Multiple Intracranial Aneurysms-The PHASES Scores Applicability in 272 Patients Harboring a Total Number of 666 Multiple Intracranial Aneurysms
- 1. Department of Neurosurgery, Otto-von-Guericke University, Germany
- 2. Department of Simulation and Graphics, Otto-von-Guericke University, Germany
- 3. Research Campus STIMULATE, Germany
Abstract
Background: The subgroup of patients with multiple intracranial aneurysms (MIA) accounts for about 20% of all patients suffering from intracranial aneurysms (IA). There are controversial findings regarding a higher rupture risk in patients with MIA, leading to uncertainties in assessment of individual risk for aneurysm rupture. Our present study aimed to perform a further external validation by applying the PHASES Score of the largest IA retrospectively to 272 patients with a total number of 666 MIA from two different German neurovascular centers.
Methods: We analyzed 272 patients harboring a total number of 666 MIA regarding the accuracy of the PHASES Score for prediction of SAH using Receiver Operating Characteristics. Additionally, we analyzed the patients with aneurysmal subarachnoid hemorrhage (SAH) more closely.
Results: The PHASES Score achieved an Area under the curve of 0.57 in prediction of SAH in patients with MIA with corresponding sensitivity of 62% and specificity of 49%. We observed the following distribution of corresponding 5-year rupture risk in patients with aneurysmal SAH: <1% risk (38%), 1-1.9% risk (33%), 2-4.9% risk (19%) and >5% risk (10%).
Conclusion: The PHASES Score shows low sensitivity and specificity in discrimination of ruptured and unruptured IAs in the important subgroup of patients with MIA; attention should be paid to applying the PHASES Score as described in the original publication. Further investigation MIA and individualized rupture risk assessment using new approaches are needed. At present, the PHASES Score remains the best evaluated clinical score for all patients with IA.
Keywords
• PHASES Score
• Multiple intracranial aneurysms
• Rupture risk
• Subarachnoid hemorrhage
Citation
Swiatek VM, Amini A, Sandalcioglu Ortuño CE, Saalfeld S, Hartmann K, et al. (2022) Multiple Intracranial Aneurysms - The PHASES Scores Applicability in 272 Patients Harboring a Total Number of 666 Multiple Intracranial Aneurysms. J Neurol Disord Stroke 9(1): 1193.
INTRODUCTION
Intracranial aneurysms (IA) are defined as vascular dilatations of brain arteries. The rupture of these pathologies leads to subarachnoid hemorrhage (SAH), which is associated with a poor prognosis [1-3] and remarkable socioeconomic consequences, indicated by a 2.7% increase in disability-adjusted life years (DALY) over the last decade [4,5]. The improving quality and availability of neuroradiological examinations leads to growing numbers of detected IA as incidental findings [6,7] and to an increased necessity for reliable individual rupture risk assessment [8]. An emerging approach is the consideration of IA as a heterogeneous disease with numerous different subgroups [9] (e.g., giant IA, small IA, familial IA) and the associated implementation of specific rupture risk assessment by means of subgroup analyses [10-15].
Multiple intracranial aneurysms (MIA, ≥2 IA) are a highly relevant subgroup of patients with IA, accounting for 20% of all patients, according to the most recent review publication by Jabbarli et al, [16]. Previously reported studies revealed controversial results regarding a possibly higher rupture risk of MIA compared to singular IA, leading to uncertainties in treatment decision of these patients [17-21]. Although some studies have made attempts to develop a scoring system based on different regression analyses [22-25], the PHASES Score continues to be the only clinically established score which can be used in patients with MIA. The PHASES score is a clinical score for the assessment of the 5-year aneurysm rupture risk, which was determined based on a pooled analysis of six prospective studies including a total number of 8.382 patients with 10.272 IAs; among these patients, 1.060 (12.6%) had MIA. In order to estimate the 5-year risk for aneurysm rupture of a patient, the PHASES Score includes the analysis of the following six parameters: population, age,presence of arterial hypertension, SAH in the patients’ history, size and the site of the IA [26].
