Indicators of High Morbidity and Poor Functional Outcome in Acute Ischemic Stroke Patients with Concurrent COVID-19
- 1. Department of Neurology, Rutgers Robert Wood Johnson University Hospital, USA
- 0. Authors contributed equally to the manuscript
Abstract
Objectives: Evidence suggests an association of increased cerebrovascular accidents frequency in patients diagnosed with the novel coronavirus disease, COVID-19. Coagulopathy resulting from the 2019 novel coronavirus (SARS-CoV-2) infection is suspected. The current study aims at evaluating inflammatory and thrombotic markers in relation to stroke severity and functional outcomes in a patient cohort of acute ischemic stroke (AIS) with concurrent COVID-19.
Materials and Methods: We performed a retrospective observational cohort study of 28 patients who tested positive for SARS-CoV-2 via polymerase chain reaction and concomitant AIS confirmed by brain imaging. We collected and analyzed data regarding initial stroke presentation, markers of coagulopathy, morbidity, and 90-day functional outcomes.
Results: The patient cohort had median NIHSS of 16 at initial presentation and median stroke volume of 52 mL. Median 90-day mRS was 4. Highest fibrinogen level recorded showed a median of 759.54 mg/dL, D-dimer and lactate dehydrogenase (LDH) showed a median of 12,463 ng/mL and 442 ng/mL closest to stroke symptoms onset, respectively. LDH (p=0.0008), D-dimer (p=0.001), and maximum fibrinogen levels (p=0.049) near the time of stroke significantly predicted final NIHSS and functional outcome 90-days after discharge.
Conclusions: Adult patients with AIS and concurrent COVID-19 disease exhibited abnormally high markers of inflammation and coagulopathy, and LDH, D-Dimer, and fibrinogen levels were predictors of morbidity and neurological disability at 90-days in this patient population. Further research is necessary to establish a definitive pattern and assess the ability to use these markers as prognostic elements of morbidity and mortality.
Keywords
• COVID-19, Cerebrovascular Accident, Acute Ischemic Stroke, Hypercoagulable State, Coagulation Markers, Inflammatory Markers
Citation
Iliceto A, Pisano TJ, Mehta DD, Rybinnik I (2022) Indicators of High Morbidity and Poor Functional Outcome in Acute Ischemic Stroke Patients with Concurrent COVID-19. J Neurol Disord Stroke 9(1): 1192.
INTRODUCTION
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) was declared a global pandemic in March 2020 by the World Health Organization [1]. The associated disease, coronavirus disease 2019 (COVID-19), was initially appreciated for causing severe respiratory symptoms, followed by extrapulmonary multiorgan involvement, including the brain [2].
Neurological COVID-19 encephalopathy, seizures, complications peripheral nervous include system involvement, and thromboembolic disease of both ischemic and hemorrhagic etiologies [3,4]. Acute ischemic strokes (AIS) account for one of the most common and feared neurological manifestations of COVID-19, including in the young age groups [5-8]. Evidence suggests that SARS-CoV-2 predisposes to severe coagulopathy resulting in a pro- thromboembolic state [4,9 11], with concurrent abnormal elevations in coagulation and inflammatory markers [12,13]. We sought to retrospectively correlate markers of coagulopathy with neurological outcomes in a cohort of patients admitted to a tertiary care center with documented AIS in the setting of active COVID-19 disease.
MATERIALS AND METHODS
Study population
We performed a retrospective observational cohort study of adult patients presenting to Rutgers Robert Wood Johnson University Hospital (New Brunswick, NJ, USA), a tertiary care comprehensive stroke center. Patients admitted between March 1, 2020 through February 28, 2021 were enrolled. The study was reviewed and approved by the Institutional Review Board (IRB) at Rutgers University and patient information and data was handled in compliance with institutional IRB guidelines. Inclusion criteria were age greater than 18 years, imaging- confirmed AIS with non-contrast Computed Tomography (NCCT) and/or Diffusion Weighted Imaging (DWI) MRI where available, and concurrent COVID-19 diagnosis by the CDC 2019- Novel Coronavirus (2019-nCoV) Real-Time RT PCR Diagnostic Panel. Fifty-five patients presented with clinical manifestations of AIS and COVID-19 in the time frame of the study among which 28 patients met inclusion criteria.
