Racial Differences in Small Vessel Disease Measured in MRI of Ischemic Stroke/TIA Patients
- 1. Department of Neurology, University of South Carolina School of Medicine, USA
Abstract
Background: White matter hyperintensities (WMH) and cerebral microbleeds (CMB) are manifestations of cerebral small vessel disease (SVD). We investigated the racial differences in SVD among African American (AA) and white patients with stroke/TIA.
Methods: Magnetic resonance imaging (MRI) of brain in consecutive patients were assessed in four years as part of a study. Deep and periventricular WMHs (DWMHs and PVWMHs, respectively) were rated visually on axial fluid-attenuated inversion recovery (FLAIR) sequence using the 3-point Fazekas scale and categorized into none/mild (grades 0 and 1) or moderate/severe (grades 2 and 3). Gradient echo imaging was used to categorize CMBs for number, location, and topography. Patient demographics were collected including vascular risk factors, periodontal disease (PD), and index event (TIA, stroke, and stroke subtype). Univariate (t-tests for continuous variables, and X2 test for categorical variables) and multivariable analyses (multiple logistic regression) were conducted to assess the association between race and SVD.
Results: A total of 861 patients, 469 AA and 360 white, were compared for moderate/severe PVWMH, DWMH, and CMB ≥5. White patients were older (67±11 vs. 60±13, p <0.001) and more likely to be male (60% vs. 52%, p = 0.02). AA patients were more likely to be hypertensive (91% vs. 85%, p = 0.006), diabetic (49% vs. 40%, p = 0.009), and have moderately severe gum disease (55% vs. 36%, p <0.001). AA race was associated with PVWMH (Adjusted OR 1.83, 95% 1.32-2.55) adjusted for age, gender, hypertension, diabetes, and PD. AA race was associated with DWMH (Adjusted OR 1.5, 95% 1.08-2.1) adjusted for age, hypertension, diabetes, and PD. AA race was associated with CMB ≥5 (Adjusted OR 3.93, 95% 1.08-14.36) adjusted for age and hypertension.
Conclusion: We report an independent association between AA race and various manifestations of SVD including moderate/severe DWMH, PVWMH, and CMB ≥5 in stroke/TIA patients.
KEYWORDS
- Racial Differences; Small Vessel Disease; White matter hyperintensities; Magnetic resonance imaging; Cerebral microbleeds
CITATION
Martin C, Pikel K, Wood S, Logue M, Mascari R, et al. (2025) Racial Differences in Small Vessel Disease Measured in MRI of Ischemic Stroke/ TIA Patients. J Neurol Disord Stroke 12(1): 1234.
INTRODUCTION
In 2020, ischemic stroke became the fifth leading cause of death in the US, accounting for more than 160,000 deaths in 2020 alone [1]. The highest proportion of strokes occur in the Southeastern US “stroke belt” with notable concentrations in Alabama, Mississippi, Georgia, and South Carolina, among other southeastern states [2]. Previous studies have established that stroke incidence and stroke- related mortality rates are higher in African American populations when compared to their white counterparts [3,4]. In an analysis of a biracial metropolitan population over 1.3 million people located in the stroke belt, Kissela et al. [5], demonstrated that AAs less than 65 years of age had two to five times greater risk of stroke than whites. Likewise, Boan et al. [6], showed that younger AAs, particularly those 45-65 years of age, had significantly greater risk of stroke and stroke-related hospitalizations than whites (50.4% vs. 29.6%, respectively). Potential explanations for these disparities in stroke risk and race-ethnicity lie within differences in the prevalence of cerebral small vessel disease (SVD) amongst the two races.
SVD is a multisystem disorder that consists of a range of pathological processes affecting the small arteries, arterioles, and capillaries of various organ systems. In relation to the brain, cerebral SVD specifically involves small subcortical cerebral arteries. SVD is a major contributor to cognitive dementia and cerebral infarction and is the primary cause of small vessel ischemic stroke [7]. Radiologically, SVD manifests as white matter hyperintensities (WMHs) and cerebral microbleeds (CMBs). WMHs represent areas in cerebral white matter that have undergone demyelination, axon loss, and gliosis; these areas appear as hyperintense lesions on T2-weighted MRI. CMBs represent areas of fixed hemorrhages, which indicate past microhemorrhages, and appear as rounded hypointense lesions with associated blooming on T2- weighted MRI [8].