More recently, two studies have investigated the discrimination reliability of the PHASES Score in patients with MIA [27,28]. However, both studies undertook methodological modifications of the PHASES Score which should at least be critically discussed or introduced completely new scoring systems based on small patient cohorts without further validation. None of the studies had a specific focus on the calculation of the PHASES Score exclusively for the largest IA as described in the original publication. Therefore, our present study aimed to address this issue and perform a further external validation by applying the PHASES Score of the largest IA retrospectively to 272 patients with a total number of 666 MIA from two different German neurovascular centers.
MATERIALS AND METHODS
After obtaining the approval of the local ethics committee of the Hanover Medical School and the Otto-von-Guericke University, we retrospectively analyzed patients with MIA treated at our neurovascular centers in Hanover and Magdeburg between 2000 and 2018.
We included 272 patients harboring a total number of 666 IA with respect to the following inclusion criteria: diagnosis of at least two intradural IA and availability of all information required for calculation of the PHASES Score, including pre interventional imaging. The rupture status of the included MIA was obtained using intraoperative findings or neuroradiological imaging. The total number of patients with aneurysmal SAH was 180. Identification of the ruptured IA was performed based on intraoperative findings in 49 cases and imaging-based in 131 cases.
Patient Cohort
Within all patients, 74% were women and 16% were men. Mean age at diagnosis of our patient cohort was 56.1 years (Range: 19-93). 65% of all patients had arterial hypertension. In the cohort presented in this study, 64 patients were treated in Hanover and 208 in Magdeburg; 180 patients had suffered SAH, while in 92 patients MIA were diagnosed incidentally. The total number of IAs of the patients was distributed as follows: 188 patients had two IA, 59 patients had three IA, 14 patients had four IA, 9 patients had five IA and two patients had six IA. Overall, 152 IA were treated surgically, 309 IA endovascularly, four IA received multimodal treatment and 200 IA were not treated during the observation period examined in this study. The mean follow-up of our cohort was 35.3 months (Range: 0-300 months). Patients’ characteristics are shown in Table 1.
|
Table 1: Patients’ characteristics. |
||||
|
Patients’ characteristics |
N |
Percentage |
Hanover |
Magdeburg |
|
Male |
72 |
26% |
17 |
55 |
|
Female |
200 |
74% |
47 |
153 |
|
SAH |
180 |
66% |
29 |
151 |
|
No SAH |
92 |
34% |
35 |
57 |
|
Hypertension |
177 |
65% |
35 |
142 |
|
No Hypertension |
95 |
35% |
29 |
66 |
|
Age <70 years |
230 |
86% |
54 |
176 |
|
Age ≥70 years |
42 |
14% |
10 |
32 |
|
Localization of the largest IA |
||||
|
Internal carotid artery |
62 |
23% |
13 |
49 |
|
Middle cerebral artery |
89 |
33% |
19 |
70 |
|
Others |
121 |
44% |
32 |
89 |
|
Number of IA per patient |
||||
|
2 IA |
188 |
69% |
52 |
136 |
|
3 IA |
59 |
22% |
7 |
52 |
|
4 IA |
14 |
5% |
0 |
14 |
|
5 IA |
9 |
3% |
4 |
5 |
|
6 IA |
2 |
1% |
1 |
1 |
|
Total number of IA |
666 |
|
|
|
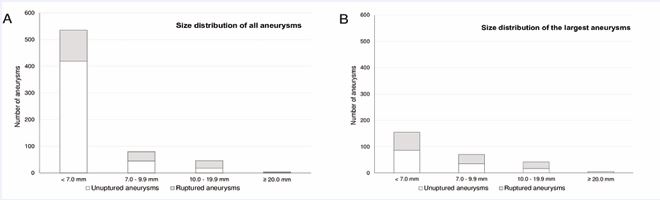
Distribution of aneurysm size of the IA in our cohort was as follows: 535 IA (80%) had a size <7.0mm (419 unruptured; 116 ruptured), 80 IA (12%) had a size between 7.0 and 9.9mm (45 unruptured; 35 ruptured), 46 IA (7%) had a size between 10.0 and 19.9mm (18 unruptured; 28 ruptured) and five IA (1%) had a size ≥ 20.0mm (one unruptured; four ruptured). The most common aneurysm localization was the middle cerebral artery (n = 230; 35%), followed by the internal carotid artery (n = 175; 26%), the anterior cerebral artery including the anterior communicating artery and the pericallosal artery (n = 114, 17%) and the basilar artery (n = 54; 8%). The exact size distribution for all IA and the largest IA in a patient are shown in Figure 1.
