence of depression in women is closely related to the changes of hormone levels in the body during periods such as pregnancy and menopause [5]. However, there is a lack of effective drugs for female depression. Although hormone replacement therapy (HRT) can reduce the severity of depression in menopausal women, it can also increase the incidence of cardiovascular disease and cancer [6]. Therefore, it is very important to develop and study the antidepressant effects and mechanisms of natural medicinal plant compounds with estrogen-like effects without side effects [7].
Resveratrol (3, 4’, 5-trihydroxystilbene) is a polyphenolic compound extracted from knotweed and is also widely found in the peels of plants such as grapes. There is growing evidence that resveratrol can relieve mood disorders [8]. In animal model studies, resveratrol has been found to have estrogen- like antidepressant effects and neuroprotective effects [9,10]. Further studies have shown that resveratrol can activate estrogen receptors in the female brain and regulate synaptic plasticity by increasing the density of dendrites and dendritic spines of CA1 pyramidal cells, especially in the hippocampus and medial prefrontal cortex (mPFC) [11]. Resveratrol can also improve the memory and emotional function of aged rats by inhibiting chronic inflammation and increasing neurogenesis [12,13].
Previous our researches have shown that in a mouse model of depression, there are many similarities and significant differences between the sexes in the effects of chronic constrain stress on neuronal plasticity [14,15]. We have investigated the antidepressant efficacy of resveratrol and its antidepressant mechanisms based on neuronal plasticity in a male mouse model of depression [14]. In the present study, we constructed a mouse model of menopausal depression by ovariectomy plus chronic restraint stress [15], and investigated the effect of resveratrol on the behavior, mood-related brain region of the mPFC and hippocampus and its regulatory signaling pathway BDNF-cofilin1, in order to explore the antidepressant effect and mechanism of resveratrol on menopause.
MATERIALS AND METHODS
Drugs and Chemicals
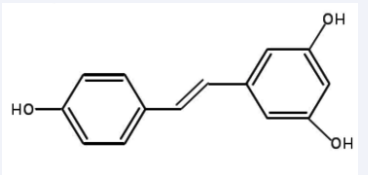
Resveratrol (3,4′,5-Trihydroxystilbene,> 98%) was purchased from Baoji Fang Sheng biological development co., LTD (Baoji, China). The chemical structures are shown in Figure1. Cofilin1 (sc-53934) and p-cofilin1 (sc-12912-R) antibodies and DAPI were purchased from Santa Cruz Biotechnology (Shanghai, China). Other chemicals were purchased from Sigma-Aldrich (Shanghai, China).
Figure 1 Molecular structure diagrams of resveratrol.
Animals and Ethical Approval of the Study
Adult female B6.Cg-TgN (Thy-YFP-H)-2Jrs (20 to 25-g) transgenic mice, which were purchased from The Jackson Laboratory (stock number 003782) and bred in the Animal Facilities at the University of Science and Technology of China (USTC), were friendly granted from Professor Jiang-Ning Zhou of USTC. The fluorescent protein (eYFP+) from these transgenic mice was constitutively expressed under the Thy1 gene promoter and was distributed in the neuronal soma, axons, and dendrites in many brain regions, including the hippocampus and mPFC [16,17]. Animals were housed under controlled environmental conditions, a 12 h dark/light cycle at 22 ± 2? and allowed free access to rodent diet and water. Animals were performed in accordance with the Guidelines of the Regulations of Experimental Animal Administration published by the State Committee of Science and Technology of the People’s Republic of China on November 14, 1988. The protocols used in this study were conducted with the approval of the Animal Use and Care Committee of Anhui Normal University (AHNU-ET2021025).
Surgery and Chronic Restraint Stress
Before starting the study, animals about 8 weeks old were adaptively housed in pairs in a normal clear plastic cage in a temperature and humidity suitability environment for 14 days. The female mices were anesthetized with 1% carbrital (intraperitoneally), and bilateral ovariectomy (OVX) was performed via small dorsal flank incisions and subsequent removal of ovaries under aseptic condition [18,19]. After surgery, the wound was topically treated with antibiotic spray to prevent infection, and mice were kept warm until they recovered from anesthesia.
Chronic restraint stress performed 14 days after ovariectomy. The protocol was performed as previously described [20,21]. In short, animals were individually placed head-first into a well- ventilated 50 ml polypropylene conical tube, which was plugged with a 3-cm-long middle tube, and finally tied with a cap of the 50 ml tube. The animals were restrained for 2 h every day for 14 days. After each session of restraint stress, the animals were returned to their home environment, where they were housed in pairs in standard plastic cages and accessed to food and water available ad libitum.
Grouping and Antidepressant Treatments
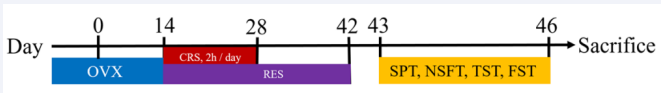
A total of 30 female mice (15 in each group) were randomly separated into two groups: chronic restraint stress group (CRS), resveratrol treatment group [RES, 100 mg/kg/d, dissolved in 5% (g/ml) carboxymethylcellulose sodium and administered by intragastric gavage (i.g.)]. Volume-matched vehicle was served as CRS. One hour before the restraint stress, the intragastric gavage was performed. Daily experiments took place between 9 am and 4 pm. An outline of the study procedures and time intervals is given in Figure 2.
Figure 2 Schematic diagram of depression models, treatment, and behavioral test. OVX, ovariectomy; CRS, chronic restraint stress; RES, resveratrol; SPT, sucrose preference test; NSFT, novelty-suppressed feeding test; TST, tail suspension test; FST, forced swim test. The mice were sacrificed on the 46th day.
Behavioral Tests
All behavioral tests were performed under the lighting conditions of 70 lx (Shadowless lights) by experimenters blinded to the treatment information.
Sucrose Preference Test (SPT): The SPT was performed using the method as described previously [22,23]. In brief, before the experiment testing, the mice were deprived of food and water for 24 hours and then given to two bottles of a 2% sucrose solution to acclimate. The second day, one of the 2% sucrose bottles was replaced with tap water. When the experiment testing, mice housed in individual behavioral cages were given two bottles with 60 ml tap water and 60 ml of a 2% sucrose solution over a 24 h period. The position of the water and sucrose solution bottles was switched every 12 h to avoid location preference after testing. The percentage [sucrose intake/(sucrose intake plus water intake)] was represented as the sucrose preference.
Novelty-Suppressed Feeding Test (NSFT): NSFT was performed following the procedure described in previous literature [21]. Shortly, after 24 h food deprivation (water was provided ad libitum), animals were assayed using the novelty- suppressed feeding (NSF) test. A single 2 × 2 cm oval food pellet was placed on a circular piece of white filter paper (150 mm diameter) positioned in the center of an open field (40 × 40 × 40 cm). Each mouse was placed in a corner of the open field. The latency (for no more than 15 minutes) to first bite the chow pellet and consumption biting the pellet was recorded. Immediately after the mouse began to bite the pellet, the animal was placed in its home cage along with a weighed food pellet for 30 min. At the end of this period, the amount of food consumed was determined by weighing the food pellet, serving as a control for change in appetite as a possible confounding factor. Anxiety was assayed by the time of latency to the first bite of the food pellet [21]. At the end of each experiment, the open field was thoroughly cleaned with 70% ethanol.
Forced Swimming Test (FST): The FST was performed as previously reported by Seo et al. [20]. This behavioral test was widely used for evaluating the antidepressant potential of current and novel molecules in experimental pharmacological studies [23,24]. Briefly, the mice were individually placed in a cylindrical container (30 cm height × 16 cm diameter), containing of water at 25 ± 1? and a depth of 14 cm. The FST lasted for 6 min, and the last five min were recorded as the session. Then, animals were dried and returned to their home cages. After each testing, the cylindrical container is cleaned and filled with fresh water for the next testing.
Tail suspension test (TST): Mice were subjected to the TST using the method described by Seo et al. [20]. In the experiment, mice were suspended individually by their tails from a metal rod fixed 50 cm above the surface of a table in a test room. The tip of the tail was fixed using adhesive tape. The mouse was suspended by the tail for six min, and the immobility time was counted during the last five min. Chronic restraint stress, treatments and the behavioral testing schedule are shown in Figure 2.