Exclusion criteria were 2019-nCoV RT PCR negativity despite clinical diagnosis of COVID-19, presence of intracerebral hemorrhage, absence of imaging-documented AIS, known coagulopathic disorder prior to the SARS-CoV-2 infection, and pregnancy.
Study design
Markers of inflammation and coagulopathy were collected at the time of stroke onset following institutional protocols for all patients included in the study as indicated in Table 2.
|
Table 2: Median and Interquartile Range (IQR) of stroke indicators, and biomarkers of inflammation and coagulopathy. |
|
|
Variable |
Median (IQR) |
|
Age |
66.50 (60-71.25) |
|
Stroke Volume (mL) |
52.24 (5.84-132.79) |
|
Initial NIHSS |
16.00 (6.75-24.50) |
|
NIHSS at 24 hours |
16.50 (6.75-22.25) |
|
Final NIHSS at discharge or death |
18.50 (3.75-28.00) |
|
90-days mRS |
4.00 (3.00-5.25) |
|
INR closest to stroke |
1.21 (1.11-1.32) |
|
PT closest to stroke (seconds) |
13.95 (12.68-15.50) |
|
PTT closest to stroke (seconds) |
28.00 (26.00-30.00) |
|
Fibrinogen closest to stroke (mg/dL) |
498.00 (351.00-364.00) |
|
Fibrinogen highest (mg/dL) |
759.54 (653.75-940.75) |
|
CRP closest to stroke (mg/dL) |
8.21 (5.43-17.73) |
|
LDH closest to stroke (IU/L) |
442.00 (277.00-545.50) |
|
D-dimer closest to stroke (ng/mL) |
12463.00 (3272.50-32752.00) |
|
D-dimer highest (ng/mL) |
24106.00 (6105.00-80165.00) |
|
ESR closest to stroke (mg/dL) |
113.50 (60.75-273.50) |
|
CPK closest to stroke (U/L) |
123.00 (98.00-326.00) |
|
Ferritin closest to stroke (ng/mL) |
792.00 (368.00-1196.00) |
|
Ferritin highest (ng/mL) |
1001.00 (599.00-1750.50) |
Fibrinogen was trended with serial collections separated on average by 12 hours over a period of 4 days (or until the time of expiration) from stroke symptom onset. Highest D-dimer and fibrinogen values during hospitalization were recorded. Clinical stroke severity was measured using the National Institutes of Health Stroke Scale (NIHSS) at initial presentation, 24 hours after the onset of stroke symptoms, and upon discharge [10,14]. Stroke volume was measured radiographically by a board certified stroke neurologist using ABC/2 method applied to the diffusion weighted imaging (DWI) MRI or NCCT [15-17]. Functional outcome at 90 days after discharge was documented for each patient using the modified Rankin Scale (mRS) [18,19].
Statistical analysis
Data analysis was performed in Python 3+ using Numpy [20] 1.18.1, SciPy [21], and Pandas [22] 1.0.1. Plotting was done with Matplotlib [23] 3.1.3 and Seaborn [24] 0.10.0. Receiver operating curve generation used Scikit-Learn [25] 0.22.1.
Coagulation markers collection were plotted against NIHSS, stroke volume, and 90-day mRS. Functional outcome classification performance, to independently predict good functional outcome (GFO), defined as mRS 0-2, was visualized using receiver operating characteristics (ROC). Confusion matrices with varying binary classification thresholds for each NIHSS predictor were used to generate ROCs [26]. Variables tested using the ROCs were analysis of initial NIHSS, LDH and D-dimer closest to stroke symptoms onset, and maximal fibrinogen value in relation to 90 day mRS.
RESULTS
Clinical Characteristics
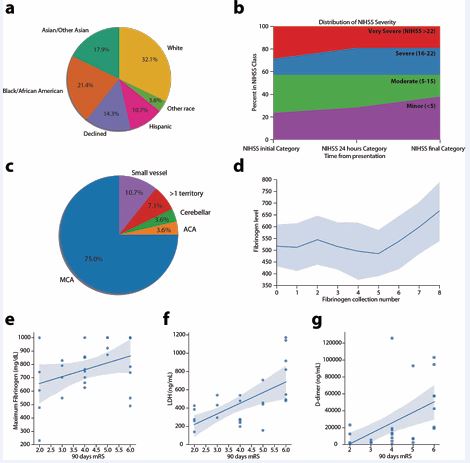
The study population comprised of 18 (64%) males and 10 females (36%) with a median age of 66 years (IQR 60-71.25 years). The ethnic background was heterogeneous with self reported demographics shown in Figure 1a.