Few studies have investigated the association between SVD and racial disparities among African American (AA) and white stroke/TIA patients. Koch et al. [9], reported significant differences in the proportion of AA and Caribbean blacks who suffered ischemic stroke due to small vessel infarction when compared to non-Hispanic whites. In a study of 194 patients with recent small vessel infarction, the proportion of CMBs was highest in Caribbean blacks and AA (38% and 18%, respectively) and lowest in non-Hispanic whites (7%). Similarly, the South London Ethnicity and Stroke Study [10], found that among 600 black and 600 white stroke patients, small vessel disease was more prevalent in black patients than whites, independent of conventional vascular risk factors. More recently, a study by Nyquist et al. [11], concluded that AA patients had significantly higher odds of having extreme deep white matter volume burden (OR 1.9, 95% 1.08-3.5) as compared with other races, contributing to the disparity in SVD prevalence among racial-ethnic groups. However, none of these studies examined the potential association of periodontal disease (PD).
Our study hopes to confirm these findings as well as investigate the effect of periodontal disease (PD) as a potential factor promoting atherosclerosis in AA. The primary purpose of this study was to confirm the association of SVD and racial disparities amongst patients with stroke/TIA admitted to a tertiary stroke center located in the stroke belt. A secondary purpose of this study was to investigate if the associations were independent of stroke risk factors and PD.
METHODS
This study is a post-hoc cross-sectional analysis of an NIH-funded randomized clinical trial called PREMIERS [12]. The STROBE guidelines were used to ensure the reporting of this observational study [13]. The PREMIERS trial investigated effects of standard versus intense periodontal treatment on the risk of recurrent vascular events among ischemic stroke and TIA patients admitted to tertiary stroke centers. This cross-sectional analysis looked at stroke/TIA patients admitted to a stroke center located in the stroke belt and assessed for any correlations between small vessel disease and racial disparity. The data presented in this study were collected as part of the PREMIERS study. Patients were consented as part of the study approved by the Prisma Health Institutional Review Board (Pro00044329).
Study Population / Demographics
Informed consent was obtained from each patient before enrollment in the study. Patient demographics were collected and used as comparison of baseline characteristics for both AA and white patients. Any race/ ethnicity other than white or AA was excluded from the study. The proportion of individuals with existing vascular risk factors, including age, gender, hypertension, diabetes mellitus, hyperlipidemia, smoking status, and periodontal disease, were assessed. Patients were considered to have hypertension iftheywerecurrently or previouslydiagnosed with hypertension, had consistent blood pressure readings of 130/80 mm Hg or higher, or if they were on medications for hypertension. Patients were considered diabetic if they were on medication for the management of type II diabetes mellitus or had a HbA1c greater than 6.5%. Patients were considered to have hyperlipidemia if they had a known history of hyperlipidemia, had an LDL of 100 mg/dL or greater, or were on a statin. To assess presence and severity of periodontal disease, oral health was examined using several measures: plaque index, gingival index, bleeding on probing, probing depth, and cementoenamel junction [12].