Tissue preparation
Following the behavioral tests, the mice were deeply anesthetized with 1% carbrital and perfused transcardially with 0.9% saline followed by euthanasia via decapitation. The brains of five mice from each group were isolated and sectioned coronally at a thickness of 20 μm using a Leica cryostat (Leica Biosystems Inc., Buffalo Grove, IL, USA). The brain sections were used for the dendritic morphological and immunohistochemical analyses. The hippocampus and mPFC tissues isolated from other mice (10 mice each group) were frozen in liquid nitrogen and stored at−80 ?C until used for qPCR.
Image Analysis and Quantification of Dendrites
The images were acquired by laser scanning confocal microscope (OLYMPUS FV1000) and analyzed by ImageJ software. Using serial sections between Bregma−2.54 mm and −3.88 mm for the ventral hippocampus (vHPC) and between Bregma +2.22 mm and +1.98 mm for the mPFC, one of every four sections was selected for analysis. No less than three fields of view were randomly selected for the statistical area in each section. The percentage of the field of view covered by dendrites was measured to estimate the dendritic density [25].
Classification and Counting of Dendritic Spines
The analysis of dendritic spine density and classification was primarily based on the methods described in previous literature [26,27]. Briefly, using serial sections between Bregma−1.22 mm and−2.54 mm for the hippocampus or between Bregma +2.22 mm and +1.98 mm for the mPFC, one of every six sections was selected for analysis. No less than three fields of view (6 × 60 objective) for each region tested were randomly selected to count the p-cofilin1 immunopositive dendritic spine density that was determined as the percentage of the field of view covered by p-cofilin1 immunopositive dendritic spines. The three areas tested included the apical proximal dendrites (less than 50 μm from the center of the neuronal body), apical distal dendrites (greater than 150 μm from the center of the neuronal body), and basal dendrites in the hippocampal CA1 and mPFC. Secondary or tertiary dendritic segments of pyramidal neurons were selected to evaluate the density of four types of dendritic spines. A minimum of three neurons were used for analysis in each section, and the total length of the dendritic segments was greater than 300 μm. Z-stacks of dendrites (up to 80 μm total on the Z-axis, with an optical section thickness of 0.5 mm, i.e., 160 images per stack) were obtained at a 6 × 60 magnification using an OLYMPUS FV1000 confocal microscope. An IMARIS 7.2.3 system (Andor Technology; Belfast, Northern Ireland) was used to reconstruct the z-stacks into 3D models for analysis. Using customized settings based on spine parameters as previously described [26,28,29], the IMARIS Filament Tracer module was used to detect, quantify, and characterize the spine structures [Table 1].
Table 1: Classification of spine morphology using IMARIS software
|
Type |
Parameters |
|
Filopodia |
Filopodia Mean width (head) <= mean width (neck) |
|
Long-Thin spines |
Mean width (neck)*2 < length (spine) AND Max width (head) >= mean width (neck) |
|
Stubby spines |
Length (spine) < 1 |
|
Mushroom spines |
Mean width (head) > mean width (neck)AND Length spine < 3μm |
Quantitative Real-time Polymerase Chain Reaction (qPCR)
Total RNA was extracted from hippocampal and mPFC tissues using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The isolated equal amounts of RNA was reverse transcribed into cDNAs using the FastQuant RT Kit according to instructions (Tiangen, Beijing, China). Amplification and real-time detection were performed by an IQ5 instrument (Bio-Rad, Hercules, CA, USA) with the SuperReal PreMix Plus reagents (Tiangen). Briefly, The improved four-step thermal cycler protocol was used, which consisted of 95? 5 min; 95? 30s, 60? 30 s, 72? 20 s, for 40 cycles; Melt Curve 65? 5 s, 95? 5 s, 72? 3 min, followed by 4?. Relative quantitative analysis of the final values was normalized to GAPDH levels by using the 2-ΔΔCt method. The primers sequences for cofilin1 and GAPDH (an internal reference) were listed in Table -2. Hippocampal and mPFC tissues of five mice each group were used for qPCR quantification.
Table 2: The primer sequences used for quantitative real-time polymerase chain reaction
|
Target gene |
Primer sequence (5?-3?) |
PCR products/bp |
|
Cofilin1 |
F:GCAACCTATGAGACCAAGGAGAG |
143 |
|
R: CTTCTTGATGGCATCCTTGGAGC |
||
|
BDNF |
F:TGGAACTCGCAATGCCGAACTAC |
88 |
|
R:TCCTTATGAATCGCCAGCCAATTCTC |
||
|
GAPDH |
F: AACTTTGGCATTGTGGAAGG |
94 |
|
R: CACATTGGGGGTAGGAACA |
Statistical Analysis
All data are presented as means ± standard error of the mean (SEM). All analyses were performed using the Statistical Package for the Social Science (SPSS) software version 22.0. Using the Levene test for homogeneity of variance, data with a P value greater than 0.05 were analyzed parametrically, and data with a P value less than 0.05 were analyzed non-parametrically. Parametric data were analyzed using independent-samples T test. Non-parametric data were analyzed using the Mann- Whitney U test. In all tests, the criterion for statistical significance was P <0.05. GraphPad Prism 8 was used for the production of all statistical graphs.
RESULTS
Effects of Resveratrol on the Depression-Like Behaviors
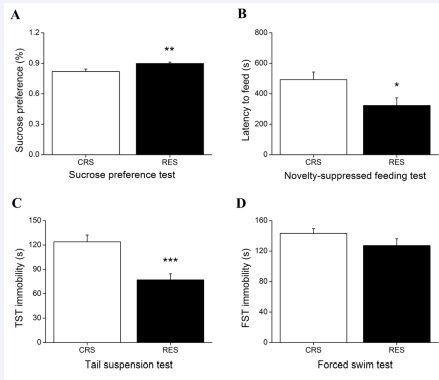
The Mann-Whitney U test showed the sucrose consumption in RES mice was more than that in CRS mice in SPT (P < 0.01) [Figure 3A]. Independent-samples T test revealed a significant difference between groups in NSFT (P < 0.05) [Figure 3B] and TST (P < 0.001) [Figure 3C]. Compared with CRS, resveratrol treatment did not result in a significant decrease in the immobility time in FST (P > 0.05) [Figure 3D].
Figure 3 Effects of resveratrol on OVX-CRS-induced depression-like behaviors. Restraint stress of 2 h daily for consecutive 2 weeks produced an decline in the sucrose consumption in SPT (A), an extended latency to feed in NSFT (B), an increased immobility time in TST (C), and a prolonged immobility time in FST (D), which of these can be alleviated by treatment with resveratrol. CRS, chronic restraint stress; TST, tail suspension test; FST, forced swim test. Data expressed as the means ± SEM (n = 15). *P < 0.05, **P < 0.01 , ***P < 0.001 versus CRS.
Effects of Resveratrol the Density of Dendrites in mPFC and Hippocampus
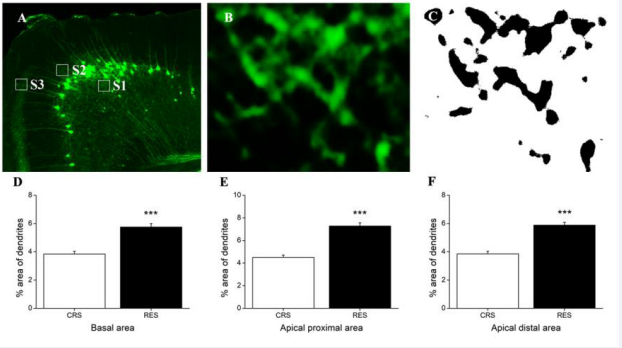
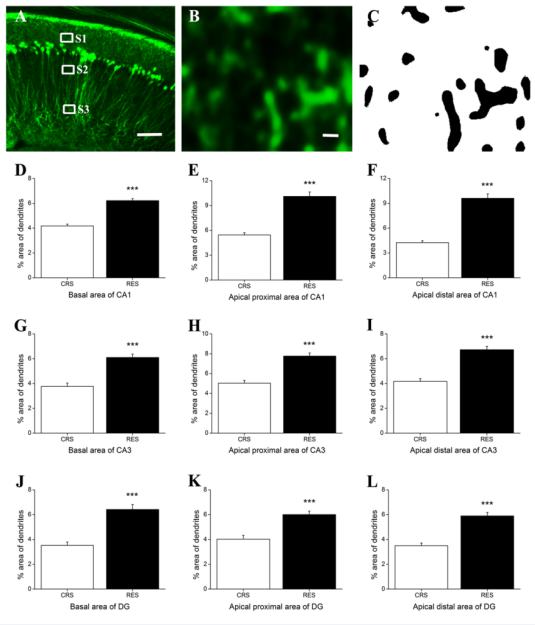
In mPFC, Independent-samples T test revealed that resveratrol treatment generated a significant upregulation in the dendrite density in basal area (P < 0.001) [Figure 4D], apical proximal area (P < 0.001) [Figure 4E], and apical distal area (P < 0.001) [Figure 4F].
