Interhemispheric Transfer of Tactile Information in Individuals with Down’s syndrome
- 1. Department of Medicine and Surgery, University of Perugia, Italy
- 2. Neurologist, Perugia, Italy
Abstract
Introduction: A dysfunction of the process that leads to maturation of corpus callosum can determine an anomalous interhemispheric interaction and so justify the appearance of cognitive disorders. The present study attempted to investigate the possible relationship between the function of the corpus callosum and intellectual disability in subjects with Down’s Syndrome.
Materials and methods: Four groups of subjects were examined: eleven individuals with Down’s Syndrome, eleven individuals with intellectual disability not due to genetic abnormality, thirteen healthy individuals matched on the basis of chronological age, and thirteen healthy individuals matched on the basis of the degree of intellectual level. A fingertip cross-localization task has been used to measure the efficiency of the interhemispheric transfer of information: since transferring tactile information from one hemisphere to the other implies a significant loss of accuracy, the degree of difference between the uni-hemispheric and bi-hemispheric conditions of the finger localization task is considered an adequate measure of the functional efficiency of the transfer processes trough the corpus callosum.
Results: Subjects with Down’s syndrome show a significantly lower performance than all other participants: their loss of accuracy corresponds to 42.9%, whereas in healthy adult controls, it is 3.7%, in healthy children, it is 17.8% and in individuals with intellectual disability not due to genetic abnormalities, it is 33.3%.
Conclusion: Individuals with Down’s syndrome present a severe deficit of interhemispheric communication. The possibility is discussed that this disconnection syndrome may play a role in the genesis of cognitive disorders and intellectual disabilities.
KEYWORDS
- Corpus callosum
- Interhemispheric transfer
- Intellectual disability
- Down’s syndrome
CITATION
Piccirilli M, D’Alessandro P (2025) Interhemispheric Transfer of Tactile Information in Individuals with Down’s syndrome. J Neurol Disord Stroke 12(1): 1233.
INTRODUCTION
The role of the corpus callosum (CC) in determining cerebral lateralization and cognitive development has long been clearly established [1-3]. Its approximately 200 million fibers allow the exchange of information between the two cerebral hemispheres and their coordination and functional integration [4-6]. Since its anatomical and functional development occurs slowly and progressively over the first few years of life, a progressive improvement in the efficiency of interhemispheric information transfer and the consequent appearance of new cognitive and behavioral acquisitions can be observed during developmental age [7-9]. The length and complexity of this process can expose the CC to numerous pathological events and cause the alteration of the full maturation of the interhemispheric communication mechanisms which in turn can justify the onset of cognitive-behavioral disorders [10-12]. Extensive studies have identified abnormalities in the shape and volume of the CC in individuals with Down’s syndrome (DS), the most common of the chromosomal conditions associated with intellectual disability [13-16]. In the present study, an attempt was made to assess the functional efficiency of CC in subjects with DS.
For this purpose, we used the fingertip cross-localization test, a task involving tactile information transfer, in which subjects have to respond to a tactile stimulus presented to one hand using either the ipsilateral or contralateral hand [17,18]. Indeed, in the assessment of CC functions, it is essential tousetestswhosecorrect performance necessarily depends on the interhemispheric transfer of information. Therefore, the experimental design must include four conditions: (a) the right hemisphere both receiving the stimulus and responding (the right hemisphere uncrossed condition); (b) the left hemisphere both receiving the stimulus and responding (the left hemisphere uncrossed condition); (c) the right hemisphere receiving the stimulus and the left hemisphere responding (the right hemisphere crossed condition); (d) the left hemisphere receiving the stimulus and the right hemisphere responding (the left hemisphere crossed condition). The crossed conditions (c and d) require interhemispheric transfer of information because one hemisphere is presented with the stimulus that the other hemisphere has to use to correctly respond. The uncrossed conditions (a and b) do not require this transfer because both the stimulus and response are lateralized to the same hemisphere. The difference in performance between uncrossed and crossed conditions (CUD: crossed– uncrossed difference) can be measured in terms of time and/or accuracy. In theory, if perfect communication were to occur, there should be no difference between the crossed and uncrossed conditions. In reality, adult subjects exhibit a loss of accuracy ranging from 0 to 10% [19,20]. On the other hand, in patients with a complete disconnection (such as split-brain subjects), the CUD varies between 70 and 80%, i.e. performance reaches the level expected from a random response (which corresponds to 25%), while in patients with partial commissurotomy, performance depends on the extent and site of the callosal section [21- 24]. Ultimately, clinical and experimental data confirm that the degree of the loss of accuracy in the crossed condition (i.e. CUD) is inversely proportional to the transfer efficiency and that fingertip cross-localization task is a reliable measure of the ability of the CC to transfer information between the two cerebral hemispheres [20,25,26].