SVD—WMHs and CMBs
To determine presence and severity of SVD, MRI scans of consecutive ischemic stroke/TIA patients admitted to a tertiary stroke center were assessed across a four-year period as part of the PREMIERS trial [12]. MRIs were performed using a Siemens 1.5 T (Symphony Tim B17; Siemens Healthcare, Erlangen, Germany) or GE 1.5 T (Discovery 450; MXR Imaging, San Diego, California, USA) machine, and imaging sequences included T2-weighted gradient echo imaging and fluid attenuated inversion recovery sequence (FLAIR) [14]. All patients’ imaging data were independently reviewed by readers who were blinded to the clinical data and the follow-up. We assessed both periventricular and deep WMHs using the axial FLAIR sequence and the three-point Fazekas scale. The location, shape, size, and number of lesions were recorded for each patient. Patients were then dichotomized into having none/mild disease, which represented Fazekas grades zero through one, or moderate/severe disease, which represented Fazekas grades two through three. CMBs were assessed using the T-2 weighted gradient echo imaging sequence. CMBs were defined according to the criteria published by Greenberg et al [8]: lesions were round or ovoid in shape, up to 10 mm in diameter, and appeared as hypointense lesions with associated blooming on T2-weighted MRI. Lesions located both in the cortex and subcortex were assessed, noting the number, location, and topography of each lesion. Previous studies have suggested that experiencing five or more microbleeds is a significant risk factor for cerebral hemorrhage [15]. For this reason, data regarding CMB obtained as part of the SVD assessment in this study were analyzed by dividing patients into two groups: those with less than five CMBs and those with five or more CMBs.
Fazeka scale and FLAIR images
WMHs as evidenced on T2-weighted gradient echo imaging were assessed using the three-point Fazekas scale. The Fazekas scale quantifies the amount of white matter lesions that appear as hyperintense foci in T2- weighted gradient echo imaging. The Fazekas scale allows for quantification of lesions both in the periventricular white matter (PVWM) and deep white matter (DWM), which are rated separately. Periventricular WMHs were rated according to the following parameters: zero = absent; one = caps or pencil-thin lining; two = smooth “halo”; three = irregular periventricular signaling extending into DWM. Deep WMHs were rated according to the following parameters: zero = absent; one = punctate foci; two = beginning confluence of foci; three = large confluent areas of foci [16]. Fazekas grades were then matched with severity of white matter disease, with grades zero and one classified as none/mild disease and grades two and three classified as moderate/severe disease.
Statistical analysis
To compare the baseline characteristics between races, student t-tests were performed to test continuous variables, and chi-squared tests were performed to compare categorical variables. Univariate analysis was used to determine the crude odds ratio of the incidence of SVD manifestations by race. Multivariable analysis was used to determine the adjusted odds ratio of the incidence of SVD manifestations by race. The multivariable odds ratio was adjusted for vascular risk factors, including age, gender, hypertension, diabetes mellitus, hyperlipidemia, and smoking status. Odds ratios were computed using multiple logistic regression. We used IBM SPSS version 28.0 (IBM, Chicago, IL) to conduct all statistical analyses.
RESULTS
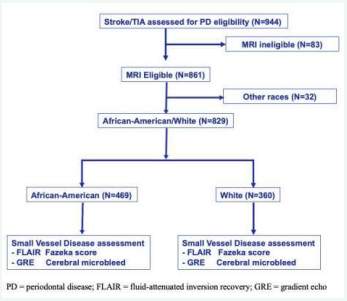
A total of 944 patients met inclusion criteria for enrollment in the study, 861 of whom were MRI eligible. Patients identifying with a race/ethnicity other than white or AA were excluded from the study. A total of 829 patients were included in data analysis, 360 of whom were white and 469 of whom were AA (Figure 1).
Figure 1: STROBE diagram for eligibility and analysis
Comparison of baseline characteristics by race is displayed in Table 1.
Table 1: Baseline characteristics, grouped by race.