Figure 4 Effects of resveratrol on the density of dendrites in mPFC. (A) showed a schematic diagram of the statistics areas in the mPFC. Magnifications (B) of the statistics area S1 in (A) was obtained in a single channel of 488 nm (OLYMPUS FV1000). Image (C) was processed using ImageJ software. The background was subtracted with a rolling value of 50, converted to 8-bit deep images and binarized using a determined threshold value (reduce noise 1, particles 1—~). The percentage of dendrite-positive area in the basal area, the apical proximal area, and the apical distal area in the pyramidal neurons of mPFC was showed in (D), (E), and (F), respectively. Data was expressed as the means ± SEM (n = 5). The boxes shown by S1, S2 and S3 represent the basal area, the apical proximal area, and the apical distal area in the mPFC, respectively. ***P < 0.001 versus CRS.
In hippocampus, Independent-samples T test showed that there was a significant difference beween CRS and RES in CA1 (basal area: P < 0.001; apical proximal area: P < 0.001; apical distal area: P < 0.001) [Figures 5D-F], CA3 (basal area: P < 0.001; apical proximal area: P < 0.001; apical distal area: P < 0.001) [Figures 5G-I], and DG (basal area: P < 0.001; apical proximal area: P < 0.001; apical distal area: P < 0.001) [Figures 5J-L].
Figure 5 Effects of resveratrol on the density of dendrites in the hippocampus. (A) showed a schematic diagram of the statistics area in the hippocampus CA1 . Magnification (B) of the statistics area S1 in (A) was obtained in a single channel of 488 nm (OLYMPUS FV1000). Image (C) was processed using ImageJ software. The percentage of dendrite-positive area in basal area, apical proximal and distal area in the hippocampal CA1, CA3, and DG was expressed in (D, E, F), (G, H, I), and (J, K, L), respectively. Data was expressed as the means ± SEM (n = 5). The boxes shown by S1, S2 and S3 represent the basal area, the apical proximal area, and the apical distal area in the hippocampal CA1, respectively. Scale bar in (A) represents 100 μm and in (C) represents 20 μm. ***P < 0.001 versus CRS.
Effects of Resveratrol on the Density of Dendritic Spines in mPFC and Hippocampus
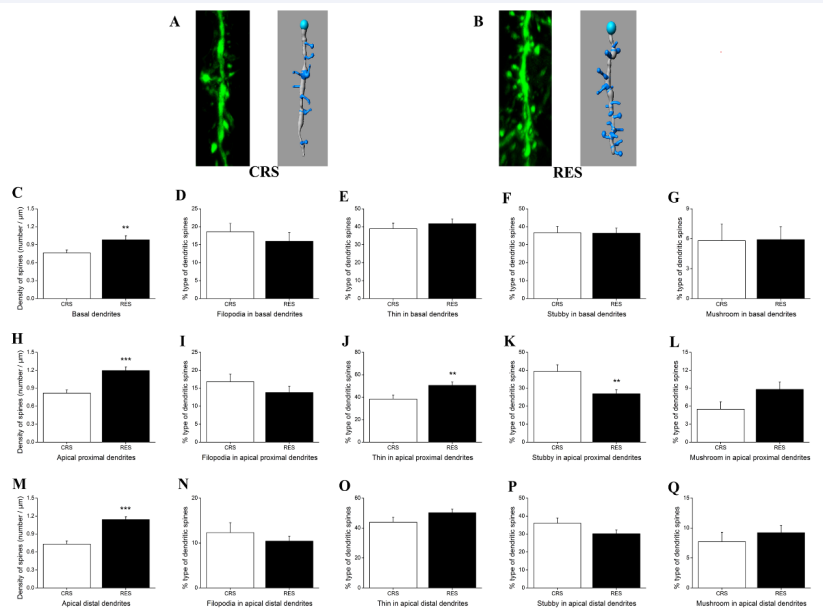
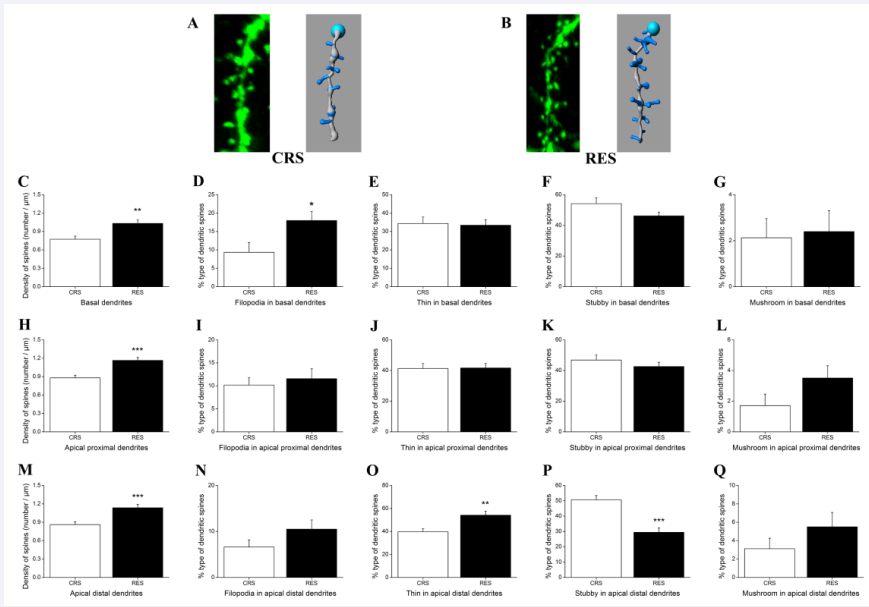
As shown in Figure 6A and B, the slice views and their traces at the apical distal dendritic segments showed the density and morphology of dendritic spines in mPFC from CRS and RES, respectively. Independent-samples T test showed that there were significant differences in the density of dendritic spines between groups in basal dendrites (P < 0.01) [Figure 6C], apical proximal dendrites (P < 0.001) [Figure 6H] and apical distal dendrites (P < 0.001) [Figure 6M]. In four types of dendritic spines, treatment with resveratrol significantly caused an upregulation of the density of dendritic spines observed only in the thin type (P < 0.01) [Figure 6J] and stubby-type (P < 0.01) [Figure 6K] dendritic spines in the apical proximal dendrites. No difference of dendritic spine types was found in the basal [Figures 6D-G] and apical distal dendrites [Figures 6N-Q] as well as in the filopodia-type [Figure 6I] and mushroom-type [Figure 6L] dendritic spines in the apical proximal dendrites in mPFC.
Figure 6 Effects of resveratrol on the density and type of dendritic spines in the pyramidal cells of mPFC. (A) and (B) respectively presented the slice views acquired by Laser scanning confocal microscope (FV1000, 60 × 6 for objective magnification) and their traces at the apical distal dendritic segments labeled by transgenic YFP protein in CRS and RES. (C), (H) and (M) showed the effects of resveratrol on the density of dendritic spines in pyramidal cells of mPFC. Effects of resveratrol on the type of dendritic spines, filopodia, long thin, stubby, and mushroom, in the basal dendrites, apical proximal dendrites, and apical distal dendrites were shown in (D-G), (I-L), and (N-Q), respectively. Data was expressed as the means ± SEM (n = 5). **P < 0.01, ***P < 0.001 versus CRS.
