Etiological ASCOD Classification of Large Vessel Occlusion Ischemic Stroke in a Retrospective Cohort of Young Adults
- 1. Cleveland Clinic Foundation, USA
- 2. Cleveland Clinic Learner College of Medicine, USA
- 3. Department of Quantitative Health Sciences, Cleveland Clinic, Cleveland, OH, USA
- 4. New York University, Ney York City, NY. USA
- 5. Virginia Tech University
Abstract
Objective: Large vessel occlusion acute ischemic stroke (LVO-AIS) is considerably less common in young (18-50 years old) adults likely due to the elusive stroke mechanisms compared to older adults. We sought to assess the etiology of LVO-AIS in young adults presenting to Cleveland Clinic Stroke Enterprise using ASCOD classification system to identify underlying stroke etiology in those labeled as cryptogenic per TOAST classification.
Methods: In this retrospective, single healthcare network study, we reviewed electronic charts of patients 18-50 years of age presenting with ischemic stroke between January 2017 and December 2021 to the Cleveland Clinic Stroke Enterprise. We identified patients who had a large vessel occlusion (LVO) on CTA or MRA at presentation. We excluded those without LVO or those who did not have intracranial vessel imaging available. We assessed demographics and stroke etiology using both the TOAST and ASCOD classification systems. We then compared those labeled as cryptogenic per TOAST classification with their respective ASCOD classification to elucidate further stroke mechanisms in these patients.
Results: Out of 1175 patients who presented with acute ischemic stroke during the study period, 220 patients were found to have LVO-AIS and were included in the study. The mean age was 40.5 ±7.0. Median (Q1, Q3) initial NIHSS was 8 (3, 16). By TOAST, stroke of undetermined etiology (34.7%) was the most common followed by stroke of other determined etiology (28.6%). However, of those labeled as “undetermined etiology,” ASCOD classification revealed 7 (9.46%) as potentially causal and 7 (9.46%) as uncertainly causal etiologies.
Conclusions: In our young adult stroke cohort, a third of patients had cryptogenic LVO-AIS. However, ASCOD classification revealed potentially causal and uncertainly causal etiologies that may have been overlooked in TOAST classification suggesting that ASCOD classification system may be more informative in establishing stroke etiology of LVO-AIS in young adults.
KEYWORDS
- Ischemic stroke
- Stroke in young adults
- Large vessel occlusion stroke
- ASCOD
- TOAST
- Etiology
CITATION
Handshoe JW, Bhayana K, Li Y, Thompson N, Martucci M, et al. (2024) Etiological ASCOD Classification of Large Vessel Occlusion Ischemic Stroke in a Retrospective Cohort of Young Adults. J Neurol Disord Stroke 11(3): 1224.
INTRODUCTION
Ischemic stroke can have devastating consequences with significant mortality and long-term morbidity [1]. Young patients with ischemic stroke, ages 18 to 49 years old, are a vulnerable subset of patients who may suffer from lasting morbidity and have increased risk of recurrent stroke if the correct etiology is not discovered and remains unaddressed. Prior studies have estimated that, in the U.S. and Europe, stroke incidence in young adults occurs from 5.8 to 11.4 per 100,000, attributing roughly 10% of all ischemic stroke to those less than 50 years of age [2,3]. While stroke in young adults has been traditionally thought of as rare, the incidence is overall increasing, possibly owing to an increase in the rise of obesity along with vascular risk factors such as hypertension, diabetes, and hyperlipidemia although the precise cause is debated [4-6]. Given the rise in traditional risk factors; one would expect a subsequent rise in large artery atherosclerosis however prior studies have shown that ‘undetermined etiology’ remains the most common stroke etiology amongst young adults [7,8].
Due to the elusive stroke mechanisms in young patients, ASCOD phenotyping (A: atherosclerosis, S: small vessel disease, C: cardiovascular, O: other causes, D: dissection) has been proposed as a novel way to clarify stroke etiology, especially in younger, more complex patients [9]. Our study aimed to utilize the ASCOD phenotyping system to compare those labeled as “undetermined etiology” by TOAST to their respective ASCOD phenotype in a cohort of young adult patients (age 18-50 years old) who presented with large vessel occlusion ischemic stroke to assess for any underlying differences that may have been missed utilizing TOAST classification system alone.