|
Risk factor |
African American N = 469 |
White N = 360 |
p-value |
|
Age |
61±13 |
68±11 |
<0.001 |
|
Male |
237 (51%) |
217 (60%) |
0.005 |
|
Hypertension |
430 (92%) |
305 (85%) |
0.002 |
|
Diabetes |
236 (50%) |
145 (40%) |
0.004 |
|
Hyperlipidemia |
332 (71%) |
260 (72%) |
0.69 |
|
Smoking |
133 (28%) |
85 (24%) |
0.12 |
|
Periodontal disease |
260 (55%) |
129 (36%) |
<0.001 |
|
Coronary artery disease |
103 (22%) |
96 (27%) |
0.12 |
|
Atrial fibrillation |
60 (13%) |
70 (19%) |
0.009 |
|
Index Event |
|||
|
TIA |
62 (13%) |
55 (15%) |
0.40 |
|
Stroke Subtype LAA Cardioembolic Small vessel occlusive Others Cryptogenic |
73 (18%) 70 (17%) 145 (40%) 9 (2%) 110 (27%) |
57 (19%) 60 (20%) 95 (31%) 11 (4%) 81 (27%) |
0.58 |
|
Moderate-severe SVD |
|||
|
PVWMH |
179 (38%) |
119 (33%) |
0.06 |
|
DWMH |
147 (31%) |
103 (29%) |
0.25 |
|
CMB ≥5 |
19 (4%) |
4 (1%) |
0.008 |
TIA = transient ischemic attack; LAA = large artery atherosclerosis; PVWMH= periventricular white matter hyperintensities; DWMH = deep white matter hyperintensities; CMB = cerebral microbleeds
White patients are older at onset of stroke/TIA (60±13 vs. 67±11, p<0.001) and are more likely to be male (52% vs. 60%, p = 0.02). AA patients are more likely to be younger at onset of stroke/TIA (60±13 vs. 67±11, p<0.001). AA patients are also more likely to suffer from vascular risk factors and comorbid conditions, including hypertension (91% vs. 85%, p = 0.006), diabetes mellitus (49% vs. 40%, p = 0.009), and PD (55% vs. 36%, p<0.001). There is no significant difference between race and other vascular risk factors, including hyperlipidemia and smoking status. Regarding index event, there is no significant difference in in white versus AA patients when comparing TIA, stroke, and stroke subtype. When comparing the incidences of different manifestations of SVD and race, we found a significant association between having at least five CMBs and AA race. Moderate-severe PVWMD and DWMD hyperintensities were more prevalent in AA patients, but there was not a significant association between these manifestations and race.
As noted in Table 2a and Table 2b,
Table 2a: Association between various manifestations of SVD and race as measured using crude odds ratio (univariate analysis).
|
Moderate-severe SVD |
Crude OR AA vs. White race |
95% CI |
p-value |
|
PVWMH |
1.32 |
0.99-1.78 |
0.06 |
|
DWMH |
1.20 |
0.88-1.62 |
0.25 |
|
CMB ≥ 5 |
3.93 |
1.32-11.65 |
0.01 |
PVWMH = periventricular white matter hyperintensities; DWMH = deep white matter hyperintensities; CMB = cerebral microbleeds
Table 2b: Association between various manifestations of SVD and race as measured using adjusted odds ratio (multivariable analysis).
|
|
Adjusted OR* AA vs. White race |
95% CI |
p-value |
|
PVWMH |
2.04 |
1.46-2.86 |
<0.001 |
|
DWMH |
1.69 |
1.20-2.36 |
0.002 |
|
CMB ≥ 5 |
4.44 |
1.43-13.77 |
0.01 |
*Adjusted for age, gender, hypertension, diabetes, hyperlipidemia, smoking PVWMH = periventricular white matter hyperintensities; DWMH = deep white matter hyperintensities; CMB = cerebral microbleeds
univariate analysis of SVD manifestations by race revealed a significant association between having at least five CMBs and AA race when analyzing the crude data (crude OR 3.82, 95% CI 1.10-13.31). Multivariable analysis of SVD manifestations by race revealed a significant association between all manifestations of moderate-severe SVD and AA race, after adjusting for vascular risk factors. When assessing odds ratio, AA patients had significantly higher odds of having moderate-severe PV and DWM manifestations (PV Adj. OR 1.83, 95% 1.32-2.55; DWM Adj. OR 1.51, 95%1.08-2.10) after adjusting for age, gender, hypertension, diabetes mellitus, hyperlipidemia, smoking status, and PD. Additionally, AA patients had significantly higher odds of having at least five CMBs (Adj. OR 3.93, 95% 1.08-14.36) after adjusting for the same vascular risk factors. These results suggest that AA race is independently associated with SVD parameters.
DISCUSSION
Our analyses demonstrate that AA race is independently associated with various vascular risk factors and manifestations of SVD in patients with ischemic stroke/ TIA. Having five or more CMB is significantly associated with African American race in stroke/TIA patients, independent of age, hypertension, and other known vascular risk factors. However, when adjusting for these same vascular risk factors, both moderate/severe PVWMH and moderate/severe DWMH become significantly associated with African American race in stroke/TIA patients.