Figure 1 A- Size distribution of all ruptured and unruptured IA; B- Size distribution of all largest IA of a patient.
Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics Version 26. In this study, the PHASES Score was approached as a diagnostic test for aneurysm rupture prediction. We explicitly point out that this procedure did not allow for the inclusion of the cumulative risk of aneurysm rupture. Receiver Operating Characteristics (ROC) was used for determining the discrimination reliability of the PHASES Score for prediction of SAH in patients with MIA. For the calculation of the PHASES Score, all patients were treated as patients with incidentally diagnosed IAs to simulate a patient consultation at a neurovascular center. Therefore, no value was assigned to the parameter “earlier SAH” in the PHASES Score for all patients, regardless of the patient’s rupture status, as this has been assumed to be unknown at the time of the patient’s presentation. For the further analysis, the rupture status of the patient was defined as the endpoint of interest. In case of the application of the PHASES Score to a patient with MIA in clinical practice, Greving et al., prescribe a calculation of the parameters for the IA with the largest diameter in order to estimate the highest possible individual rupture risk [29]. Therefore, we analyzed the IA with the largest diameter with respect to its PHASES Score, regardless of its known rupture status. An illustration of an illustrative case and the selection of the IA to be calculated in our study can be found in Figure 2.
Figure 2 Demonstration of an illustrative case and the calculation of the PHASES Score in this study. All necessary parameters for the calculation of the PHASES Score were retrospectively obtained and the size of all IAs of the patient was determined. The largest aneurysm of the patient was then identified and included in the analysis (highlighted here in grey or red). (ICA = internal carotid artery, MCA = middle cerebral artery).
The predictive value of the PHASES Score was described by the Area under the curve (AUC) of the obtained ROC-curve. Unruptured IA was identified as such at the time of patients’ admittance to hospital. Since most of these patients directly underwent treatment instead of just being kept under observation, there is no information on the natural progression of these IA, which might result in some ruptured IA being classified as unruptured IA. For this reason, we performed a closer analysis of the patients with confirmed aneurysmal SAH in equivalence to previously published studies in order to avoid bias due to unknown natural progression of the unruptured IA. The distribution of PHASES Score values and corresponding 5-year risk for aneurysm rupture were identified and visualized.
RESULTS
A total number of 272 patients harboring 666 MIA were enrolled in this study. All patients were Europeans and underwent treatment or consulted for clinical advice at our neurovascular centers in Hanover and Magdeburg. Among the patients examined, 180 suffered SAH due to aneurysm rupture. In a total number of 46 (26%) of these 180 patients, the largest IA was not the cause of the SAH. In 43 (24%) patients, the largest IA analyzed did not achieve the highest number of points in the PHASES Score and therefore did not indicate the highest possible potential rupture risk of the patient.
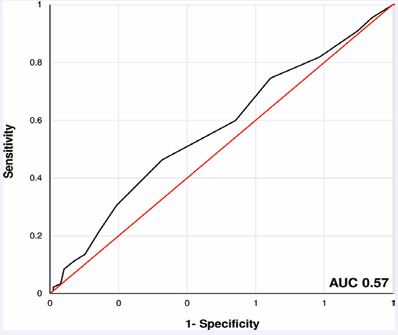
The statistical analysis revealed an AUC of 0.57 (95% confidence interval: 0.5 - 0.64) for the PHASES Score in prediction of SAH in patients with MIA (Figure 3).
Figure 3 ROC - curve showing the precision of the PHASES Score for prediction of SAH in patients with MIA. In our cohort, an AUC of 0.57 was achieved (sensitivity 62% and specificity 49% using a cut-off at 4.5 PHASES Score values).