METHODS
The study was completed retrospectively via manual electronic chart review after IRB approval was obtained. Using Slicer Dicer software in EPIC, we obtained medical record numbers of patients with ICD-10 codes for “ischemic stroke” who were seen in the Cleveland Clinic health system from January 2017 to December 2021. This initially yielded 6,383 patients. We excluded those with no brain imaging available (n=1,153) and those who had their stroke event prior to January 2017 (n=5,230). 1,175 patients met the inclusion criteria for the Cleveland Clinic Young Adult Stroke database i.e., were between ages 18-50 years, had brain and vessel imaging available and had their stroke event between 01/2017 and 12/2021. Of those, 220 patients presented with a large vessel occlusion ischemic stroke and were included in our study cohort. For these 220 patients with LVO-AIS, further data were collected on relevant demographics, risk factors, imaging characteristics and follow-up. For each patient, the TOAST classification was collected if previously documented and the ASCOD phenotyping was determined by the study physicians reviewing the available retrospective data. Following data collection, this information was categorized, and the “undetermined” TOAST classification was compared to their respective ASCOD phenotyping. Demographics and clinical characteristics were summarized by mean with standard deviation, or median with interquartile range for continuous variables, and counts with percentage for categorical variables.
The Cleveland Clinic Foundation IRB reviewed our study design, under the ID 22-206 and was found to be exempt. Given retrospective nature and de-identified data, consent was not obtained for data collection. The study did not receive any internal or outside funding and all authors do not have any financial or personal disclosures.
RESULTS
Table 1a summarizes demographics and clinical characteristics of the study sample (N=220).
Table 1a: Demographics and clinical characteristics of the study sample (N=220).
|
|
Total (N=220) |
|
Age |
40.5 ± 7.0 |
|
Gender |
|
|
Male |
111 (50.5) |
|
Female |
109 (49.5) |
|
Race |
|
|
White |
141 (64.1) |
|
African American |
57 (25.9) |
|
Other |
22 (10.0) |
|
BMI* |
31.1 ± 7.7 |
|
TOAST* |
|
|
Large Artery Atherosclerosis (embolus/thrombosis) |
37 (17.4) |
|
Cardioembolism |
41 (19.2) |
|
Stroke of Other determined etiology |
61 (28.6) |
|
Stroke of Undetermined etiology |
74 (34.7) |
|
Initial NIHSS* |
8.0 [3.0, 16.0] |
|
Initial mRS* |
0.0 [0.0, 0.0] |
|
Discharge NIHSS* |
2.0 [0.0, 7.0] |
|
Discharge mRS* |
2.0 [1.0, 4.0] |
|
90-day NIHSS* |
0.0 [0.0, 2.0] |
|
90-day mRS* |
1.0 [1.0, 3.0] |
|
Hours from last known well to admission* |
7.0 [2.0, 21.0] |
|
ICH* |
22 (10.9) |
|
Symptomatic ICH* |
4 (2.0) |
|
Imaging Data |
|
|
Arterial territory |
|
|
Anterior circulation* |
193 (90.6) |
|
Posterior circulation* |
20 (9.4) |
|
Topography of infarcts |
|
|
Cortical* |
140 (65.7) |
|
Subcortical* |
109 (51.2) |
|
Brainstem* |
2 (0.94) |
|
Cerebellar* |
8 (3.8) |
|
Borderzone/watershed* |
5 (2.3) |
|
Infarct pattern* |
|
|
Lacunar |
3 (1.5) |
|
Embolic |
91 (44.4) |
|
Athero-occlusive |
103 (50.2) |
|
Hypoperfusion |
4 (2.0) |
|
Vasculitic |
4 (2.0) |
|
Infarct count* |
|
|
Focal (1) |
163 (79.5) |
|
Multifocal (>1) |
42 (20.5) |
|
Core infarct size* |
19.5 [5.0, 42.0] |
The mean age was 40.5 ± 7.0 with 111 males (50.5%) and 109 females (49.5%). Of those, 141 were white (64.1%), 57 African American (25.9%), and 22 (10%) labeled as “other”. The TOAST classification (number, percentage) was as follows: large artery atherosclerosis (37, 17.4%), cardio-embolism (41, 19.2%), stroke of other determined etiology (61, 28.6%), stroke of undetermined etiology (74, 34.7%). Table 1b summarizes demographics and clinical characteristics of the study sample, stratified by TOAST category. Seven patients who were missing their TOAST classification were not included in Table 1b.