In this study, the fingertip cross-localization task was employed to examine the hypothesis that the interhemispheric transfer process is less efficient in individuals with DS. To this end, the performance of subjects with DS was compared with the performance of three control groups: healthy subjects of the same chronological age, healthy subjects of the same mental age, and subjects with intellectual disability of a similar degree but not attributable to DS.
MATERIALS AND METHODS
Subjects
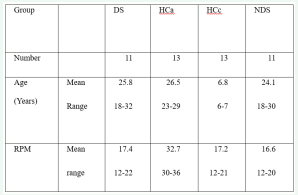
Eleven individuals, diagnosed with Down’s syndrome, living in residential care center for people with intellectual disabilities in Perugia, Italy, were selected as participants in this study according to the following criteria: male gender, right-handedness, sufficient sensorimotor skills to perform the task, and adequate cognitive level to cooperate with the examiner. Handedness was examined using the Edinburgh Inventory and intellectual level was tested using Raven’s Colored Progressive Matrices (RPM); to be eligible for the study, a subject had to perform at least seven tasks on the manual dexterity questionnaire with its right hand and to achieve a score of at least 12 points on the RPM [27,28].
Based on these same criteria, eleven subjects with intellectual disability that were not due to Down’s syndrome or to other genetic abnormalities (NDS subjects), were selected from the same residential care center. Subjects with focal brain lesions documented by CT and/or MRI, epileptic seizures, treatment with psychotropic drugs, were excluded.
Additionally, two groups of healthy subjects were examined. One of these groups was comparable in chronological age (HCa subjects), while the other was comparable according to intellectual level (HCc subjects). As a result, compared to individuals with DS, age-selected subjects presented a higher RPM score, whereas those selected on the basis of their RPM score had a lower chronological age.
None of the 48 study subjects had to undergo additional medical investigations; clinical information was obtained from the medical records of the residential center and the clinical interview of the control subjects and parents of the children examined.
Table 1 shows the main characteristics of the four groups of subjects participating in the study.
Table 1: Main characteristics of the study participants.
DS = subjects with Down’s syndrome; HCa = healthy control subjects with comparable chronological age; HCc = healthy control subjects with comparable mental age; NDS = subjects with intellectual disability not due to Down’s syndrome; RPM = Raven Coloured Progressive Matrices (maximum score = 36)
Procedure
The subjects were seated with their arms resting on a table and their hands placed facing each other, thumbs up. They looked at a blank screen so that their hands were out of view. The experimenter touched the tip of one of the four fingers with the pencil tip and asked the subject to then touch the same finger with the thumb of the same hand (uncrossed condition) or the corresponding finger of the other hand with the thumb of that opposite hand (crossed condition). The task was first demonstrated with the subject’s hand in full view until he understood what was required.
Ninety-six trials were performed, in series of twenty- four for each hand/task combination (right-to-right, right-to-left, left-to-left, and left-to-right). The sequences of trials for each condition were different. Each finger was stimulated the same number of times (12 times each for the four hand/task conditions). Subjects responded immediately after stimulation, and the examiner recorded each response on his/her answer sheet. No feedback was given concerning the accuracy of performance. All subjects first performed the trials of the uncrossed condition and then the trials of the crossed condition. The choice of hand on which the stimulation was performed was counterbalanced in all subjects.