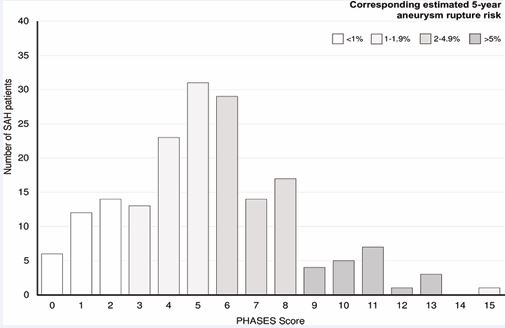
The maximum values for sensitivity (62%) and specificity (49%) were achieved at an optimal threshold value for classification in patients with and without SAH of a PHASES Score of 4.5. Closer analysis of the 180 patients with SAH showed that 128 (71%) of these patients had a PHASES Score of 0-6 points with an estimated 5 year rupture risk of <2%. In addition, we observed the following distribution of corresponding 5-year rupture risk in our patient cohort with patients suffering from aneurysmal SAH: 38% of all patients (n = 68) had a 5-year rupture risk of <1%, 33% of all patients (n = 60) had a 5-year rupture risk of 1-1.9%, 19% of all patients (n = 35) had a 5-year rupture risk of 2-4.9% and 10% of all patients (n = 17) had a 5-year rupture risk of >5% (Figure 4).
Figure 4 Distribution of PHASES Score values of 180 patients with SAH and MIA. Visualization of the corresponding 5-year aneurysm rupture risk which showed that 38% of all patients had a risk of 5%.
DISCUSSION
The PHASES Score was introduced to provide a manageable and reliable score in advising patients with IA, including patients harboring MIA by prescribing the score calculation for the largest IA [29]. Our results show that the classification of patients with MIA into the categories “patient with SAH” and “patient without SAH” by the PHASES Score achieved an AUC of 0.57. Further analysis of the ROC–curve revealed a sensitivity (62%) and specificity (49%). In addition, the deeper analysis of patients with SAH from MIA in our cohort showed that 71% would have been estimated with a 5-year rupture risk of <2%.
Two recently published studies already evaluated the discrimination reliability of the PHASES Score between ruptured and unruptured IAs [27,28]. In general, the AUCs achieved by the PHASES Score for the discrimination between ruptured and unruptured IAs obtained by these studies are consistent with the results reported in this study (Feng et al.: AUC = 0.57 and Neulen et al.: AUC = 0.75 vs. AUC = 0.57) [27,28]. However, these studies undertook methodological modifications of the PHASES Score or introduced completely new scoring systems based on small patient cohorts without further validation.
Feng et al., analyzed various modifications of the PHASES Score in a patient cohort of 701 patients harboring 1.673 IA. The modifications of the PHASES Score were carried out in relation to the following aspects: aneurysm-based calculation of the PHASES Score, calculation of the highest PHASES Score, calculation of the sum PHASES Score of all IA and calculation of the mean PHASES Score of all IAs [27,28]. In this context, it is important to point out those Greving et al., already evaluated whether such modifications of the PHASES Score are beneficial for the prediction of rupture probability. An aneurysm-based analysis was performed, and the results were the same as for the patient-based analysis, therefore a patient-based approach was finally chosen [26]. During the prospective follow-up of 29.166 person-years studied, aneurysm rupture occurred in only 230 patients; in 220 of these patients, the largest IA was proven to be the source of SAH, proving that in these patients the largest IA was at highest risk of rupture [26. Thus, it can be assumed that in most patients the calculation of the largest aneurysm also leads to the identification of the highest risk of rupture.
Interestingly and in accordance to previously published results [27,30-32], the analysis of our patient cohort showed a high amount of ruptured MIA with a diameter of <7.0 mm. The size of ruptured IA has decreased by 15% in the last 20 years and the average size of ruptured IAs decreased from 10.1 mm to 6.6mm [10,32]. In addition, many studies stated that in 12.5 29% the largest IA is not the one to cause SAH in patients with MIA [27,33,34], which is consistent with our data collected here. However, these results are all based on retrospective investigations and therefore have only limited significance compared to the PHASES Score.