Table 1b. Demographics and clinical characteristics of the study sample, stratified by TOAST classifications
|
|
Total (N=213) |
Large Artery Atherosclerosis (embolus thrombosis) (N=37) |
Cardioembolism (N=41) |
Stroke of Other determined etiology (N=61) |
Stroke of Undetermined etiology (N=74) |
p-value |
|
Age |
40.4 ± 7.0 |
43.2 ± 5.6 |
39.3 ± 7.4 |
40.2 ± 7.1 |
39.8 ± 7.0 |
0.050a |
|
Gender |
|
|
|
|
|
0.45c |
|
Male |
109 (51.2) |
19 (51.4) |
18 (43.9) |
29 (47.5) |
43 (58.1) |
|
|
Female |
104 (48.8) |
18 (48.6) |
23 (56.1) |
32 (52.5) |
31 (41.9) |
|
|
Race |
|
|
|
|
|
0.14d |
|
White |
141 (66.2) |
22 (59.5) |
26 (63.4) |
46 (75.4) |
47 (63.5) |
|
|
African American |
57 (26.8) |
11 (29.7) |
11 (26.8) |
15 (24.6) |
20 (27.0) |
|
|
Other |
15 (7.0) |
4 (10.8) |
4 (9.8) |
0 (0.00) |
7 (9.5) |
|
|
BMI* |
31.1 ± 7.7 |
31.6 ± 8.1 |
31.3 ± 9.0 |
30.3 ± 6.5 |
31.2 ± 7.9 |
0.84a |
|
Initial NIHSS* |
8.0 [3.0, 16.0] |
6.0 [3.0, 10.0] |
11.0 [6.0, 20.0] |
11.0 [3.0, 18.0] |
6.0 [3.0, 12.0] |
0.019b |
|
Initial mRS* |
0.0 [0.0, 0.0] |
0.0 [0.0, 0.0] |
0.0 [0.0, 1.0] |
0.0 [0.0, 1.0] |
0.0 [0.0, 0.0] |
0.38b |
|
Discharge NIHSS* |
2.0 [0.0, 7.0] |
4.0 [1.0, 11.0] |
1.0 [0.0, 6.0] |
2.0 [0.0, 10.0] |
1.0 [0.0, 6.0] |
0.45b |
|
Discharge mRS* |
2.0 [1.0, 4.0] |
2.0 [1.0, 4.0] |
1.5 [1.0, 4.0] |
2.5 [1.0, 5.0] |
1.0 [0.0, 3.0] |
0.078b |
|
90 day NIHSS* |
0.0 [0.0, 2.0] |
1.0 [0.0, 3.0] |
0.0 [0.0, 2.0] |
0.5 [0.0, 4.0] |
0.0 [0.0, 0.5] |
0.28b |
|
90 day mRS* |
1.0 [1.0, 3.0] |
1.5 [1.0, 3.0] |
1.0 [1.0, 4.0] |
2.0 [1.0, 4.0] |
1.0 [0.0, 2.0] |
0.28b |
|
Hours from last known well to admission* |
7.0 [2.0, 21.0] |
10.0 [4.0, 26.0] |
5.0 [2.0, 13.0] |
11.0 [2.0, 24.0] |
4.0 [1.0, 37.0] |
0.50b |
|
ICH* |
22 (10.9) |
2 (5.6) |
6 (15.0) |
5 (8.8) |
9 (13.0) |
0.51d |
|
Symptomatic ICH* |
4 (2.0) |
0 (0.00) |
2 (5.0) |
2 (3.5) |
0 (0.00) |
0.14d |
|
Imaging Data |
|
|
|
|
|
|
|
Arterial territory |
|
|
|
|
|
|
|
Anterior circulation |
193 (90.6) |
36 (97.3) |
36 (87.8) |
53 (86.9) |
68 (91.9) |
0.31d |
|
Posterior circulation |
20 (9.4) |
1 (2.7) |
5 (12.2) |
8 (13.1) |
6 (8.1) |
0.31d |
|
Topography of infarcts |
|
|
|
|
|
|
|
Cortical |
140 (65.7) |
17 (45.9) |