Figure 1 (a) Distribution of patients self or family-reported race upon admission. (b) Initial NIHSS by severity. (c) Distribution of stroke locations. (d) Fibrinogen collections over time with serial collection every 4 hours from stroke symptom onset. (e) Linear regression of maximum fibrinogen level in relation to 90-day mRS. (f) Linear regression of LDH level closest to stroke in relation to 90-day mRS. (g) Linear regression of D-dimer level closest to stroke in relation to 90- day mRS.
22 patients (79%) had at least 1 vascular risk factor with the most common being hypertension (HTN), diabetes (DM), hyperlipidemia (HLD), and atrial fibrillation (Afib; Table 1). 18 patients (64.3%) had two or more vascular risk factors. 18 patients (64%) presented with symptoms of AIS on arrival to the hospital, while 10 patients (36%) presented with symptoms related to COVID-19 disease and subsequently developed stroke symptoms during the hospital admission (Table 1).
|
Table 1: Patient demographic, symptoms at presentation, AIS features, and interventions. |
|
|
Variable |
Number of patients (%) n = 28 |
|
Gender Female |
10 (35.7) |
|
Atrial Fibrillation |
3 (10.7) |
|
DM |
11 (39.3) |
|
HLD |
9 (32.1) |
|
HTN |
21 (75.0) |
|
Initial presentation COVID-19 symptoms |
11 (39.3) |
|
Initial presentation stroke symptoms |
18 (64.3) |
|
Cough |
8 (28.6) |
|
Diarrhea |
1 (3.6) |
|
Dyspnea |
14 (50.0) |
|
Fatigue |
1 (3.6) |
|
Fever |
7 (25.0) |
|
Generalized Weakness |
5 (17.9) |
|
Intervention IV tPA alone |
2 (7.1) |
|
Intervention Mechanical Thrombectomy alone |
3 (10.7) |
|
Intervention IV tPA + mechanical thrombectomy |
5 (17.9) |
Cerebrovascular Evaluation
Strokes were severe with median NIHSS score of 16 and interquartile range (IQR) of 17.5 at stroke symptom discovery (Table 2). 10 patients (35.7%) qualified for acute reperfusion therapies with 2 patients (7.1%) receiving only tissue plasminogen activator (tPA), 3 patients (10.7%) only clot retrieval via mechanical thrombectomy (MT), and 5 patients (17.9%) treated with both tPA and MT. 14 patients (50%) presented with an emergent large vessel occlusion (ELVO).
18 patients (64.3%) did not receive any reperfusion therapy. Major reasons for ineligibility were presentation outside of therapeutic window (15 patients) [26], large completed ischemic lesion on initial imaging defined as Alberta Stroke Program Early CT (ASPECT) [27] score below 6 (10 patients), absence of emergent large vessel occlusion (6 patients), and anticoagulation therapy and/or bleeding diathesis (6 patients). Median final ischemic core volume was 52.24 mL and IQR 126.94 mL. Middle cerebral artery (MCA) territory accounted for 75% of the stroke locations, while 7.1% of patients had strokes in multiple vascular territories (Figure 1c). Other vascular distributions involved included subcortical (11%), anterior cerebral artery (ACA) (3.6%), and cerebellum (3.6%) (Figure 1c). With respect to outcomes, only 5 patients (18%) achieved functional independence at 90 days defined as mRS score 0-2, and median mRS score was 4. 90-day mortality in this cohort was 25%.
Markers of coagulopathy
26 patients (93%) had fibrinogen collections above the normal range (reference range 200-400 mg/dL). Elevations in other markers were notable for 24 patients (86%) with an elevated LDH (reference range 125-220 International Units/Litre; IU/L) and 28 patients (100%) with an elevated D-dimer (reference range 0-500 ng/mL; Table 2).
71% of patients reached fibrinogen levels greater than 700 mg/dL, 5 of whom surpassed the upper limit of the quantification assay for fibrinogen with values greater than 1000 mg/dL. Trends of fibrinogen collection over time showed a gradual and significant increase in overall fibrinogen levels from stroke symptoms onset (Figure 1d).