Figures 7A and B showed the slice views and their traces at the apical distal dendritic segments in the hippocampal CA1 from CRS and RES, respectively. Independent-samples T test indicated that resveratrol treatment formed a significant increased density of dendrites in all hippocampal areas tested (basal dendrites, P < 0.01, [Figure 7C]; apical proximal dendrites, P < 0.001, [Figure 7H]; apical distal dendrites, P < 0.001, [Figure 7M]. There was a significant difference between groups detected in the filopodia type in the basal dendrites (P < 0.05) [Figure 7D] as well as in the thin type (P < 0.01) [Figure 7O] and stubby type (P < 0.001) [Figure 7P] in the apical distal dendrites. In other hippocampal areas tested, there was no significant difference between CRS and RES in the dendritic spine density of different types [Figures 7E- G, 7I-L, 7N and Q].
Figure 7 Effects of resveratrol on the density and type of dendritic spines in the pyramidal cells of hippocampal CA1. (A) and (B) respectively presented the slice views and their traces at the apical distal dendritic segments in CRS and RES. (C), (H) and (M) showed the effects of resveratrol on the density of dendritic spines in the pyramidal cells of hippocampal CA1. Effects of resveratrol on the type of dendritic spines, filopodia, long thin, stubby, and mushroom, in the basal dendrites, apical proximal dendrites, and apical distal dendrites were shown in (D-G), (I-L), and (N-Q), respectively. Data was expressed as the means ± SEM (n = 5). *P < 0.05, **P < 0.01, ***P < 0.001 versus CRS.
Effects of Resveratrol on Cofilin1 in mPFC and
Hippocampus
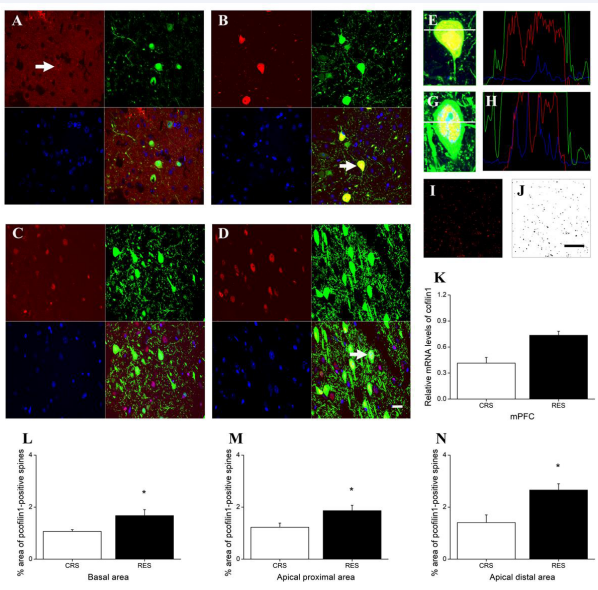
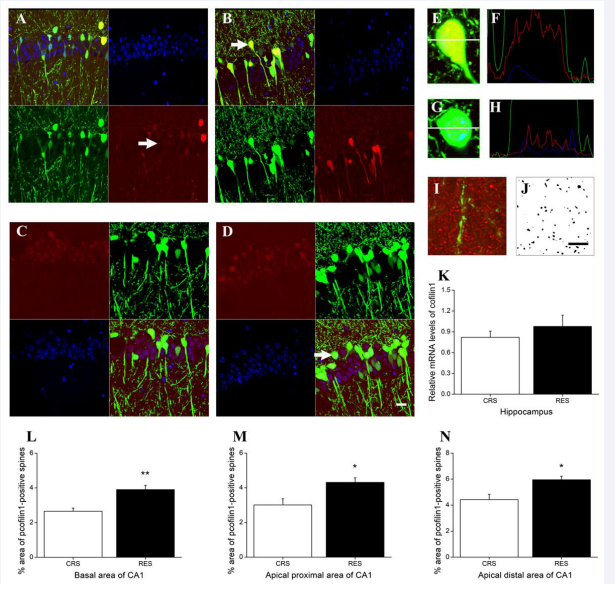
In mPFC, cofilin1 immunoreactivity was mainly distributed in the inner peri-membrane in CRS [Figure 8A], but was diffusively distributed mainly in the cytoplasm and nucleus, also rarely in some process, in RES [Figures 8B,E and F]. Independent-samples T test showed that no difference between CRS and RES was detected in the levels of cofilin1 using qPCR quantification (P > 0.05) [Figure 8K]. Immunoreactivity of p-cofilin1 was mainly distributed in the nucleus in both RES and CRS [Figures 8C,D,G and H]. P-cofilin1 in RES showed stronger immunofluorescence intensity and more immunopositive cells than that in CRS [Figures 8C and D]. Independent-samples T test revealed that the p-cofilin1 immunofluorescence positive dendrite/dendritic spine density in RES was higher than in CRS in all hippocampal areas tested, including the basal dendrites (P < 0.05) [Figure 8L]. apical proximal dendrites (P < 0.05) [Figure 8M], and apical distal dendrites (P < 0.05) [Figure 8N].
Figure 8 Effects of resveratrol on the expression level and distribution of cofilin1 and p-cofilin1 in mPFC. (A) in CRS group and (B) in RES group showed the red Cy3-labeled cofilin1, green YFP-labeled excitatory neurons, blue DAPI-labeled nuclei, and their merged images, respectively. The distribution of p-cofilin1 in CRS and RES was presented in (C) and (D), respectively. The florescence intensity profile plots (F) are shown by the white line across the neuron in (E) indicated by the arrow in (B). The florescence intensity profile plots (H) are shown by the white line across the neuron in (G) indicated by the arrow in (D). (J) was from (I) processed by ImageJ software. Quantification of cofilin1 by qPCR was presented in (K). The effects of resveratrol on the density of p-cofilin1 immunopositive dendritic spines in the basal area, the apical proximal area and the apical distal area were counted in (L), (M), and (N), respectively. Scale bar in (D) represents 20 μm. Data was expressed as the means ± SEM (n = 3). *P < 0.05 versus CRS.
In hippocampus, immunofluorescence revealed that cofilin1 immunoreactivity was found in the nucleus, cytoplasm, and process [Figures 9A and B]. Among these cofilin1 immunopositive cells, in the CRS, more cells showed cofilin1 immunopositive distribution in the inner peri-membrane [Figure 9A]; in the RES, more cells showed cofilin1 immunopositive diffuse distribution in the cytoplasm [Figures 9B, E, and F]. Immunoreactivity of p-cofilin1 was mainly distributed in the nucleus in both RES and CRS [Figures 9C and D]. P-cofilin1 in RES [Figures 9D, G and
H] showed stronger immunofluorescence intensity and more immunopositive cells than that in CRS [Figure 9C]. Independent- samples T test showed that between CRS and RES, no obvious difference was detected in cofilin1 mRNA levels (P > 0.05) [Figure 9K], but significant differences were found in the density of p-cofilin1 immunopositive dendritic spines observed in basal dendrites (P < 0.01) [Figure 9L], apical proximal dendrites (P < 0.05) [Figure 9M], and apical distal dendrites (P < 0.05) [Figure 9N].
Figure 9 Effects of resveratrol on the expression level and distribution of cofilin1 and p-cofilin1 in hippocampus. (A) and (B) showed cofilin1 distribution from CRS and RES. (C) and (D) displayed the distribution of p-cofilin1 in CRS and RES, respectively. The florescence intensity profile plots (F) are shown by the white line across the neuron in (E) indicated by the arrow in (B). The florescence intensity profile plots (H) are shown by the white line across the neuron in (G) indicated by the arrow in (D). No co-location with p-cofilin1 positive red fluorecence and YFP positive red fluorescence was found in the dendritic arborization and dendritic spines (I). (J) was from (I) by removing the green fluorescence and processing with ImageJ software. Quantification of cofilin1 by qPCR was presented in (K). The effects of resveratrol on the density of p-cofilin1 immunopositive dendritic spines in the basal area, the apical proximal area and the apical distal area were counted in (L), (M), and (N), respectively. Scale bar in (D) represents 20 μm. Data was expressed as the means ± SEM (n = 3). *P < 0.05, **P < 0.01 versus CRS.