In our study, we found some important differences in baseline risk factors, which may explain the racial disparity in SVD. AA were significantly younger at stroke onset compared to whites and were more likely to suffer from comorbid diseases, including hypertension and diabetes. These findings concur with those of previous studies, which demonstrated that AA are younger at stroke onset [18,19], and that the prevalence of SVD risk factors, including hypertension and diabetes, were significantly higher in AA compared to whites [17,20]. This suggests that SVD occurs at a younger age in AA patients, which may explain why AA patients are younger at stroke onset.
Few previous studies have investigated racial-ethnic differences in small vessel disease, particularly differences in WMH and CMB burden. In 2001, the Northern Manhattan Stroke Study (NOMASS) [21], found that stroke risk factors, including hypertension and atrial fibrillation, were more prevalent in AA patients. The increased prevalence of these risk factors contributes to the disparity seen in ischemic stroke and mortality rates among different racial-ethnic groups. A similar study by Castello et al. [22], investigated the association between racial-ethnical differences and MRI-defined cerebral SVD in patients who experienced intracerebral hemorrhage. Minority patients had greater global cerebral SVD (p = 0.011) and hypertensive arthropathy burden (p = 0.021) on MRI than white patients. This corroborates our findings that there are racial-ethnical differences which underly cerebral SVD severity.
Our study also showed a significantly higher prevalence of PD as measured by tooth loss in AA patients residing in the stroke belt. This correlates with findings of previous studies investigating the increased risk of PD in AA [23, 24]. The REGARDS study [25], demonstrated that the chance of tooth loss, a marker of PD, was 1.5 times greater in AA than whites. Patients with significant tooth loss had higher levels of systemic inflammatory markers, including C-reactive protein (CRP) and white blood cell (WBC) count. These patients also had higher rate of stroke (OR 1.28, 95% 1.09-1.49). More recently, Arabi et al. [26], reviewed the association between periodontitis and SVD-associated cerebrovascular disorders and suggested systemic inflammation as a possible mediator. The increased prevalence of PD may also be an explanation for increased chances of SVD in AA.
Some literature suggests that WMH burden only partially explains why AA patients experience different manifestations of cerebral SVD, including post-stroke cognitive impairment [27,28]. A recent study by El Husseini et al. [29], suggests that the prevalence of cognitive impairment secondary to small vessel stroke is increased in AA patients because of an alteration in systemic immune function. Both cognitive function and serum inflammatory/ endothelial dysfunction biomarkers were measured at least six weeks post-stroke in a small cohort of AA patients. Of the biomarkers analyzed, chronic elevation of vascular cadherin adhesion molecule-1 (VCAM-1) was independently associated with a decline in post-stroke cognitive function and an increase in WMH volume. These findings support previous evidence which demonstrated that serum VCAM-1 levels remain elevated in AA patients for at least six weeks after lacunar infarct [30]. These chronic elevations in VCAM-1 suggest that VCAM-1 plays a role in modifying the response at the endothelial level in response to vascular risk factors. These findings indicate that AA patients experience a prolonged and ineffective immune resolution during the chronic phase of stroke recovery.
Our study is not without limitations. First, this study was a secondary analysis of trial participants within PREMIERS; therefore, the generalizability to the US population may be reduced. Second, although the study took steps to measure PD, there may be other unmeasured confounders, such as socioeconomic background. Finally, the data presented only reflect those of either white or AA race/ethnicity. We are therefore unable to extrapolate the data to other races. However, our study differs from previous trials in unique ways. First, periodontal disease was included as an additional vascular risk factor for ischemic stroke. The data presented in this study were collected as part of the screening process for the PREMIERS trial [12], which prospectively investigated the effect of periodontal treatment on recurrent stroke risk. According to the CDC [31], severe periodontal disease is most common in Mexican Americans and non-Hispanic Black adults. This substantiates the idea that periodontal disease may be an additional factor contributing to SVD. Our study adds to the much-needed body of evidence that suggests known differences in SVD across racial-ethnic groups or geography [32].