Neulen et al., performed a statistically highly extensive analysis of the PHASES Score, the UIATS Score and a new scoring system in a patient cohort of 40 patients with a total of 101 MIA [26,28,35]. They were able to show that the PHASES Score estimated a low 5-year rupture risk in a larger proportion of unruptured IA than of the ruptured IAs [28]. The achieved AUC of the PHASES Score and the UIATS Score were 0.75 and 0.54, respectively [28]. Aneurysm size was found to the most robust predictor of aneurysm rupture in this small patient cohort [28]. As a result of these unfavorable results achieved by these PHASES and the UIATS Score, a modification of the UIATS Score was made by adding aneurysm-specific factors [28]. This approach causes concern since the UIATS Score was not designed for predicting the aneurysm rupture risk, but to provide a recommendation for or against aneurysm treatment. Furthermore, its design is not based on prospective data, but on a Delphi consensus of numerous experts in the field of cerebrovascular disease [35].
It becomes clear that the application of the PHASES Score in clinical practice is challenging, especially for the specific subgroup of patients with MIA, as the retrospective application of the score does not provide us with convincing results. A potential approach, which has been introduced by several studies, to further evolve the rupture risk assessment of patients with MIA as the most frequent subgroup of patients with IA, could be to develop regression models based on multiple factors [22,24,36]. These alternative approaches may also be able to reflect the cumulative rupture risk of patients more accurately with MIA.
Therefore, the need for further investigation of the rupture risk assessment of MIA is needed. Either present models do not address the specifics of the subgroup of IA, or the conception of the model is considered too complex for the application in daily clinical evaluation of patients. Several advanced analytical techniques and new imaging modalities, such as Computational Fluid Dynamics and high - resolution Magnetic Resonance Imaging, provide the opportunity to gain a better understanding of the pathophysiology leading to rupture of MIA and consequently to improve the prediction accuracy of aneurysm rupture [35-37].
LIMITATIONS
However, the following limitations need to be pointed out and considered for the interpretation of this study’s results: First, the determination of the ruptured IA in patients with MIA is always challenging and may be subject to a certain degree of uncertainty. This must be highlighted especially for those cases where the ruptured IA has not been verified by intraoperative findings, which was the case in 73% of the here enrolled patients. Furthermore, this retrospective study investigating the PHASES Score’s applicability in patients with MIA is not able to include the cumulative rupture risk of IA, which is why the aspect of increasing ruptures risk over the lifetime of a patient included by the PHASES Score has not been reflected. Further analysis of this aspect needs to be done on a prospective cohort under consideration of possible ethical conflicts and potentially resulting biased data.
CONCLUSION
Retrospectively, the PHASES Score shows low sensitivity and specificity in discrimination of ruptured and unruptured IAs in the important subgroup of patients with MIA. However, when using the score retrospectively, attention should be paid to applying it as described in the original publication while avoiding modifications. These findings underline the importance of further investigation of this subgroup and the need for more individualized rupture risk assessment. The use of new analytical techniques in investigation of IA might present a possible approach towards more reliable prediction of aneurysms rupture. At present, the PHASES Score remains the best evaluated clinical score for all patients with IA.
ETHICS APPROVAL
Ethical approval was waived by the local Ethics Committees of the Hanover Medical School and the Otto-von-Guericke University in view of the retrospective nature of the study and all the procedures being performed were part of the routine care.
AUTHORS’ CONTRIBUTIONS
BN, VMS, IES, CAD and KPS contributed to the study conception and design. Literature search and data collection were performed by VMS, BN, AA and CESO. Data analysis was performed by VMS, BN, SS, AR, MS and KH; writing of the first manuscript draft was performed by BN and VMS. All authors commented on previous versions of the manuscript and approved the final manuscript.
- Data acquisition: VMS, BN, AA, CESO
- Data analysis: VMS, BN, SS, AR, MS, KH
- Conception and design of study: BN, VMS, IES, CAD, KPS
- Writing of manuscript: BN, VMS
REFERENCES
- Backes D, Vergouwen MDI, Velthuis BK, van der Schaaf IC, Bor ASE, Algra A, et al. Difference in Aneurysm Characteristics Between Ruptured and Unruptured Aneurysms in Patients With Multiple Intracranial Aneurysms. Stroke. 2014; 45: 1299–303.