Effects of Resveratrol on BDNF mRNA in mPFC and Hippocampus
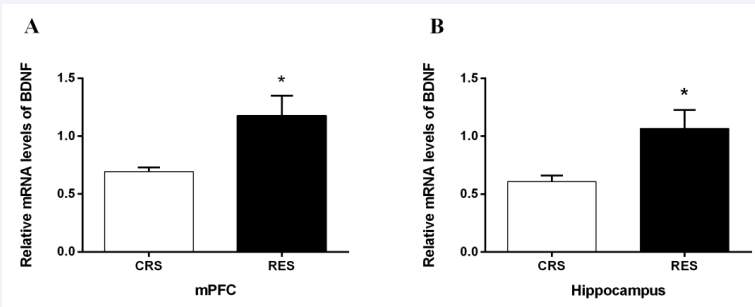
The qPCR quantitative study showed that the BDNF mRNA levels in the mPFC (P < 0.05) [Figure 10A] and hippocampus (P < 0.05) [Figure 10B] were significantly higher in the resveratrol- treated group than in the CRS group.
Figure 10 Effects of resveratrol on the expression level of BDNF in mPFC (A) and hippocampus (B). Data was expressed as the means ± SEM (n = 5). *P < 0.05 versus CRS.
DISCUSSION
In order to deeply understand the pathogenesis of MDD and develop drugs to treat the disease, the animal model of MDD is essential [30,31]. However, due to the heterogeneity of depression, there is currently a lack of recognized genetic animal models. Most of the animal models of depression are induced by stress, including chronic restraint stress [20], chronic mild unpredictable stress [32], chronic social frustration model [33] and so on. When constructing animal models of menopausal depression, some models were constructed with OVX [34,35], some with OVX+CRS [15], and some with OVX+CUMS [36]. In the previous study, we successfully constructed the model of menopausal depression with OVX+CRS [15]. The grouping in this study, with OVX as the control, the difference between the RES group and the CRS group can be regarded as the effect of resveratrol treatment in alleviating CRS stress-induced treatment.
Numerous clinical studies have confirmed that the incidence of depression in women is nearly twice as high as that of men at all ages [37-39]. The reasons for the sex difference can be multifaceted and may include depression-related gene expression, neuroplasticity, and immune signatures [39-41], as well as some biomarkers of depression such as BDNF and genetic polymorphisms associated with risk for depression [39,42,43]. However, the exact reasons for the sex difference are unclear.
Accumulating evidence has shown that chronic stress or/and psychosocial trauma-induced neuronal plasticity injury, which can be achieved by altering the BDNF-coffilin1 pathway [44,45], may be one of the key mechanisms of depression pathogenesis [23,46-49]. However, there are few studies on the sex differences in the pathogenesis, and some results are often inconsistent and contradictory. Studies have revealed that depression is strongly associated with multiple brain regions such as hippocampus and mPFC [15,50-56].
In mPFC, postmortem studies of individuals with MDD have demonstrated a reduction in the size of pyramidal neurons in the dorsal lateral PFC [15,29]. In rats, the dendritic arbors of pyramidal neurons of mPFC are less complex in normal adult females than in males [53,57-59]. In male rats, chronic stress can cause atrophy of mPFC neuronal apical dendrites [53,57,60- 68]. Animal model studies have indicated that in mPFC, chronic stress can reduce the length and number of branches of the apical dendrites in males, and increase the length of branches of the apical dendrites in females, while chronic stress has no significant effect on the length and number of branches of the basal and apical dendrites in OVX rats [57]. Our previous studies have shown that chronic restraint stress for 2 weeks (2 hours/ day) can significantly reduce the density of mPFC dendrites and dendritic spines in male mice [14], and can also reduce the density of mPFC dendrites and dendritic spines in OVX mice [15], but there are significant differences between male and OVX mice in the effect of CRS on dendritic spine types. Chronic restraint stress had no significant effect on the density of different types of dendritic spines in the mPFC of male mice [14], but CRS could significantly reduce the density of thin-type dendritic spines in basal, apical proximal and distal dendrites, as well as the density of mushroom-type dendritic spines in apical proximal and distal dendrites in the mPFC of OVX mice [15]. In addition, CRS could change the distribution of cofilin1 in neurons of mPFC both in male and OVX mice, i.e., in the control mice, coflin1 immunoreactivity was difusively distributed mainly in the cytoplasm and nucleus, also rarely in some process; in the depression model mice, coflin1 immunoreactivity was mainly distributed in the inner peri-membrane. However, in the mPFC of male mice, p-coflin1 in control group showed stronger immunofuorescence intensity and more immunopositive cells, and higher expression levels, which were demonstrated by western blot, than that in depression model group [14]. In contrast, in the mPFC of OVX mice, no significant difference in p-cofilin1 levels and p-cofilin1- immunopositive dendritic spine density was found between control group and depression model group [15].
In hippocampus, postmortem brain tissue studies revealed a significant reduction in neuronal cell body size and neuropil in MDD [15,48,69]. In rats, CRS reduced the density of dendritic spines of pyramidal neurons in the CA3 in females, but had no effect in males, while CRS reduced the density of dendritic spines of pyramidal neurons in the CA1 [57,70], and minished the apical dendritic arborization of pyramidal neurons in the CA3 in males, but had no significant effect in females [39,71]. Our previous studies showed that, similar to the situation in mPFC, CRS for 2 weeks (2 hours/day) significantly reduced the density of hippocampal dendrites and dendritic spines in male mice [14], and also reduced the density of hippocampal dendrites and dendritic spines in OVX mice [15], but there were significant differences between male and OVX mice in the effect of CRS on dendritic spine types. Chronic restraint stress only significantly reduced the density of stubby-type dendritic spines in the basal dendrites of CA1 in male mice [14], but reduced the density of filopodia-type dendritic spines in the basal and apical distal dendrites as well as the density of thin-type dendritic spines in apical distal dendrites of CA1 in OVX mice [15]. There were also obvious sex differences in the effects of CRS on the distribution of coflin1 immunoreactivity in hippocampal neurons. In male mice, in the control group, more cells showed coflin1 immunopositive distribution in the inner peri-membrane; in the model group, more cells showed coflin1 immunopositive difuse distribution in the cytoplasm [14]. However, in OVX mice, both in the control and model group, cofilin1 was distributed diffusely in the cytoplasm, nucleus, and processes in the pyramidal neurons. In addition, distribution of cofilin1 in dendrites was uniform and continuous in control mice, but exhibited an uneven distribution in model mice, in which some dendritic segments lacked cofilin1 labeling [15]. On the other hand, there was no significant difference in the p-coflin1 immunopositive distribution between sexes, which was mainly distributed in the nucleus, but the phosphorylation level of coflin1 was significantly different between sexes. In male mice, CRS remarkably downregulated the p-coflin1 immunofuorescence intensity and expression level in the hippocampus [14], while in OVX mice, CRS only significantly decreased the p-cofilin1 immunopositive dendritic spine density in the CA1 [15]. Our results suggest that the neuroplasticity mechanism of depression has many similarities and significant differences between genders.
At present, there is still a great controversy in the research on the efficacy of depression drugs. Most studies suggest that there is a difference between the sexes, or that women are better than men [72-81], or that men are better than women [72,80,82-84], although there are also studies that say that there are no differences between the sexes [72,78,85-95]. A growing number of animal studies have shown antidepressant efficacy of resveratrol [96-100], but these studies are based on male rodent models. One randomized placebo-controlled study showed that supplementing with equol and resveratrol can improve some symptoms in menopausal women [101]. In the early study, we showed that resveratrol significantly alleviated depressive- like behaviors in male mice induced by CRS [14]. The results of this study revealed that in the mouse model of menopausal depression induced by OVX plus CRS, three of the same four behavioral assessments were similar to those of the male model of depression, i.e., resveratrol increased the sucrose consumption in SPT, shortened the latency time in NSFT, and decreased the duration of TST immobility, but one was different, that is, resveratrol did not significantly reduce the immobility time in the FST. Our results suggest that there are also some differences in the efficacy of resveratrol antidepressants between genders.