The score was based on the number of correct responses (with 48 as the maximum value for each condition). The CUD (difference in the overall number of errors between uncrossed and crossed tasks) was calculated by using the following formula: (uncrossed test on the right hand − crossed test on the right hand) + (uncrossed test on the left hand − crossed test on the left hand).
Statistical analysis
A mixed ANOVA with Group [DS individuals, healthy controls compared for chronological age (HCa), healthy controls compared for intellectual level (HCc), individuals with intellectual disability not due to DS (NDS)] as the between factor and Hand (right vs. left) and Condition (uncrossed-hand vs. crossed-hand) as repeated measures was run using the number of correct responses.
RESULTS
Table 2 shows the percentage point obtained in each combination of hand/task by the four groups of subjects.
Table 2: Fingertip cross-localization task: mean correct scores (%) for each hand condition.
DS = subjects with Down’s syndrome; HCa = healthy control subjects with comparable chronological age; HCc = healthy control subjects with comparable intellectual level; NDS = subjects with intellectual disability not due to Down’s syndrome; df = difference between crossed and uncrossed tasks.
|
Hand |
|
right |
left |
both |
||||||
|
Condition |
|
UC |
CR |
df |
UC |
CR |
df |
UC |
CR |
df |
|
DS |
Mean |
87.1 |
46.4 |
40.7 |
89 |
43.9 |
45.1 |
88 |
45.2 |
42.9 |
|
SD |
5.9 |
16.9 |
11 |
7.4 |
14.8 |
7.4 |
6.6 |
15.8 |
9.2 |
|
|
HCa |
Mean |
99.3 |
94.8 |
4.5 |
98.2 |
95.3 |
2.9 |
98.8 |
95.1 |
3.7 |
|
SD |
13 |
7 |
5.7 |
2.7 |
4.5 |
2.3 |
1.7 |
5.7 |
4 |
|
|
HCc |
Mean |
98.7 |
79.2 |
19.5 |
98.7 |
82.7 |
16 |
98.7 |
80.9 |
17.8 |
|
SD |
1.6 |
9.7 |
8.1 |
1.8 |
7.3 |
5.5 |
1.7 |
8.5 |
6.8 |
|
|
NDS |
Mean |
87.9 |
56.4 |
31.5 |
88.8 |
53.8 |
35 |
88.3 |
55.1 |
32.2 |
|
SD |
6.7 |
12.3 |
5.6 |
7.5 |
13.9 |
6.4 |
7.1 |
13.1 |
6 |
|
There were a significant effect of the factors related to group [F(3,176)=131.1, p<0.0001], and task [F(1,176)=341.2, p<0.0001], and of the interaction between group and task [F(3,176)=46.9, p<0.0001]. The four groups differed significantly from each other in terms of the number of correct answers (DS=66.6, HCa=96.9, HCc=89.8, NDS=71.7). Post hoc analyses demonstrated the difference between the groups [DS vs HCa: F(1,94)=292.8, p<0.0001; DS vs HCc: F(1,94)=171.5, p<0.0001; DS vs NDS: F(1,86)=8.9, p<0.003; NDS vs HCa: F(1,94)=195.7, p<0.0001; NDS vs HCc: F(1,94)=99.5, p<0.0001; HCa vs HCc: F(1,102)=17.6, p<0.0001).
The crossed task was significantly different from the uncrossed task, regardless of which hand was used (uncrossed task = 93.7, crossed task = 70.1; F(1,190)=379.1, p<0.0001).
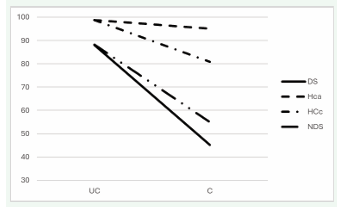
The task-group interaction indicated that the four groups differed from each other in relation to the scores obtained (Figure 1).