- Bender MT, Wendt H, Monarch T, Beaty N, Lin L-M, Huang J, et al. Small Aneurysms Account for the Majority and Increasing Percentage of Aneurysmal Subarachnoid Hemorrhage: A 25-Year, Single Institution Study. Neurosurgery. 2018; 83: 692–9.
- Berg P, Beuing O. Multiple intracranial aneurysms: a direct hemodynamic comparison between ruptured and unruptured vessel malformations. Int J Comput Assist Radiol Surg. 2018; 13: 83–93.
- Björkman J, Frösen J, Tähtinen O, Backes D, Huttunen T, Harju J, et al. Irregular Shape Identifies Ruptured Intracranial Aneurysm in Subarachnoid Hemorrhage Patients With Multiple Aneurysms. Stroke. 2017; 48: 1986–9.
- Broderick JP, Brown RD, Sauerbeck L, Hornung R, Huston J, Woo D, et al. Greater rupture risk for familial as compared to sporadic unruptured intracranial aneurysms. Stroke. 2009; 40:1952–7.
- Brown RD, Broderick JP. Unruptured intracranial aneurysms: epidemiology, natural history, management options, and familial screening. Lancet Neurol. 2014; 13:393–404.
- Burns JD, Huston J, Layton KF, Piepgras DG, Brown RD. Intracranial aneurysm enlargement on serial magnetic resonance angiography: frequency and risk factors. Stroke. 2009; 40:406–11.
- Dengler J, Heuschmann PU, Endres M, Meyer B, Rohde V, Rufenacht DA, et al. The rationale and design of the Giant Intracranial Aneurysm Registry: a retrospective and prospective study. Int J Stroke. 2011; 6: 266–70.
- Detmer FJ, Chung BJ, Mut F, Slawski M, Hamzei-Sichani F, Putman C, et al. Development and internal validation of an aneurysm rupture probability model based on patient characteristics and aneurysm location, morphology, and hemodynamics. Int J Comput Assist Radiol Surg. 2018; 13:1767–79.
- Etminan N, Brown RD, Beseoglu K, Juvela S, Raymond J, Morita A, et al. The unruptured intracranial aneurysm treatment score: a multidisciplinary consensus. Neurology. 2015; 85: 881–9.
- Feng X, Tong X, Chen J, Peng F, Niu H, Xia J, et al. External Validation of the PHASES Score in Patients with Multiple Intracranial Aneurysms. J Stroke Cerebrovasc Dis Off J Natl Stroke Assoc. 2021; 30: 105643.
- Gabriel RA, Kim H, Sidney S, McCulloch CE, Singh V, Johnston SC, etal. Ten-year detection rate of brain arteriovenous malformations in alarge, multiethnic, defined population. Stroke. 2010; 41: 21–6.
- GBD 2016 Lifetime Risk of Stroke Collaborators, Feigin VL, Nguyen G, Cercy K, Johnson CO, Alam T, et al. Global, Regional, and Country- Specific Lifetime Risks of Stroke, 1990 and 2016. N Engl J Med. 2018; 379: 2429–37.
- Greving JP, Wermer MJH, Brown RD, Morita A, Juvela S, Yonekura M, et al. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. Lancet Neurol. 2014; 13: 59–66.
- Guan J, Karsy M, Couldwell WT, Schmidt RH, Taussky P, MacDonald JD, et al. Factors influencing management of unruptured intracranial aneurysms: an analysis of 424 consecutive patients. J Neurosurg. 2017; 127: 96–101.
- Hilditch CA, Brinjikji W, Tsang AC, Nicholson P, Kostynskyy A, Tymianski M, et al. Application of PHASES and ELAPSS scores to ruptured cerebral aneurysms: how many would have been conservatively managed? J Neurosurg Sci. 2021; 65: 33-37.
- Huttunen T, von und zu Fraunberg M, Frösen J, Lehecka M, Tromp G, Helin K, et al. Saccular intracranial aneurysm disease: distribution of site, size, and age suggests different etiologies for aneurysm formation and rupture in 316 familial and 1454 sporadic eastern Finnish patients. Neurosurgery. 2010; 66: 631–8.