29 (70.7) |
44 (72.1) |
50 (67.6) |
0.044c |
|
Subcortical |
109 (51.2) |
24 (64.9) |
21 (51.2) |
26 (42.6) |
38 (51.4) |
0.21c |
|
Brainstem |
2 (0.94) |
0 (0.00) |
0 (0.00) |
1 (1.6) |
1 (1.4) |
0.99d |
|
Cerebellar |
8 (3.8) |
0 (0.00) |
1 (2.4) |
4 (6.6) |
3 (4.1) |
0.48d |
|
Borderzone/watershed |
5 (2.3) |
3 (8.1) |
0 (0.00) |
0 (0.00) |
2 (2.7) |
0.046d |
|
Infarct count* |
|
|
|
|
|
0.68c |
|
Focal (1) |
163 (79.5) |
28 (75.7) |
33 (84.6) |
45 (76.3) |
57 (81.4) |
|
|
Multifocal (>1) |
42 (20.5) |
9 (24.3) |
6 (15.4) |
14 (23.7) |
13 (18.6) |
|
|
Core Infarct Size (ml) |
19.5 [5.0, 42.0] |
8.5 [2.5, 28.0] |
42.0 [7.0, 71.0] |
21.0 [11.0, 30.0] |
19.0 [5.0, 27.0] |
0.50b |
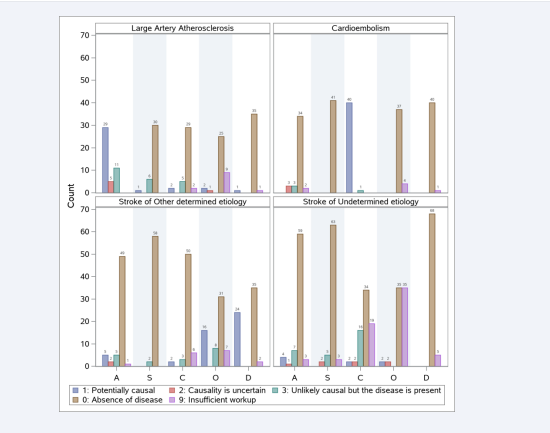
Initial NIHSS differed significantly across TOAST classifications (p=0.019). Patients with cardio- embolism and other determined etiology were noted to have higher initial NIHSS compared to other groups (median 11.0 vs median 6.0). Figure 1 shows bar charts of ASCOD classifications, for patients in each TOAST category. This does not restrict to the highest grade. For example, if a patient has ASCOD classification of A1+S0+C0+O0+D0, then it contributes to the count of all 5 categories. Those with the TOAST classification “stroke of undetermined etiology” were further analyzed compared to their ASCOD classification. Table 2 summarizes ASCOD classifications for patients with “Stroke of Undetermined etiology” based on TOAST (N=74).
Table 2. ASCOD classifications for patients with “Stroke of Undetermined etiology” based on TOAST (N=74).
|
ASCOD classification |
Count |
|
A1 |
4 |
|
A2 |
1 |
|
A3 |
7 |
|
A0 |
59 |
|
A9 |
3 |
|
S1 |
0 |
|
S2 |
2 |
|
S3 |
5 |
|
S0 |
63 |
|
S9 |
3 |
|
C1 |
2 |
|
C2 |
2 |
|
C3 |
16 |
|
C0 |
34 |
|
C9 |
19 |
|
O1 |
2 |
|
O2 |
2 |
|
O3 |
0 |
|
O0 |
35 |
|
O9 |
35 |
|
D1 |
0 |
|
D2 |
0 |
|
D3 |
0 |
|
D0 |
68 |
|
D9 |
5 |
Numbers in Table 2 correspond to the bottom right plot in Figure 1.
Figure 1: ASCOD classifications for patients in each TOAST category
Table 3 breaks down ASCOD classifications in more detail,
Table 3: Detailed ASCOD classifications for patients with “Stroke of Undetermined etiology” based on TOAST (N=74).