Figure 1: Hand/task interaction: The four groups of subjects differ from each other in terms of the scores obtained in the crossed and uncrossed tasks of the fingertip localization test. DS = subjects with Down’s syndrome; HCa = healthy control subjects with comparable chronological age; HCc = healthy control subjects with comparable mental age; NDS = subjects with intellectual disability not due to genetic abnormalities.
Crossed and uncrossed tasks present different difficulties for the four groups: the loss of accuracy was 42.9% for DS, 3.7% for HCa, 17.8% for HCc and 33.3% for NDS.
DISCUSSION
Our study attempted to evaluate the efficiency of interhemispheric communication in individuals with Down’s syndrome, as measured by the fingertip cross- localization task. Firstly, it is worth mentioning that this task has proven to be a reliable behavioral index of the functional effectiveness of interhemispheric communication. Since the scores obtained from the crossed test were significantly lower than the scores obtained from the uncrossed test, regardless of the hand used, the experimental paradigm suggests that the transfer of information from one hemisphere to the other implies a significant loss of accuracy. As documented in previous literature [17-26], transferring information between hemispheres involves a significant reduction in accuracy; therefore, the degree of difference between the uncrossed and crossed conditions can be considered an adequate measure of the functional efficiency of information transfer processes.
The main result of our study reveals that DS individuals exhibit considerable challenges in the tactile cross- localization task, suggesting a potential dysfunction in communication between the hemispheres. From this perspective, the behavior of these individuals can be likened to the interhemispheric disconnection syndrome of split- brain subjects that have undergone partial callosotomy [29,30]. In fact, DS subjects seem to correctly utilize just slightly more than half (57.1%) of the information coming from the contralateral hemisphere. Moreover, they show a significantly lower performance than all other participants: whereas the loss of accuracy in DS corresponds to 42.9, in healthy adult controls, it is 3.7%, in healthy children, it is 17.8% and in NDS, it is 33.3%.
Since control subjects with comparable chronological age had a much higher RPM score, subjects with an equivalent intellectual level were also examined, but the results showed that the efficiency of interhemispheric communication in Down syndrome is also lower than would be expected with regard to intellectual level. One has to consider that the callosal efficiency develops with age and that the typical performance of adult subjects is reached between the age of 10 and 13 [20,25]; according to data available in the literature, the degree of accuracy equivalent to the score obtained by DS is observed in healthy control subjects who are 4 years old [25,31,32]. Thus, in DS individuals, there does not appear to be a direct relationship between the degree of maturation of the interhemispheric commissures and intellectual level, as measured by RPM [33,34]. This lack of relationship between overall cognitive efficiency and CC dysfunction could suggest the existence of some compensatory mechanisms capable of overcoming the limitations imposed by imperfect communication between the two cerebral hemispheres. In this regard, agenesis of the CC could represent a useful model [29,35]: it is known that these individuals can present extremely variable intellectual development ranging from values in the normal range to scores indicating severe intellectual disability, and it has been theorized that this wide variability could be due precisely to the functional efficiency of compensation mechanisms [36-38]. These findings therefore suggest that alterations in the normal development of the CC and thus in the processes of hemispheric lateralization may only partially contribute to the cognitive deficits typical of DS [39]. On the other hand, the partial disconnection syndrome could also merely be an epiphenomenon of other factors involved in the genesis of intellectual disability.
Furthermore, although both DS and NDS subjects appear to be characterized by a deficit in the capacity to transfer information, our study also shows that there is a significant difference between these two groups. Callosal function appears to be especially compromised in DS subjects. One possible interpretation could be offered by the criteria used for selecting the sample examined. That is, it can be hypothesized that the presence of a callosal disconnection is not a constant characteristic among subjects with intellectual disability and that there are pathologies that lead to intellectual disability without presenting any impairment of callous dysfunction. In this light, neuroimaging studies comparing subjects with DS and with William syndrome have indeed led to these conclusions [40,41]. It is likely that, although not specific to DS, callosal dysfunction may be one of its salient features, whereas the degree of its impairment may be variable in other clinical conditions characterized by intellectual disability.