- Jabbarli R, Dinger TF, Darkwah Oppong M, Pierscianek D, Dammann P, Wrede KH, et al. Risk Factors for and Clinical Consequences of Multiple Intracranial Aneurysms: A Systematic Review and Meta- Analysis. Stroke. 2018; 49: 848–55.
- Jing L, Fan J, Wang Y, Li H, Wang S, Yang X, et al. Morphologic and Hemodynamic Analysis in the Patients with Multiple Intracranial Aneurysms: Ruptured versus Unruptured. PloS One. 2015; 10: e0132494.
- Juvela S, Poussa K, Lehto H, Porras M. Natural history of unruptured intracranial aneurysms: a long-term follow-up study. Stroke. 2013; 44: 2414–21.
- Kashiwazaki D, Kuroda S, Sapporo SAH Study Group. Size ratio can highly predict rupture risk in intracranial small (<5 mm) aneurysms. Stroke. 2013; 44: 2169–73.
- Matsumoto K, Oshino S, Sasaki M, Tsuruzono K, Taketsuna S, YoshimineT. Incidence of growth and rupture of unruptured intracranial aneurysms followed by serial MRA. Acta Neurochir (Wien). 2013; 155: 211–6.
- Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002; 360: 1267–74.
- Neulen A, Pantel T, König J, Brockmann MA, Ringel F, Kantelhardt SR. Comparison of Unruptured Intracranial Aneurysm Treatment Score and PHASES Score in Subarachnoid Hemorrhage Patients With Multiple Intracranial Aneurysms. Front Neurol. 2021; 12: 616497.
- Neyazi B, Sandalcioglu IE, Maslehaty H. Evaluation of the risk of rupture of intracranial aneurysms in patients with aneurysmal subarachnoid hemorrhage according to the PHASES score. Neurosurg Rev. 2019; 42: 489–92.
- Neyazi B, Swiatek VM, Skalej M, Beuing O, Stein K-P, Hattingen J, et al. Rupture risk assessment for multiple intracranial aneurysms: why there is no need for dozens of clinical, morphological and hemodynamic parameters. Ther Adv Neurol Disord. 2020; 13: 1756286420966159.
- Nieuwkamp DJ, Setz LE, Algra A, Linn FHH, de Rooij NK, Rinkel GJE.Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol. 2009; 8: 635–42.
- Pagiola I, Mihalea C, Caroff J, Ikka L, Chalumeau V, Iacobucci M, et al. The PHASES score: To treat or not to treat? Retrospective evaluation of the risk of rupture of intracranial aneurysms in patients with aneurysmal subarachnoid hemorrhage. J Neuroradiol 2020; 47: 349-352.
- Rinkel GJE. Intracranial aneurysm screening: indications and advice for practice. Lancet Neurol. 2005; 4: 122– 8.
- Rutledge C, Jonzzon S, Winkler EA, Raper D, Lawton MT, Abla AA. Small Aneurysms with Low PHASES Scores Account for Most Subarachnoid Hemorrhage Cases. World Neurosurg. 2020; 139: e580–4.
- Samaniego EA, Roa JA, Hasan D. Vessel wall imaging in intracranial aneurysms. J Neurointerventional Surg. 2019; 11: 1105–12.
- Strother CM, Jiang J. Intracranial aneurysms, cancer, x-rays, and computational fluid dynamics. AJNR Am J Neuroradiol. 2012; 33: 991–2.
- Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol. 2011; 10: 626–36.
- Wiebers DO, Whisnant JP, Huston J, Meissner I, Brown RD, Piepgras DG, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003; 362: 103–10.
- Xiang J, Tutino VM, Snyder KV, Meng H. CFD: computational fluid dynamics or confounding factor dissemination? The role of hemodynamics in intracranial aneurysm rupture risk assessment. AJNR Am J Neuroradiol. 2014; 35: 1849–57.
- Xiang J, Natarajan SK, Tremmel M, Ma D, Mocco J, Hopkins LN, et al. Hemodynamic-morphologic discriminants for intracranial aneurysm rupture. Stroke. 2011; 42: 144–52.
- Yasui N, Suzuki A, Nishimura H, Suzuki K, Abe T. Long-term follow-up study of unruptured intracranial aneurysms. Neurosurgery. 1997; 40: 1155–9.