|
|
N (%) |
Detail list of ASCOD |
|
Highest grade: 1 |
7 (9.46) |
|
|
A1 |
4 |
A1+S0+C0+O0+D0 (2), A1+S0+C9+O0+D0 (1), A1+S0+C0+O9+D0 (1) |
|
C1 |
1 |
A0+S0+C1+O9+D9 (1) |
|
O1 |
1 |
A0+S0+C0+O1+D0 (1) |
|
C1+O1 |
1 |
A0+S0+C1+O1+O9+D0 (1) |
|
Highest grade: 2 |
7 (9.46) |
|
|
A2 |
1 |
A2+S0+C9+O0+D0 (1) |
|
S2 |
2 |
A3+S2+C9+O9+D9 (1), A0+S2+C3+O0+D0 (1) |
|
C2 |
2 |
A0+S0+C2+O0+D0 (2) |
|
O2 |
2 |
A0+S0+C0+O2+D0 (2) |
|
Highest grade: 3 |
23 (31.08) |
|
|
A3 |
4 |
A3+S0+C9+O0+D0 (1), A3+S0+C9+O9+D0 (1), A3+A0+S0+C0+O0+D0 (1), A3+S0+C0+O9+D0 (1) |
|
S3 |
4 |
A0+S3+C0+O9+D0 (3), A0+S3+C0+O0+D0 (1) |
|
C3 |
12 |
A0+S0+C3+O0+D0 (9), A0+S0+C3+O9+D0 (2), A0+S0+C3+O9+D9 (1) |
|
A3+C3 |
2 |
A3+S0+C3+O9+D0 (2) |
|
S3+C3 |
1 |
A0+S3+C3+O0+D0 (1) |
|
Highest grade: 0 |
35 (47.30) |
|
|
Highest grade: 9 |
1 (1.35) |
|
|
Missing |
1 (1.35) |
|
|
TOTAL |
74 (100) |
|
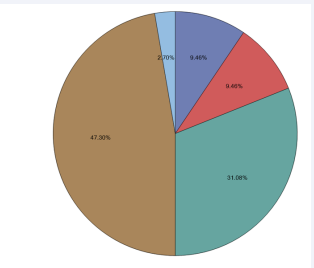
for patients with “Stroke of Undetermined etiology” based on TOAST (N=74). Each of the 74 patients’ complete ASCOD classifications can be found in Table 3. Seven (9.46%) patients had “1” (potentially causal) as the highest ASCOD grade, including 4 with atherosclerosis, 1 with cardiac pathology, 1 with other cause, and 1 with both cardiac pathology and other cause. Seven (9.46%) had “2” (causality is uncertain) as the highest ASCOD grade, including 1 with atherosclerosis, 2 with small-vessel disease, 2 with cardiac pathology and 2 with other causes. Twenty-three (31.08%) patients had “3” (unlikely causal but the disease is present) as the highest ASCOD grade, including 4 with atherosclerosis, 4 with small-vessel disease, 12 with cardiac pathology, 2 with both atherosclerosis and cardiac pathology and 1 with both small-vessel disease and cardiac pathology. Thirty-five (47.30%) patients had “0” (absence of disease) as the highest ASCOD grade. “Detail list of ASCOD” column shows complete ASCOD classifications for the corresponding patients. Figure 2 shows the pie chart of ASCOD highest grade,
Figure 2: Pie chart of ASCOD highest grade for patients with “Stroke of Undetermined etiology” based on TOAST (N=74).
for patients with “Stroke of Undetermined etiology” based on TOAST (N=74). Nearly 10% of patients with LVO-AIS who had “undetermined etiology” based on TOAST, were noted to have likely causal etiologies when they were categorized based on the ASCOD classification system.
DISCUSSION
The incidence of LVO-AIS in our young adult cohort was 18.7%, with cryptogenic stroke or “stroke of undetermined etiology” being the most common stroke etiology per TOAST classification system. Although this is lower than the LVO-AIS incidence of 24%-36% reported in adults 18-80 years of age, these results are consistent with prior studies of LVO-AIS in young adults and still constitute a significant proportion of AIS in young adults who otherwise are considered less likely to have typical risk factors for LVO-AIS [5,10,11-13]. The Athens stroke registry, involving 258 patients with first ischemic stroke less than 45 years of age, showed stroke etiology to be most commonly ‘undetermined’ (33.6%), followed by ‘other’ (25.6%), and cardioembolic (15.8%). Of those classified as ‘other’, cervical dissection represented 6.7% [8]. The Helsinki registry involved a larger patient population, 1008 patients, aged 15 to 49 years old with first ischemic stroke. Their analysis showed the most common etiology to be ‘other determined’ (26%), followed by ‘undetermined, extensive evaluation’ (22.4%), ‘cardio-embolism’ (19.6%), ‘small vessel occlusion’ (13.8%), and ‘large artery atherosclerosis’ (7.5%) [7]. A larger scale observational study involving pooled data from multiple European centers and 3331 patients (ages 18 to 49) with first-ever ischemic stroke from 1988-2010 showed the most classified etiology as undetermined (39.6%) followed by ‘other’ (22%), (of which cervical artery dissection was highly represented (12.8%)), small vessel occlusion (12.4%) and ‘large artery atherosclerosis’ (9%) [2].