A similar trend was observed for D-dimer, which showed a median value of 12,463 ng/mL at the time of stroke symptom discovery and a median value of 24,106 mg/mL representing the highest recorded value during the hospitalization (Table 2). The aggregate net elevation in D-dimer level of 14,765 ng/mL between the initial and peak D-dimer collections corresponded to an increase in serum level by 65.4% across the two time points.
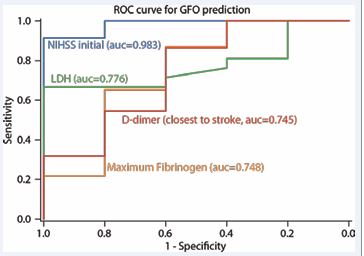
Independent linear regressions of coagulation markers with 90-day mRS showed a significant correlation of maximum LDH (p=0.0008), D-dimer (p=0.001) collected after stroke symptom discovery and highest recorded value, and maximum fibrinogen levels (p=0.049) during the hospitalization (Table 3). Of the aforementioned biomarkers, ROC analysis of LDH resulted be the highest predictor of GFO with area under the curve (AUC) of 0.776.
Among serial fibrinogen collections, highest fibrinogen level showed to be the most predictive of poor outcome both discharge and at 90 days with area under the curve (AUC) of 0.748 (Figure 2).
Figure 2 Test performance for predicting good functional outcomes (GFO). Receiver operator curves (ROC) to predict GFO (defined as mRS 0-2) in respect to LDH closest to stroke, D-dimer closest to stroke, and maximum fibrinogen values. Initial NIHSS as predictor of GFO used as control measure. Area under the curve calculations (AUCs) are included.
Initial NIHSS, which is a well validated predictor of GFO, was used as a quality control measure to ensure data reliability for the patient cohort with an AUC of 0.983. No significant correlation was observed between functional outcome and C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), creatinine, creatine phosphokinase (CPK), international normalized ratio (INR), prothrombin time (PT), or partial thromboplastin time (PTT) (Table 3).
|
Table 3: Linear regression of clinical and laboratory indicators with respect to 90-day mRS. Statistically significant values (p<0.05) are highlighted. |
|||
|
Variable |
r value |
p val |
R slope |
|
Age |
0.1679 |
0.393065 |
0.0221 |
|
Stroke Volume (mL) |
0.5589 |
0.001991 |
0.0057 |
|
NIHSS initial |
0.6943 |
4.16E-05 |
0.1027 |
|
NIHSS 24 hours |
0.7412 |
6.40E-06 |
0.1053 |
|
NIHSS final |
0.8983 |
8.82E-11 |
0.0891 |
|
INR closest to stroke |
0.3 |
0.120851 |
1.4083 |
|
PT closest to stroke (seconds) |
0.3105 |
0.107771 |
0.1265 |
|
PTT closest to stroke (seconds) |
-0.1625 |
0.427821 |
-0.0674 |
|
Fibrinogen closest to stroke (mg/dL) |
-0.1473 |
0.463589 |
-0.0009 |
|
Fibrinogen highest (mg/dL) |
0.3756 |
0.048859 |
0.0027 |
|
CRP closest to stroke (mg/dL) |
0.12 |
0.559385 |
0.0019 |
|
LDH closest to stroke (ng/mL) |
0.618 |
0.000767 |
0.0033 |
|
D-dimer closest to stroke (ng/mL) |
0.4941 |
0.008807 |
0 |
|
Creatinine level closest to stroke mg/dL) |
0.2664 |
0.198063 |
0.4283 |
|
ESR closest to stroke (mg/dL) |
0.0733 |
0.803392 |
0.0003 |
|
CPK closest to stroke (U/L) |
0.117 |
0.654603 |
0.001 |
|
Ferritin closest to stroke (ng/mL) |
0.3166 |
0.141014 |
0.0003 |
DISCUSSION
Patients with AIS and COVID-19 have high stroke severity both by clinical markers (NIHSS and mRS) and radiographic markers (median stroke volume), which is consistent with multinational cohorts and United States-based mortality database reviews [28,29]. The reasons for abnormally high morbidity and mortality in patients with AIS and COVID-19 are likely multifactorial. While atypical symptomatology, delayed presentation, and underlying vascular comorbidities are well- recognized poor prognostic markers, SARS-CoV-2 induced coagulopathy is also a concern.