Nevertheless, it is imperative to recognize that the findings of our study cannot be generalized. Firstly, it is important to bear in mind that our population comprises only right-handed male individuals and that there is no agreement as to how these individual variables intervene in determining the pattern of cerebral organization [42,43]. Secondly, it is worthwhile to state explicitly that the demonstration of a deficit in the transfer of tactile information does not imply that there also exist deficits in the other functions carried out by the cerebral commissures. There is sufficient anatomical and physiological evidence to state that the CC is composed of several functionally distinct sub-units [44,45]. The maturation process of each of these functional sub-units occurs in different ways and according to different time frames [4,8]. Consequently, the use of different instruments to measure callosal function could lead to different results than the ones we obtained in our study [46,47]. In more general terms, it is important to consider that the CC is made up of nerve fibers that differ greatly in terms of type, caliber and myelination; so, the relationship between the anatomical and functional aspects of the CC cannot be established with certainty: for example, a larger size of the CC could indicate either greater or lesser interhemispheric connectivity, depending on the inhibitory or facilitatory function of its fibers [48].
Finally, a comment deserves the lower efficiency of DS and NDS in the uncrossed task compared to healthy controls. This could also suggest a functional alteration of the parietal structures, which are thought to be responsible for the ability to perform the tactile localization task [23]. In any case, the determining factor as far as this study is concerned seems to be represented by the existence of a significant difference between the uncrossed and crossed tasks. Therefore, even the possible presence of a parietal dysfunction would not change the interpretation of our data.
CONCLUSION
In conclusion, the results of our investigation suggest that DS subjects present a severe functional deficit of the corpus callosum. It is plausible to hypothesize that these defects of interhemispheric communication contribute to the cognitive anomalies of the syndrome [33,34]. However, further investigations are needed to gain a better understanding of the relationships between the etiopathogenesis of intellectual disability, the morphological and functional characteristics of the corpus callosum and individual cognitive and behavioral profiles. Specifically, it might be useful to establish whether similar interhemispheric anomalies are also present in individuals with a higher intellectual level than those analyzed in our sample [49-51]. Furthermore, an adequate recognition of the role of callosal dysfunction could inform specific rehabilitation strategies for subjects with intellectual disability.
ACKNOWLEDGEMENT
The authors would like to thank the staff of the Sereni Rehabilitation Centre in Perugia for their assistance and cooperation in conducting this study.
REFERENCES
- Gazzaniga MS. Forty-five years of split-brain research and still going strong. Nat Rev Neurosci. 2005; 6: 653-659.
- Gazzaniga MS. Cerebral specialization and interhemispheric communication: does the corpus callosum enable the human condition? Brain. 2000; 123: 1293-1326.
- Myers JJ, Sperry RW. Interhemispheric communication after section of the forebrain commissures. Cortex. 1985; 21: 249-260.
- Aboitiz F, Montiel J. One hundred million years of interhemispheric communication: The history of the corpus callosum. Braz J Med Biol Res. 2003; 36: 409-420.
- Hinkley LB, Marco EJ, Findlay AM, Honma S, Jeremy RJ, Strominger Z, et al. The role of corpus callosum development in functional connectivity and cognitive processing. PLoS One. 2012; 7: e39804.
- Schulte T, Müller-Oehring EM. Contribution of callosal connections to the interhemispheric integration of visuomotor and cognitive processes. Neuropsychol Rev. 2010; 20: 174-190.
- Johansen-Berg H, Della Maggiore V, Behrens TE, Smith SM, Paus TJ. Integrity of white matter in the corpus callosum correlates with bimanual co-ordination skills. Neuroimage. 2007; 36: T16-T21.
- Innocenti GM. The development of interhemispheric connections. Trends Neurosci. 1981; 4: 142-144.
- Finlayson MAJ. Behavioral manifestation of the development of interhemispheric transfer of learning in children. Cortex. 1976; 12: 290-295.