The pharmacological mechanism of depression can be roughly divided into two categories: one is the mechanism study of the current monoamine drugs against different target hypotheses, and the other is the antidepressant mechanism of potential antidepressant drugs [39-41]. However, studies on sex differences in these antidepressant mechanisms are rare. Accumulated evidence shows that resveratrol can achieve antidepressant efficacy through a variety of pathways, such as anti-oxidant, anti-inflammatory, and anti-apoptotic effects, etc [96-100]. In terms of the mechanism of neuronal plasticity in depression, our previous studies showed that in a mouse model of male depression, resveratrol significantly increased the density of dendrites and dendritic spines in the CA1 and mPFC, and increased the density of stubby-type dendritic spines in basal dendrites in the CA1, but had no significant effect on the density of dendritic spines in the mPFC. In addition, resveratrol altered the coflin1distribution in hippocampus and mPFC. In the hippocampus, resveratrol can change the more cells showing coflin1 immunopositive difuse distribution in the cytoplasm into the more cells showing coflin1 immunopositive distribution in the inner peri-membrane. In mPFC, the distribution was opposite to that in the hippocampus. Moreover, resveratrol can increase the phosphorylation levels of coffilin1 and BDNF expression levels both in hippocampus and mPFC [14]. In the present study, in a mouse model of menopausal depression induced by OVX plus CRS, resveratrol significantly increased the density of CA1 and mPFC dendrites and dendritic spines and increased the density of filopodia-type dendritic spines in the basal dendrites and thin-type and stubby-type dendritic spines in the apical distal dendrites in CA1, as well as increased the density of thin-type dendritic spines in the apical proximal dendrites, but decreased the density of stubby-type dendritic spines in the apical proximal dendrites in mPFC. Simultaneously, resveratrol can change the distribution of cofilin1 in neurons. Both in hippocampus and mPFC, resveratrol treatment can change the more cells showing cofilin1 immunopositive distribution in the inner peri- membrane into the more cells showing cofilin1 immunopositive diffuse distribution in the cytoplasm. In addition, resveratrol cannot significantly alter the mRNA levels of cofilin1 in hippocampus and mPFC, but it can significantly increase the density of p-coffilin1 immunopositive dendritic spines and BDNF expression levels in these brain regions. Our results suggest that there are many similarities and some differences between sexes in the antidepressant mechanism of resveratrol based on neuronal plasticity.
CONCLUSION
In conclusion, although there are some sex differences in the efficacy and antidepressant mechanism of resveratrol based on neuronal plasticity, our study suggests that resveratrol may reduce the loss of dendrites and dendritic spines in the hippocampus and mPFC of mice through the BDNF-cofilin1 signaling pathway, thereby alleviating depressive-like behavior in mice.
Acknowledgments
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Hui Xu, Zhen-Qiang Zhang, Geng Chen, Ming-Jun Ge, Zong-Hao Yu, Jun-Xian Shen, Chuan Pan, Fei Han, and Xiu-Ling Zhu. The first draft of the manuscript was written by Hui Xu and Ya-Ping Lu, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
We thank Prof Jiang-Ning Zhou for providing us with transgenic mice (B6.Cg-TgN (Thy-YFP-H) 2Jrs), Mr. Ke-Qing Zhou for help in the experimental methods, Ms. Wen-Qing Mei, Yan Zhang, Xiu-Ling Zhang, Xin Chen, Yue Cheng, Yu-Xuan Wang, and Min Wu for help in the experiment, and Mr. Hao Yan and Ms. Xiao- Yan Ma for help in the confocal photomicrographs.
FUNDING
This work was supported by the National Natural Science Foundation of China (No. 30470537), The University Synergy Innovation Program of Anhui Province (GXXT-2020-030), The Natural Science Foundation of the Department of Education, Anhui Province (ZD2008006-1), Anhui Provincial Key Laboratory of the Conservation and Exploitation of Biological Resources, Anhui Provincial Key Laboratory of Molecular Enzymology and Mechanism of Major Diseases, Key Laboratory of Biomedicine in Gene Diseases and Health of Anhui Higher Education Institutes and the Innovation Team of Scientific Research Platform in Anhui Universities.
REFERENCES
- Seney ML, Huo Z, Cahill K, French L, Puralewski R, Zhang J, et al. Opposite Molecular Signatures of Depression in Men and Women. Biol Psychiatry. 2018; 84: 18-27.
- Labonte B, Engmann O, Purushothaman I, Menard C, Wang J, Tan C, et al. Sex-specific transcriptional signatures in human depression. Nat Med. 2017; 23: 1102-1111.
- Kessler RC. Epidemiology of women and depression. J Affect Disord. 2003; 74: 5-13.
- Kornstein SG, Schatzberg AF, Thase ME, Yonkers KA, McCullough JP, Keitner GI, et al. Gender differences in chronic major and double depression. J Affect Disord. 2000; 60: 1-11.
- Parry BL, Sorenson DL, Meliska CJ, Basavaraj N, Zirpoli GG, Zirpoli GG, et al. Hormonal basis of mood and postpartum disorders. Curr Womens Health Rep. 2003; 3: 230-235.
- Bredemann TM, McMahon LL. 17beta Estradiol increases resilience and improves hippocampal synaptic function in helpless ovariectomized rats. Psychoneuroendocrinology. 2014; 42: 77-88.
- Echeverria V, Echeverria F, Barreto GE, Echeverría J, Mendoza C. Estrogenic Plants: to Prevent Neurodegeneration and Memory Loss and Other Symptoms in Women After Menopause. Front Pharmacol. 2021; 12: 644103.
- Ge JF, Xu YY, Cheng JQ, Qin G, Chen FH. Resveratrol Ameliorates the Anxiety- and Depression-Like Behavior of Subclinical Hypothyroidism Rat: Possible Involvement of the HPT Axis, HPA Axis, and Wnt/β- Catenin Pathway. Front Endocrinol (Lausanne). 2016; 7: 44.
- Rege SD, Geetha T, Griffin GD, Broderick TL, Babu JR. Neuroprotective effects of resveratrol in Alzheimer disease pathology. Front Aging Neurosci. 2014; 6: 218.
- Sharma M, Gupta YK. Chronic treatment with trans resveratrol prevents intracerebroventricular streptozotocin induced cognitive impairment and oxidative stress in rats. Life Sci. 2002; 71: 2489- 2498.
- Evans HM, Howe PR, Wong RH. Effects of Resveratrol on Cognitive Performance, Mood and Cerebrovascular Function in Post- Menopausal Women; A 14-Week Randomised Placebo-Controlled Intervention Trial. Nutrients. 2017; 9: 27.
- Kodali M, Parihar VK, Hattiangady B, Mishra V, Shuai B, Shetty AK. Resveratrol prevents age-related memory and mood dysfunction with increased hippocampal neurogenesis and microvasculature, and reduced glial activation. Sci Rep. 2015; 5: 8075.
- Nemeroff CB, Owens MJ. Treatment of mood disorders. Nature Neurosci. 2002; 5: 1068-1070.
- Chen JJ, Shen JX, Yu ZH, Pan C, Han F, Zhu XL, et al. The Antidepressant Effects of Resveratrol are accompanied by the Attenuation of Dendrite/Dendritic Spine Loss and the Upregulation of BDNF/p- cofilin1 Levels in Chronic Restraint Mice. Neurochem Res. 2021; 46: 660-674.
- Xu H, Yu ZH, Ge MJ, Shen JX, Han F, Pan C, et al. Estradiol attenuates chronic restraint stress-induced dendrite and dendritic spine loss and cofilin1 activation in ovariectomized mice. Horm Behav. 2021; 135: 105040.
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000: 28: 41-51.
- Porrero C, Rubio-Garrido P, Avendaño C, Clascá F. Mapping of fluorescent protein-expressing neurons and axon pathways in adult and developing Thy1-eYFP-H transgenic mice. Brain Res. 2010; 1345: 59-72.
- Bekku N, Yoshimura H. Animal model of menopausal depressive-like state in female mice: prolongation of immobility time in the forced swimming test following ovariectomy. Psychopharmacol (Berl). 2005; 183: 300-307.
- Zhang K, Wang Z, Pan X, Pan X, Pan X. Antidepressant-like effects of Xiaochaihutang in perimenopausal mice. J Ethnopharmacol. 2020; 248: 112318.
- Seo JS, Park JY, Choi J, Kim TK, Shin JH, Lee JK, et al. NADPH oxidase mediates depressive behavior induced by chronic stress in mice. J Neurosci. 2012; 32: 9690-9699.
- Seo JS, Wei J, Qin L, Kim Y, Yan Z, Greengard P. Cellular and molecular basis for stress-induced depression. Mol Psychiatry. 2017; 22: 1440-1447.
- Willner P, Towell A, Sampson D, Sampson D, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacol. 1987; 93: 358-364.