An association between COVID-19, increased risk of hypercoagulability, and a high incidence of severe AIS has been reported [30,31]. Emerging literature suggests elevations in coagulation and inflammatory markers that are seen in patients with COVID-19 may trigger a coagulopathic cascade promoting clot formation and disease burden [32]. In our study, there is a clear early upward trend in fibrinogen on multiple serial collections over time as well as LDH and D-dimer after stroke symptom discovery. All patients had elevated D-dimer levels during their hospitalization. Additionally, elevated fibrinogen levels were recorded in 92.9% of our patients, and levels exceeding 700 mg/dL correlated with poor outcome. A predictive relationship was not observed between functional outcome and CRP, ESR, creatinine, CPK, INR, PT, or PTT levels. However, similar to other studies [32,33], elevated levels of ESR, CRP, CPK and ferritin were noted, suggestive of a pro-inflammatory state. Our findings correlate well with recently described cohorts by McAlpine et al. [33], and Goyal et al. [34].
This study has several limitations. Given retrospective observational methodology, there may be a significant selection bias, although outcomes noted in our study parallel those of larger multinational cohorts and mortality databases, suggesting that our patient population was reasonably representative28,29. Small sample size, lack of a control group and challenges in recognizing stroke onset and deriving the exact timing of biomarker values limits the strength of our correlations.
Our study calls attention to the possibility that inflammatory and coagulation markers may be independent predictors of outcome in patients with AIS and concurrent COVID-19. LDH and D- dimer levels were shown to be reliable predictor of poor outcomes in patients with intracerebral hemorrhage and acute ischemic stroke in the literature [35,36]. In our cohort, fibrinogen emerged as another possible biomarker relevant to prognosis in this patient population.
In our study, adult patients with AIS and concurrent COVID-19 disease exhibited abnormally high markers of coagulopathy, and LDH, D-Dimer, and fibrinogen levels were predictors of morbidity and neurological disability at 90-days in this patient population. This finding should be validated in future studies comparing COVID-19 patients with AIS to those with non-stroke complications. Also, more research is needed to define exact biomarker thresholds predictive of outcomes in patients with AIS and concurrent COVID-19 disease.
REFERENCES
- Cucinotta D, Vanelli M. WHO Declares COVID-19 a Pandemic. ActaBiomed. 2020; 91: 157-160.
- Zhou F, Yu T, Du R, Guohui Fan, Ying Liu, Zhibo Liu, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020; 395: 1054–1062.
- Mao L, Jin H, Wang M, Yu Hu, Shengcai Chen, Quanwei He, et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020; 77: 683-690.
- Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are
associated with poor prognosis in patients with novel coronavirus
- Pisano TJ, Hakkinen I, Rybinnik I. Large Vessel Occlusion Secondary to COVID-19 Hypercoagulability in a Young Patient: A Case Report and Literature Review. J Stroke Cerebrovasc Dis. 2020; 29: 105307.
- Tan Y-K, Goh C, Leow AST, Paul A Tambyah, Alicia Ang, Eng-Soo Yap, et al. COVID-19 and ischemic stroke: a systematic review and meta- summary of the literature. J Thromb Thrombolysis. 2020; 50: 587– 595.
- Parsay S, Vosoughi A, Khabbaz A, Sadigh-Eteghad S. The Incidence and Mortality Ratio of Ischemic Cerebrovascular Accidents in COVID-19 Cases: A Systematic Review and Meta-Analysis. J Stroke Cerebrovasc Dis. 2021; 30: 105552.
- Oxley TJ, Mocco J, Majidi S, Christopher P Kellner, Hazem Shoirah, I Paul Singh, et al. Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. N Engl J Med. 2020; 382: e60.
- Whyte CS, Morrow GB, Mitchell JL, Chowdary P, Mutch NJ. Fibrinolytic abnormalities in acute respiratory distress syndrome (ARDS) and versatility of thrombolytic drugs to treat COVID-19. J Thromb Haemost. 2020; 18: 1548–1555.
- Klok FA, Kruip MJHA, van der Meer NJM, MS Arbous, DAMPJ Gommers, KM Kant, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020; 191: 145-147.
- Helms J, Severac F, Merdji H, Anglés-Cano E, Meziani F. Prothrombotic phenotype in COVID-19 severe patients. Intensive Care Med. 2020. p. 1502–1503.
- Singhania N, Bansal S, Nimmatoori DP, Ejaz AA, McCullough PA, Singhania G. Current Overview on Hypercoagulability in COVID-19. Am J Cardiovasc Drugs. 2020; 20: 393–403.