- Giedd JN, Blumenthal J, Jeffries NO, Rajapakse JC, Vaituzis C, Liu H, et al. Development of the human corpus callosum during childhood and adolescence: a longitudinal MRI study. Prog Neuropsychopharmachol Biol Psychiatr. 1999; 23: 571-588.
- Joseph R, Gallagher RE, Holloway W, Kahn J. Two brains one child: interhemispheric information transfer deficits and confabulatory responding in children aged 4, 7, 10. Cortex. 1984; 20: 317-331.
- Gazzaniga MS. Cognitive and neurological aspects of hemisphere disconnection in the human brain. Discuss Neurosci. 1987; 4: 52-53.
- Bull MJ. Down syndrome. N Engl J Med. 2020; 382: 2344- 2352.
- White NS, Alkire MT, Haier RJ. A voxel-based morphometric study of nondemented adults with Down syndrome. Neuroimage. 2003; 20: 393-403.
- Teipel SJ, Alexander GE, Schapiro MB, Möller HJ, Rapoport SI, Hampel H. Age-related cortical grey matter reductions in non-demented Down’s syndrome adults determined by MRI with voxel-based morphometry. Brain. 2004; 127: 811-824,
- Koenig KA, Oh SH, Stasko MR, Roth EC, Taylor HG, Ruedrich S, et al. High resolution structural and functional MRI of the hippocampus in young adults with Down syndrome. Brain Commun. 2021; 19: 3: fcab088.
- Galin D, Diamond R, Herron J. Development of crossed and uncrossed tactile localization on the fingers. Brain Lang. 1977; 4: 588-590.
- Piccirilli M, D’Alessandro P, Germani A, Boccardi V, Pigliautile M, Ancarani V, et al. Age-related decline in interhemispheric transfer of tactile information: The fingertip cross-localization task. J Clin Neurosci. 2020; 77: 75-80.
- Bogen JE. The Callosal Syndrome. In Heilman KM, Valenstein E, eds. Clinical Neuropsychology. New York: Oxford University Press. 1979: 295-338.
- Quinn K, Geffen G. The development of tactile transfer of information. Neuropsychologia. 1986; 24: 793-804.
- Geffen G, Nillson J, Quinn K, Teng EL. The effect of lesion of the corpus callosum on finger localization. Neuropsychologia. 1985; 23: 497-514.
- Bentin S, Sahar A, Moscovitch M. Intermanual information transfer in patients with lesions of the corpus callosum. Neuropsychologia. 1984; 22: 601-611.
- Satomi K, Kinoshita Y, Hirakawa S. Disturbances of cross-localization of fingertips in a callosal patient. Cortex. 1991. 27: 327-331.
- Caillé S, Sauerwein HC, Schiavetto A, Villemure JG, Lassonde M. Sensory and motor interhemispheric integration after section of different portions of the anterior corpus callosum in nonepileptic patients. Neurosurg. 2005; 57: 50-59.
- Galin D, Johnstone J, Nakell L, Herron J. Development of the capacity for tactile information transfer between hemispheres in normal children. Science. 1979; 204: 1330-1332.
- Piccirilli M, Palermo MT, Germani A, Bertoli ML, Ancarani V, Buratta L, et al. Music Playing and Interhemispheric Communication: Older Professional Musicians Outperform Age-Matched Non-Musicians in Fingertip Cross-Localization Test. J Int Neuropsychol Soc. 2021; 27: 282-292.
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia. 1971; 9: 97-113.
- Raven JC. Guide to using the Coloured Progressive Matrices. London: Lewis, 1965.
- Lassonde M, Jeeves MA. Callosal agenesis. A natural split-brain? New York: Plenum Press. 1994.
- Fabri M, Polonara G, Del Pesce M, Quattrini A, Salvolini U, Manzoni T. Posterior corpus callosum and interhemispheric transfer of somatosensory information: an fMRI and neuropsychological study of a partially callosotomized patient. J Cogn Neurosci. 2001; 13: 1071- 1079.