CONCLUSIONS
In summary, our study demonstrates that the prevalence of cerebral SVD is higher in AA patients with prior stroke/ TIA versus their white counterparts. AA patients are more likely to suffer from moderate-severe white matter disease and cerebral microbleeds, resulting in increased risk for ischemic stroke. This may be explained partially by an increased prevalence of vascular risk factors and periodontal disease, leading to endothelial dysfunction and ineffective immune responses. Future studies could be aimed at investigating the association between racial- ethnical differences and post-stroke cognitive impairment.
REFERENCES
- Murphy SL, Kochanek KD, Xu JQ, Arias E. Mortality in the United States, 2020. NCHS Data Brief, no 427. Hyattsville, MD: National Center for Health Statistics. 2021.
- Centers for Disease Control and Prevention. Stroke death rates, total population 35 and older. Centers for Disease Control and Prevention. 2022.
- Gillum RF. Stroke in blacks. Stroke. 1988; 19: 1-9.
- Carnethon M, Pu J, Howard G, Albert M, Anderson C, Bertoni A, et al. Cardiovascular Health in African Americans: A Scientific Statement From the American Heart Association. Circulation. 2017; 136: e393-e423.
- Kissela B, Schneider A, Kleindorfer D, Khoury J, Miller R, Alwell K, et al. Stroke in a biracial population. Stroke. 2004; 35: 426-431.
- Boan AD, Feng WW, Ovbiagele B, Bachman DL, Ellis C, Adams RJ, et al. Persistent racial disparity in stroke hospitalization and economic impact in young adults in the buckle of stroke belt. Stroke. 2014; 45: 1932-1938.
- Berry C, Sidik N, Pereira AC, Ford TJ, Touyz RM, Kaski JC, et al. Small- vessel disease in the heart and Brain: Current knowledge, unmet therapeutic need, and future directions. J Am Heart Assoc. 2019: 8.
- Greenberg S, Vernooij MW, Cordonnier C, Viswanathan A, Salman R, Warach S, et al. Cerebral Microbleeds: A Field Guide to their Detection and Interpretation. Lancet Neurol. 2009; 8: 165-174.
- Koch S, Gupta R, McClendon MS, Romano JG. Racial-Ethnic Differences in Lacunar Infarction in a Multiethnic Stroke Population. J Stroke Cerebrovasc Dis. 2013; 22: 107-112.
- Markus H, Khan U, Birns J, Evans A, Kalra L, Rudd A, et al. Differences in stroke subtypes between black and white patients with stroke: the South London Ethnicity and Stroke Study. Circulation. 2007; 116: 2157-2164.
- Nyquist PA, Bilgel MS, Gottesman R, Yanek LR, Moy TF, Becker LC, et al. Extreme Deep White Matter Hyperintensity Volumes are Associated with African American Race. Cerebrovasc Dis. 2014; 37: 244-250.
- Sen S, Redd KT, Phillips ST, McMillian B, Giamberardino L, Hardin J, et al. PeRiodontal Treatment to Eliminate Minority Inequality and Rural Disparities in Stroke (PREMIERS): A Multicenter, randomized, controlled study. Int J Cerebrovasc Dis Stroke. 2019; 2: PMC7064156.
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. PLOS Med. 2007; 4: e296.
- Paolini S, Burdine J, Verenes M, Webster J, Faber T, Graham CB, et al. Rapid Short MRI Sequence Useful in Eliminating Stroke Mimics Among Acute Stroke Patients Considered for Intravenous Thrombolysis. J Neurol Disord. 2013; 1: 137.
- Dannenberg S, Scheitz JF, Rozanski M, Erdur H, Brunecker P, Werring DJ, et al. Number of cerebral microbleeds and risk of intracerebral hemorrhage after intravenous thrombolysis. Stroke. 2014; 45: 2900-2905.
- Zeng W, Chen Y, Zhu Z, Gao S, Xia J, Chen X, et al. Severity of white matter hyperintensities: Lesion patterns, cognition, and microstructural changes. J Cereb Blood Flow Metab. 2020; 40: 2454-2463.