- Zhu XL, Chen JJ, Han F, Pan C, Zhuang TT, Zhuang TT, et al. Novel antidepressant effects of Paeonol alleviate neuronal injury with concomitant alterations in BDNF, Rac1 and RhoA levels in chronic unpredictable mild stress rats. Psychopharmacol. 2018; 235: 2177-2191.
- Porsolt RD, Le Pichon M, Jalfre M. Depression:a new animal model sensitive to antidepressant treatments. Nature. 1977; 266: 730-732.
- Han F, Zhuang TT, Chen JJ, Zhu XL, Cai YF, Lu YP. Novel derivative of Paeonol, Paeononlsilatie sodium, alleviates behavioral damage and hippocampal dendritic injury in Alzheimer’s disease concurrent with cofilin1/phosphorylated-cofilin1 and RAC1/CDC42 alterations in rats. PLoS One. 2017; 12: e0185102.
- Foster Olive M, Del Franco AP, Gipson CD. Diolistic Labeling and Analysis of Dendritic Spines. Methods Mol Biol. 2018; 1727: 179-200.
- Sebastian V, Estil JB, Chen D, Schrott LM, Serrano PA. Acute physiological stress promotes clustering of synaptic markers and alters spine morphology in the hippocampus. PLoS One. 2013; 8: e79077.
- Wang F, Cui N, Yang L, Shi L, Li Q, Zhang G, et al. Resveratrol Rescues the Impairments of Hippocampal Neurons Stimulated by Microglial Over-Activation In Vitro. Cell Mol Neurobiol. 2015; 35: 1003-1015.
- Deleglise B, Lassus B, Soubeyre V, Alleaume-Butaux A, Hjorth JJ, Vignes M, et al. Synapto-protective drugs evaluation in reconstructed neuronal network. PLoS One. 2013; 8: e71103.
- Song J, Kim YK. Animal models for the study of depressive disorder. CNS Neurosci Ther. 2021; 27: 633-642.
- Dai W, Feng K, Sun X, Xu L, Wu S, Rahmand K, et al. Natural products for the treatment of stress-induced depression: Pharmacology, mechanism and traditional use. J Ethnopharmacol. 2022; 285: 114692.
- Wang YL, Wu HR, Zhang SS, Xiao HL, Ma YY, Yu J, et al. Catalpol ameliorates depressive-like behaviors in CUMS mice via oxidative stress-mediated NLRP3 inflammasome and neuroinflammation. Transl Psychiatry. 2021; 11: 353.
- Dang R, Wang M, Li X, Wang H, Liu L, Wu Q, et al. Edaravone ameliorates depressive and anxiety-like behaviors via Sirt1/Nrf2/ HO-1/Gpx4 pathway. J Neuroinflammation. 2022; 19: 41.
- Lim DW, Kim M, Yoon M, Lee J, Lee C, Um MY. 1,3-Dicaffeoylquinic Acid as an Active Compound of Arctium lappa Root Extract Ameliorates Depressive-Like Behavior by Regulating Hippocampal Nitric Oxide Synthesis in Ovariectomized Mice. Antioxidants (Basel). 2021; 10: 1281.
- Moreira SF, Nunes EA, Kuo J, de Macedo IC, Muchale A, de Oliveira C, et al. Hypoestrogenism alters mood: Ketamine reverses depressive- like behavior induced by ovariectomy in rats. Pharmacol Rep. 2016; 68: 109-115.
- Ma J, Guo CY, Li HB, Wu SH, Li GL. Prophylactic Effects of Hemp Seed Oil on Perimenopausal Depression: A Role of HPA Axis. J Oleo Sci. 2023; 72: 939-955.
- 37. Twenge JM, Nolen-Hoeksema S. Age, gender, race, socioeconomic status, and birth cohort differences on the children’s depression inventory: a meta-analysis. J Abnorm Psychol. 2002; 111: 578-588.
- Salk RH, Hyde JS, Abramson LY. Gender differences in depression in representative national samples: Meta-analyses of diagnoses and symptoms. Psychol Bull. 2017; 143: 783-822.
- Eid RS, Gobinath AR, Galea LAM. Sex differences in depression: Insights from clinical and preclinical studies. Prog Neurobiol. 2019; 176: 86-102.
- Altemus M, Sarvaiya N, Neill Epperson C. Sex differences in anxiety and depression clinical perspectives. Front Neuroendocrinol. 2014; 35: 320-330.
- Bangasser DA, Valentino RJ. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front Neuroendocrinol. 2014; 35: 303-319.
- Labaka A, Goñi-Balentziaga O, Lebeña A, Pérez-Tejada J. Biological Sex Differences in Depression: A Systematic Review. Biol Res Nurs. 2018; 20: 383-392.
- Perry LM, Goldstein-Piekarski AN, Williams LM. Sex differences modulating serotonergic polymorphisms implicated in the mechanistic pathways of risk for depression and related disorders. J Neurosci Res. 2017; 95: 737-762.
- Castren E, Kojima M. Brain-derived neurotrophic factor in mood disorders and antidepressant treatments. Neurobiol Dis. 2017; 97: 119-126.
- Carniel BP, da Rocha NS. Brain-derived neurotrophic factor (BDNF) and inflammatory markers: Perspectives for the management of depression. Prog Neuropsychopharmacol Biol Psychiatr. 2021; 108: 110151.
- Liu X, Chan CB, Jang SW, Pradoldej S, Huang J, He K, et al. A synthetic 7,8-dihydroxyflavone derivative promotes neurogenesis and exhibits potent antidepressant effect. J Med Chem. 2010; 53: 8274-8286.
- Rantamaki T, Yalcin I. Antidepressant drug action--From rapid changes on network function to network rewiring. Prog Neuropsychopharmacol Biol Psychiatry. 2016; 64: 285-292.
- Duman RS, Aghajanian GK. Synaptic Dysfunction in Depression: Potential Therapeutic Targets. Science. 2012; 338: 68-72.
- McEwen BS, Eiland L, Hunter RG, Miller MM. Stress and anxiety: structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacol. 2012; 62: 3-12.
- McKlveen JM, Morano RL, Fitzgerald M, Zoubovsky S, Cassella SN, Scheimann JR, et al. Chronic Stress Increases Prefrontal Inhibition: A Mechanism for Stress-Induced Prefrontal Dysfunction. Biol Psychiatry. 2016; 80: 754-764.
- Liu RJ, Ota KT, Dutheil S, Duman RS, Aghajanian GK. Ketamine Strengthens CRF-Activated Amygdala Inputs to Basal Dendrites in mPFC Layer V Pyramidal Cells in the Prelimbic but not Infralimbic Subregion, A Key Suppressor of Stress Responses. Neuropsychopharmacol. 2015; 40: 2066-2075.
- Lei Y, Wang J, Wang D, Li C, Liu B, Fang X, et al. SIRT1 in forebrain excitatory neurons produces sexually dimorphic effects on depression-related behaviors and modulates neuronal excitability and synaptic transmission in the medial prefrontal cortex. Mol Psychiatry. 2020; 25: 1094-1111.
- Farrell MR., Sengelaub DR, Wellman CL. Sex differences and chronic stress effects on the neural circuitry underlying fear conditioning and extinction. Physiol Behav. 2013; 122: 208-215.
- Weniger G, Lange C, Irle E. Abnormal size of the amygdala predicts impaired emotional memory in major depressive disorder. J Affect Disord. 2006; 94: 219-229.
- Fossati P, Radtchenko A, Radtchenko A. Neuroplasticity: from MRI to depressive symptoms. Eur Neuropsychopharmacol. 2004; 5: 503-510.
- Tronson NC, Corcoran KA, Jovasevic V, Radulovic J. Fear conditioning and extinction: emotional states encoded by distinct signaling pathways. Trends Neurosci. 2012; 35: 145-155.
- Garrett JE, Wellman CL. Chronic stress effects on dendritic morphology in medial prefrontal cortex: sex differences and estrogen dependence. Neuroscience. 2009; 162: 195-207.
- Markham JA, Juraska JM. Aging and sex influence the anatomy of the rat anterior cingulate cortex. Neurobiol Aging. 2002; 23: 579-588.
- Kolb B, Stewart J. Sex-related differences in dendritic branching of cells in the prefrontal cortex of rats. J Neuroendocrinol. 1991; 3: 95- 99.
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004; 60: 236-248.