- Salamy A. Commissural transmission: maturational changes in humans. Science. 1978; 200: 1409-1411.
- O’Leary DS. A development study of interhemispheric transfer in children aged 5 to 10. Child Dev. 1980; 51: 743-750.
- Luders E, Narr KL, Bilder RM, Thompson PM, Szeszko PR, Hamilton L, et al. Positive correlations between corpus callosum thickness and intelligence. Neuroimage. 2007; 37: 1457-1464.
- Hutchinson AD, Mathias JL, Jacobson BL, Ruzic L, Bond AN, Banich MT. Relationship between intelligence and the size and composition of the corpus callosum. Exp Brain Res. 2009; 192: 455-464.
- Paul LK, Brown WS, Adolphs R, Tyszka JM, Richards LJ, Mukherjee P, et al. Agenesis of the corpus callosum: genetic, developmental and functional aspects of connectivity. Nat Rev Neurosci. 2007; 8: 287- 299.
- Brown WS, Paul LK. The neuropsychological syndrome of agenesis of the corpus callosum. J Int Neuropsychol Soc. 2019; 25: 324-330.
- Fischer M, Ryan SB, Dobyns WB. Mechanisms of interhemispheric transfer and pattern of cognitive functions in acallosal patients of normal intelligence. Arch Neurol. 1992; 49: 271-277.
- Lassonde M, Sauerwein H, Lepore F. Extent and limits of callosal plasticity: presence of disconnection symptoms in callosal agenesis. Neuropsychologia. 1995; 33: 989-1007.
- Lepore F, Ptito M, Jasper HH. Two Hemisphere-One Brain: Functions of the Corpus Callosum. New York: Liss ed; 1986
- Jernigan TL, Bellugi U. Anomalous brain morphology on Magnetic Resonance mages in William’s Syndrome and Down’s syndrome. Arch Neurol. 1990; 47: 529-533
- Wang PP, Doherty S, Hesselink JR, Bellugi U. Callosal Morphology Concurs with Neurobehavioral and Neuropathological Findings in Two Neurodevelopmental Disorders. Arch Neurol. 1992; 49: 407-411.
- Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989; 112: 799-835.
- Piccirilli M, Finali G, Sciarma T. Negative evidence of difference between right and left handers in interhemispheric transfer of information. Neuropsychologia. 1989; 27: 1023-1026.
- de Lacoste MC, Kirkpatrick JB, Ross ED. Topography of the human corpus callosum. J Neuropathol Exp Neurol. 1985; 44: 578-591.
- Fabri M, Polonara G. Functional topography of human corpus callosum: an FMRI mapping study. Neural Plast. 2013; 2013: 251308.
- Scally B, Burke MR, Bunce D, Delvenne JF. Visual and visuomotor interhemispheric transfer time in older adults. Neurobiol Aging. 2018; 65: 69-76.
- Poffenberger AT. Reaction time to retinal stimulation with special reference to time lost in conduction through nerve centers. Archs Psychol. 1912; 23: 1-73.
- van der Knaap LJ, van der Ham IJ. How does the corpus callosum mediate interhemispheric transfer? A review. Behav. Brain Res. 2011; 223: 211-221.
- Piccirilli M, D’Alessandro P, Mazzi P, Sciarma T, Testa A. Cerebral organization for language in Down’s Syndrome patients. Cortex. 1991; 27: 41-47.
- Channell MM, Mattie LJ, Hamilton DR, Capone GT, Mahone EM, Sherman SL, et al. Down Syndrome Cognition Project. Capturing cognitive and behavioral variability among individuals with Down syndrome: a latent profile analysis. J Neurodev Disord. 2021; 13: 16.
- Karmiloff-Smith A, Al-Janabi T, D’Souza H, Groet J, Massand E, Mok K, et al. The importance of understanding individual differences in Down syndrome. F1000Res. 2016; 5: F1000 Faculty Rev-389.