- The ATTACC, ACTIV-4a, and REMAP-CAP Investigators, Lawler PR, Goligher EC, et al. Therapeutic anticoagulation in non-critically ill patients with Covid-19 [online]. bioRxiv medRxiv; 2021.
- Lyden P, Raman R, Liu L, Emr M, Warren M, Marler J. National Institutes of Health Stroke Scale certification is reliable across multiple venues. Stroke. 2009; 40: 2507–2511.
- Kothari RU, Brott T, Broderick JP, LR Sauerbeck, M Zuccarello, J Khoury, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996; 27: 1304–1305.
- Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993; 24: 987–993.
- Sims JR, Gharai LR, Schaefer PW, M Vangel, ES Rosenthal, MH Lev, et al. ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology. 2009; 72: 2104–2110.
- Rankin J. Cerebral Vascular Accidents in Patients over the Age of 60: II. Prognosis [online]. Scott Med J. 1957; 2: 200-15.
- van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988; 19: 604–607.
- Oliphant TE. Guide to NumPy. academia.edu; Epub 2006.
- Jones E, Oliphant T, Peterson P. SciPy: Open source scientific tools forPython. 2001.
- McKinney W, Others. Data structures for statistical computing in python. Proceedings of the 9th Python in Science Conference. Austin, TX; 2010. 51–56.
- Hunter JD. Matplotlib: A 2D Graphics Environment. Comput Sci Eng. IEEE Computer Society. 2007; 9: 90–95.
- Waskom M, Botvinnik O, Hobson P, et al. seaborn: v0.5.0 (November2014) [online]. 2014.
- Pedregosa F, Varoquaux G, Gramfort A, et al. Scikit-learn: Machine Learning in Python. J Mach Learn Res. 2011; 12: 2825–2830.
- Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019; 50: e344–e418.
- Mokin M, Primiani CT, Siddiqui AH, Turk AS. ASPECTS (Alberta Stroke Program Early CT Score) Measurement Using Hounsfield Unit Values When Selecting Patients for Stroke Thrombectomy. Stroke. 2017; 48: 1574–1579.
- Sharma R, Kuohn LR, Weinberger DM, Joshua L Warren, Lauren H Sansing, Adam Jasne, et al. Excess Cerebrovascular Mortality in the United States During the COVID-19 Pandemic. Stroke. 2021; 52: 563– 572.
- Mathew T, John SK, Sarma G, Raghunandan Nadig, Shiva Kumar R, Uday Murgod, et al. COVID-19-related strokes are associated with increased mortality and morbidity: A multicenter comparative study from Bengaluru, South India. Int J Stroke. 2021; 16: 429-436.
- Divani AA, Andalib S, Di Napoli M, Simona Lattanzi, M Shazam Hussain,José Biller, et al. Coronavirus Disease 2019 and Stroke: Clinical
Manifestations and Pathophysiological Insights. J Stroke Cerebrovasc
- Helms J, Tacquard C, Severac F, Ian Leonard-Lorant, Mickaël Ohana, Xavier Delabranche, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020; 46: 1089–1098.
- Zeng F, Huang Y, Guo Y, Mingzhu Yin, Xiang Chen, Liang Xiao, et al. Association of inflammatory markers with the severity of COVID- 19: A meta-analysis. Int J Infect Dis. 2020; 96: 467–474.
- McAlpine LS, Zubair AS, Maran I, Pola Chojecka, Paul Lleva, Adam S Jasne, et al. Ischemic Stroke, Inflammation, and Endotheliopathy in COVID-19 Patients. Stroke. 2021; 52: e233–e238.
- Goyal N, Sodani AK, Jain R, Ram H. Do Elevated Levels of Inflammatory Biomarkers Predict the Risk of Occurrence of Ischemic Stroke in SARS-CoV2 ?: An Observational Study. J Stroke Cerebrovasc Dis. 2021; 30: 106063.
- Chu H, Huang C, Dong J, Xiaobo Yang, Jun Xiang, Qiang Dong, et al. Lactate Dehydrogenase Predicts Early Hematoma Expansion and Poor Outcomes in Intracerebral Hemorrhage Patients. Transl Stroke Res. 2019; 10: 620–629.
- Zhang J, Liu L, Tao J, Yanlin Song, Yimeng Fan, Maling Gou, et al. Prognostic role of early D-dimer level in patients with acute ischemic stroke. PLoS One. 2019; 14: e0211458