- Martin KP, Wellman CL. NMDA receptor blockade alters stress- induced dendritic remodeling in medial prefrontal cortex. Cereb Cortex. 2011. 21: 2366-2373.
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neurosci. 2004; 125: 1-6.
- Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006; 16: 313-320.
- Radley JJ, Rocher AB, Janssen WG, Hof PR, McEwen BS, Morrison JH. Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Exp Neurol. 2005; 196: 199-203.
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006; 26: 7870-7874.
- Cerqueira JJ, Mailliet F, Almeida OF, Jay TM, Sousa N. The prefrontal cortex as a key target of the maladaptive response to stress. J Neurosci. 2007; 27: 2781-2787.
- Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J Neurosci. 2006; 26: 5733-5738.
- Shansky RM, Hamo C, Hof PR, McEwen BS, Morrison JH. Stress- induced dendritic remodeling in the prefrontal cortex is circuit specific. Cereb Cortex. 2009; 19: 2479-2484.
- Sadowski MJ, Pankiewicz J, Scholtzova H, Mehta PD, Prelli F. Blocking the apolipoprotein E/amyloid-beta interaction as a potential therapeutic approach for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2006; 103: 18787-18792.
- McLaughlin KJ, Baran SE, Wright RL, Conrad CD. Chronic stress enhances spatial memory in ovariectomized female rats despite CA3 dendritic retraction: possible involvement of CA1 neurons. Neurosci. 2005; 135: 1045-1054.
- Galea LA, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neurosci. 1997; 81: 689-697.
- Sramek JJ, Murphy MF, Cutler NR. Sex differences in the psychopharmacological treatment of depression. Dialogues Clin Neurosci. 2016; 18: 447-457.
- Yang SJ, Kim SY, Stewart R, Kim JM, Shin IS, Jung SW, et al. Gender differences in 12-week antidepressant treatment outcomes for a naturalistic secondary care cohort: the CRESCEND study. Psychiatry Res. 2011; 189: 82-90.
- Young EA, Kornstein SG, Marcus SM, Harvey AT, Warden D, Wisniewski SR, et al. Sex differences in response to citalopram: a STAR*D report. J Psychiatr Res. 2009; 43: 503-511.
- Naito S, Sato K, Yoshida K, Higuchi H, Takahashi H, Kamata M, et al. Gender differences in the clinical effects of fluvoxamine and milnacipran in Japanese major depressive patients. Psychiatry Clin Neurosci. 2007; 61: 421-427.
- Berlanga C, Flores-Ramos M. Different gender response to serotonergic and noradrenergic antidepressants. A comparative study of the efficacy of citalopram and reboxetine. J Affect Disord. 2006; 95: 119-123.
- Khan A, Brodhead AE, Schwartz KA, Kolts RL, Brown WA. Sex differences in antidepressant response in recent antidepressant clinical trials. J Clin Psychopharmacol. 2005; 25: 318-324.
- Quitkin FM, Stewart JW, McGrath PJ, Taylor BP, Tisminetzky MS, Petkova E, et al. Are there differences between women’s and men’s antidepressant responses? Am J Psychiatry. 2002; 159: 1848-1854.
- Martenyi F, Dossenbach M, Mraz K, Metcalfe S. Gender differences in the efficacy of fluoxetine and maprotiline in depressed patients: a double-blind trial of antidepressants with serotonergic or norepinephrinergic reuptake inhibition profile. Eur Neuropsychopharmacol. 2001; 11: 227-232.
- Kornstein SG, Schatzberg AF, Thase ME, Yonkers KA, McCullough JP, Keitner GI, et al. Gender differences in treatment response to sertraline versus imipramine in chronic depression. Am J Psychiatry. 2000; 157: 1445-1452.
- Haykal RF, Akiskal HS. The long-term outcome of dysthymia in private practice: clinical features, temperament, and the art of management. J Clin Psychiatry. 1999; 60: 508-518.
- Frank E, Carpenter LL, Kupfer DJ. Sex differences in recurrent depression: are there any that are significant? Am J Psychiatry. 1988; 145: 41-45.
- How long should the elderly take antidepressants? A double-blind placebo-controlled study of continuation/prophylaxis therapy with dothiepin. Old Age Depression Interest Group. Br J Psychiatry. 1993; 162: 175-182.
- Raskin A. Age-sex differences in response to antidepressant drugs. J Nerv Ment Dis. 1974; 159: 120-130.
- Kornstein SG, Pedersen RD, Holland PJ, Nemeroff CB, Rothschild AJ, Thase ME, et al. Influence of sex and menopausal status on response, remission, and recurrence in patients with recurrent major depressive disorder treated with venlafaxine extended release or fluoxetine: analysis of data from the PREVENT study. J Clin Psychiatry. 2014; 75: 62-68.
- Cuijpers P, Weitz E, Twisk J, Kuehner C, Cristea I, David D, et al. Gender as predictor and moderator of outcome in cognitive behavior therapy and pharmacotherapy for adult depression: an “individual patient data” meta-analysis. Depress Anxiety. 2014; 31: 941-951.
- Kornstein SG, Clayton AH, Soares CN, Padmanabhan SK, Guico-Pabia CJ. Analysis by age and sex of efficacy data from placebo-controlled trials of desvenlafaxine in outpatients with major depressive disorder. J Clin Psychopharmacol. 2010; 30: 294-299.
- Pinto-Meza A, Usall J, Serrano-Blanco A, Suárez D, Haro JM. Gender differences in response to antidepressant treatment prescribed in primary care. Does menopause make a difference? J Affect Disord. 2006; 93: 53-60.
- Thiels C, Linden M, Grieger F, Leonard J. Gender differences in routine treatment of depressed outpatients with the selective serotonin reuptake inhibitor sertraline. Int Clin Psychopharmacol. 2005; 20: 1-7.
- Wohlfarth T, Storosum JG, Elferink AJ, van Zwieten BJ, Fouwels A, et al. Response to tricyclic antidepressants: independent of gender? Am J Psychiatry. 2004; 161: 370-372.
- Baca E, Garcia-Garcia M, Porras-Chavarino A. Gender differences in treatment response to sertraline versus imipramine in patients with nonmelancholic depressive disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2004; 28: 57-65.
- Parker G, Parker K, Austin MP, Mitchell P, Brotchie H. Gender differences in response to differing antidepressant drug classes: two negative studies. Psychol Med. 2003; 33: 1473-1477.
- Hildebrandt MG, Steyerberg EW, Stage KB, Passchier J, Kragh- Soerensen P, et al. Are gender differences important for the clinical effects of antidepressants? Am J Psychiatry. 2003; 160: 1643-1650.
- Entsuah AR, Huang H, Thase ME. Response and remission rates in different subpopulations with major depressive disorder administered venlafaxine, selective serotonin reuptake inhibitors, or placebo. J Clin Psychiatry. 2001; 62: 869-877.
- Himmelhoch JM, Thase ME, Mallinger AG, Houck P. Tranylcypromine versus imipramine in anergic bipolar depression. Am J Psychiatry. 1991; 148: 910-916.
- Menegas S, Keller GS, Possamai-Della T, Aguiar-Geraldo JM, Quevedo J, et al. Potential mechanisms of action of resveratrol in prevention and therapy for mental disorders. J Nutr Biochem. 2023; 121: 109435.
- Moore A, Beidler J, Hong MY. Resveratrol and Depression in Animal Models: A Systematic Review of the Biological Mechanisms. Molecules. 2018; 23: 2197.
- Xu YY, Liang J, Xia QR. Novel insights into the pharmacological effects of resveratrol on the management of depression: a short review. Pharmazie. 2017; 72: 499-502.
- Diaz-Gerevini GT, Repossi G, Dain A, Tarres MC, Das UN, et al. Beneficial action of resveratrol: How and why? Nutrition. 2016; 32: 174-178.
- de Oliveira MR, Chenet AL, Duarte AR, Scaini G, Quevedo J. Molecular Mechanisms Underlying the Anti-Depressant Effects of Resveratrol: A Review. Mol Neurobiol. 2018; 55: 4543-4559.
- Davinelli S, Scapagnini G, Marzatico F, Nobile V, Ferrara N, et al. Influence of equol and resveratrol supplementation on health- related quality of life in menopausal women: A randomized, placebo- controlled study. Maturitas. 2017; 96: 77-83.