Those with large artery atherosclerosis tended to be older compared to other TOAST categories (p=0.05) which is likely due to the rise of vascular risk factors and premature atherosclerosis in these young adults [4]. No significant differences were found in regard to race or gender with respect to TOAST etiology in our cohort, although previous data suggests that males are more likely to present with atherosclerosis [2]. Initial NIHSS differed significantly (p=0.019) with respect to TOAST etiology in those presenting with cardio-embolism or stroke of other determined etiology, which may be related to larger clot burdens in these
groups secondary to central embolism or large artery dissection. There was also a tendency for infarct core size to be larger in those labeled cardio-embolic, however when compared across groups this was not significant (p=0.5). The ASCOD classification for each TOAST category was congruent, with most scoring “1: potentially causal” in their respective categories. Those in the “stroke of other determined etiology” group were found to have potentially causal grading most heavily in the “dissection” category followed by “other causes”. In those labeled “stroke of undetermined etiology” by TOAST, their respective ASCOD classification showed that nearly 10% of those had etiologies that were classified as “potentially causal” and nearly 10% had etiologies in which “causality is uncertain”. The most common “potentially causal” classification in this group was in the “atherosclerosis” category, followed by “cardio-embolic” and “other causes”. This may suggest that the ASCOD classification system may prove beneficial in providing additional information on stroke etiology information in young patients with stroke of “undetermined etiology” by TOAST, as it offers greater insight into potentially causal stroke mechanisms not accounted for in the TOAST classification system.
Etiologies for ischemic stroke in young patients are known to be variable. Most studies utilize the TOAST criteria for classifying etiology of stroke occurring in young patients: ‘large artery atherosclerosis’, ‘cardio-embolism’, ‘small vessel occlusion’, ‘stroke of other determined etiology’, and ‘stroke of undetermined etiology’ [3,14]. Within the ‘other determined etiology’, entities such as non-atherosclerotic vasculopathies, inflammatory vasculitides, hematologic disorders, and hypercoagulable states exist [14]. Young patients are prone to various risk factors that are not as commonly seen in older individuals such as oral contraceptive use, substance abuse, pregnancy-related hypercoagulability disorder and paradoxical cardio-embolism in the setting of a patent foramen ovale. In addition, systemic genetic diseases, arteriopathies, and/or coagulopathy may manifest earlier in life leading to increased risk of stroke at an early age [12]. Given the myriad of conditions that can lead to ischemic stroke in the young, time to discovery of the exact etiology may be delayed when compared to older individuals.
While previous studies help elucidate the respective etiologies that are commonly seen in young adults, they are limited in their generalizability given that they are primarily representative of white, European populations. Our study provides further generalizability as the mid-western population of the United States is a mix of Caucasian, African American and Asian communities. Within certain stroke subtypes, such as those who presented with large vessel occlusion, research into etiology among young patients is limited. A subgroup analysis of the MR CLEAN database examining etiology for those ages 18-49 showed that embolic stroke of undetermined source (ESUS) represented 31%, carotid dissection 16%, cardio-embolism 15%, and large artery atherosclerosis 10% of all strokes. Younger patients also seemed to have improved outcomes compared to older groups [10].
Commonly seen etiologies for stroke in the young often include ‘other determined etiology.’ This can be attributed to cervical dissection incidence in young adults and complications of non-atherosclerotic vasculopathy and coagulopathy early in life. Cardio-embolism is also highly represented with provoking factors such as atrial fibrillation/flutter, cardiomyopathy, patent foramen ovale (PFO), and atrial septal aneurysm (ASA) [2,7,8]. While less frequent than in older individuals, large artery atherosclerosis remains an important consideration especially considering that vascular risk factors often increase with older age [2]. The etiology ‘undetermined’ with either complete or incomplete evaluation was highly represented as well in our study. This is likely due to the complex conditions that can occur in young patients, time needed for diagnosis, and misattribution of PFO/ASA to this classification [15]. Demographics and medical co-morbidities are evolving among young adults. Advances in diagnostic tools and genetic testing in the last decade may lead to more clarification for stroke etiology and decrease in time to diagnosis.