- Ohira T, Shahar E, Chambless LE, Rosamond W, Mosley T, Folsom A. Risk Factors for Ischemic Stroke Subtypes: The Atherosclerosis Risk in Communities Study. Stroke. 2006; 37: 2493-2498.
- Noorbakhsh-Sabet N, Tsivgoulis G, Shahjouei S, Hu Y, Goyal N, Alexandrov AV, et al. Racial difference in cerebral microbleed burden among a patient population in the Mid-South United States. J Stroke Cerebrovasc Dis. 2018; 27: 2657-2661.
- Shahjouei S, Tsivgoulis G, Singh M, McCormack M, Noorbakhsh-Sabet N, Goyal N, et al. Racial Difference in Cerebral Microbleed Burden among Ischemic Stroke Patients. J Stroke Cerebrovasc Dis. 2007; 26: 2680-2685.
- Wolfe C, Rudd A, Howard R, Coshall C, Stewart J, Lawrence E, et al. Incidence and case fatality rates of stroke subtypes in a multiethnic population: the South London Stroke Register. J Neurol Neurosurg Psychiatr. 2002; 72: 211-216.
- Sacco R, Boden-Albala B, Abel G, Fin I-F, Elkind M, Hauser WA, et al. Race-Ethnic Disparities in the Impact of Stroke Risk Factors: The Northern Manhattan Stroke Study. Stroke. 2001; 32: 1725-1731.
- Castello JP, Pasi M, Abramson JR, Rodriguez-Torres A, Marini S, Demel S, et al. Contribution of racial and ethnic differences in cerebral small vessel disease subtype and burden to risk of cerebral hemorrhage recurrence. Neurology. 2021; 96: e2469-e2480.
- Marchesan JT, Moss K, Morelli T, Teles FR, Divaris K, Styner M, et al. Distinct Microbial Signatures between Periodontal Profile Classes. J Dent Res. 2021; 100: 1405-1413.
- Eke P, Dye B, Wei L, Slade G, Thornton-Evans G, Borgnakke W, et al. Update on Prevalence of Periodontitis in Adults in the United States: NHANES 2009 to 2012. J Periodontol. 2015; 86: 611-622.
- You Z, Cushman M, Jenny N, Howard G. Tooth loss, systemic inflammation, and prevalent stroke among participants in the reasons for geographic and racial difference in stroke (REGARDS) study. Atherosclerosis. 2009; 203: 615-519.
- Arabi G, Thomalla G, Heydecke G, Seedorf U. Chronic oral infection: An emerging risk factor of cerebral small vessel disease. Oral Diseases. 2018; 25: 710-719.
- Jorgensen D, Shaaban CE, Wiley C, Gianaros P, Mettenburg J, Rosano C. A population neuroscience approach to the study of cerebral small vessel disease in midlife and late life: an invited review. Am J Physiol Heart Circulatory Physiol. 2018; 314: H1117-H1136.
- Brickman A, Schupf N, Manly J, Luchsinger J, Andrews H, Tang M, et al. Brain morphology in elderly African Americans, Caribbean Hispanics, and Caucasians from Northern Manhattan. Arch Neurol. 2008; 65: 1053-1061.
- El Husseini N, Bushnell C, Brown CM, Attix D, Rost NS, Samsa GP, et al. Vascular Cellular Adhesion Molecule-1 (VCAM-1) and Memory Impairment in African-Americans after Small Vessel-Type Stroke. J Stroke Cerebrovasc Dis. 2020; 29.
- Brown C, Bushnell C, Samsa G, Goldstein L, Colton C. Chronic systemic immune dysfunction in African Americans with small vessel-type ischemic stroke. Transl Stroke Res. 2015; 6: 430-436.
- Centers for Disease Control and Prevention. Disparities in Oral Health. Centers for Disease Control and Prevention. 2022.
- Cannistraro RJ, Badi M, Eidelman BH, Dickson DW, Middlebrooks EH, Meschia JF. CNS small vessel disease: A clinical review. Neurology. 2019; 92: 1146-1156.