LIMITATIONS
Our study has several limitations. The data collected in our study was through retrospective chart review and therefore only available data in medical charts was used to confirm stroke etiology and classification based on TOAST and ASCOD classification systems. Stroke work up for all patients may not have been standardized and therefore those with incomplete evaluation or missing data may have been categorized as “undetermined” etiology therefore increasing the number of patients in this category. However, despite that, our results are similar to prior studies suggesting that one third of stroke in young adults is cryptogenic.
CONCLUSION
In conclusion, our study highlights that LVO is prevalent in young adults with AIS, however the most common etiology of LVO-AIS in these patients is cryptogenic. The ASCOD classification system may allow for additional insights into potentially causal stroke mechanisms in young adults presenting with LVO- AIS. Further studies are needed to better understand stroke mechanisms in young adults.
REFERENCES
- Feigin VL, Owolabi MO; World Stroke Organization–Lancet Neurology Commission Stroke Collaboration Group. Pragmatic solutions to reduce the global burden of stroke: a World Stroke Organization- Lancet Neurology Commission. Lancet Neurol. 2023; 22: 1160-1206.
- Yesilot Barlas N, Putaala J, Waje-Andreassen U, Vassilopoulou S, Nardi K, Odier C, et al. Etiology of first-ever ischaemic stroke in european young adults: The 15 Cities Young Stroke Study. Eur J Neurol. 2013; 20: 1431–1439.
- Maaijwee NA, Rutten-Jacobs LC, Schaapsmeerders P, van Dijk EJ, de Leeuw F-E. Ischaemic stroke in young adults: Risk factors and long- term consequences. Nat Rev Neurol. 2014; 10: 315–325.
- George MG, Tong X, Bowman BA. Prevalence of cardiovascular risk factors and strokes in younger adults. JAMA Neurol. 2017; 74: 695- 703.
- George MG. Risk Factors for Ischemic Stroke in Younger Adults: A Focused Update. Stroke. 2020; 51: 729-735.
- Burke JF, Skolarus LE. Are More Young People Having Strokes?-A Simple Question With an Uncertain Answer. JAMA Neurol. 2017; 74: 639-641.
- Putaala J, Metso AJ, Metso TM, Konkola N, Kraemer Y, Haapaniemi E, et al. Analysis of 1008 consecutive patients aged 15 to 49 with first-ever ischemic stroke: the Helsinki young stroke registry. Stroke. 2009; 40: 1195-1203.
- Spengos K, Vemmos K. Risk factors, etiology, and outcome of first- ever ischemic stroke in young adults aged 15 to 45 - the Athens young stroke registry. Eur J Neurol. 2010; 17: 1358-1364.
- Amarenco P, Bogousslavsky J, Caplan LR, Donnan GA, Wolf ME, Hennerici MG. The ASCOD Phenotyping of Ischemic Stroke (Updated ASCO Phenotyping). Cerebrovasc Dis. 2013; 36: 1-5.
- Brouwer J, Smaal JA, Emmer BJ, de Ridder IR, van den Wijngaard IR, de Leeuw FE, et al. Endovascular Thrombectomy in Young Patients With Stroke: A MR CLEAN Registry Study. Stroke. 2022; 53: 34-42.
- Sweid A, Hammoud B, Ramesh S, Wong D, Alexander TD, Weinberg JH, et al. Acute ischaemic stroke interventions: large vessel occlusion and beyond. Stroke Vasc Neurol. 2019; 5: 80-85.
- Malhotra K, Gornbein J, Saver JL. Ischemic strokes due to large- vessel occlusions contribute disproportionately to stroke-related dependence and death: a review. Front Neurol. 2017; 8: 651.
- Dozois A, Hampton L, Kingston CW, Lambert G, Porcelli TJ, Sorenson D, et al. PLUMBER study (prevalence of large vessel occlusion strokes in Mecklenburg County emergency response). Stroke. 2017; 48: 3397-3399.
- Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993; 24: 35-41.
- Ferro JM, Massaro AR, Mas JL. Aetiological diagnosis of ischaemic stroke in young adults. Lancet Neurol. 2010; 9: 1085-1096.