Rationale and Design of Naoxueshu Oral Liquid in Patients with Spontaneous Intracerebral Hemorrhage: A Multicentre, Randomized, Placebo-Controlled, Double-Blind Trial (ENSTAR)
- 1. Department of Neurology, Capital Medical University, China
- 2. China National Clinical Research Center for Neurological Diseases, China
- 3. Department of Neurology, The Affiliated Hospital of Qingdao University, China
- 4. Department of Neurology, Beijing University of Chinese Medicine, China
- 5. Institute for Brain Disorders, Beijing University of Chinese Medicine, China
Abstract
Background: Naoxueshu oral liquid (NXSOL) shows promise as a treatment for intracerebral hemorrhage (ICH), but its effectiveness lacks confirmation from large-scale randomized controlled trials. Our study aims to assess the efficacy and safety of NXSOL in non-surgical spontaneous ICH patients.
Methods and design: NXSOL in patients with spontaneous ICH (ENSTAR) is a multicenter, prospective, randomized, double-blind, placebo-controlled clinical trial. Adult supratentorial SICH patients whose hematoma volume ≤40ml, baseline National Institutes of Health Stroke Scale (NIHSS) ≥ 6 and ≤ 25, and Glasgow coma scale (GCS) ≥9 within 72 hours after symptom onset are included. Patients who are scheduled for or have already undergone surgery treatment are excluded. Participants are randomly assigned, in a 1:1 ratio, to receive either NXSOL or placebo 10ml 3 times a day for 30 consecutive days. Both NXSOL and control groups will get standard western medical treatment. All will be followed up for 90 days. Primary efficacy outcome is the proportion of mRS ≤2 at 90 days. Secondary efficacy outcomes include mortality within 90 days, changes in brain edema volume on computed tomography (CT) and NIHSS change at 7 and 14 days, cognitive function assessment and economic evaluation of drugs at 90 days. Safety outcomes are rate of hematoma expansion at 7 and 14 days, severe or moderate bleeding at 90 days.
Discussion: ENSTAR will provide evidence for the efficacy and safety of NXSOL in patients with SICH.
Strengths and limitations: Naoxueshu Oral Liquid in patients with Spontaneous Intracerebral Hemorrhage (ENSTAR) is a multicenter, prospective, randomized, double-blind, placebo-controlled clinical trial and will evaluate the efficacy and safety of NXSOL in patients with SICH. Data will provide evidence for NXSOL use in spontaneous ICH patients. Insufficient sample size may be a limitation of this study.
KEYWORD
- Intracerebral hemorrhage
- Traditional Chinese medicine
- Nao-Xue-Shu
- Hematoma
- MRI
CITATION
Wang W, Jia J, Zhang Y, Wang A, Xie A, et al. (2024) Rationale and Design of Naoxueshu Oral Liquid in Patients with Spontaneous Intracerebral Hemorrhage: A Multicentre, Randomized, Placebo-Controlled, Double-Blind Trial (ENSTAR). J Neurol Disord Stroke 11(4): 1229.
INTRODUCTION AND RATIONALE
Intracerebral hemorrhage (ICH) is a severe cerebrovascular disease with high disability and mortality rates. About 14.9% of acute stroke hospitalizations are ICH with in-hospital death/ discharge against medical advice rate of 19.5% which is higher than that of 5.8% for ischemic stroke [1]. The mortality of ICH in 3 months is 20∼30% and 46% of patients experiencing death or severe disability within a year [2]. The brain injury after ICH primarily comprises the primary injury as well as the secondary injury. The primary injury is induced by the direct pressure effects of an acutely expanding mass lesion and hematoma growth. The secondary injury is induced by the physiological and cellular pathways triggered by the hematoma and its metabolized blood products, including cerebral edema, inflammation, and biochemical toxicity of blood products such as hemoglobin, iron, and thrombin [3,4]. 2022 Guideline for the Management of Spontaneous ICH provides us with standardized western medical treatment protocols [5]. According to the guideline the blood pressure lowering and reversal of anticoagulation are the major medical therapies for SICH aimed at limiting hematoma enlargemen. But the search for effective medical treatments for protecting tissue from secondary post-ICH injury has to date been unsuccessful.
In traditional Chinese medicine (TCM) theory, ICH belongs to the category of “stroke”. The main pathogenesis was qi stagnation and blood stasis: “All bleeding must leave the meridian, and the blood from the meridian is blood stasis, which always needs removing.” The blood of the meridian will block the brain, leading to the original spirit becoming trapped and blinded. Blood flow is not smooth. Qi deficiency and fluid deficiency lead to blockage of meridian vessels and paralysis of limbs. Naoxueshu oral liquid (NXSOL) is the first Chinese patent medicine for the treatment of ICH. NXSOL has the effects of tonifying qi, promoting blood circulation and removing blood stasis and is mainly used for hemorrhagic stroke in patients with Qi deficiency and blood stasis.
NXSOL consists of Astragalus membranaceus (Fisch.), Hirudo nipponica Whitman (Hirudinidae), Bunge. (Leguminosae), Acorus tatarinowii Schott (Acoraceae), Paeonia suffruticosa Andr. (Paeoniaceae), Rheum palmatum L. (Paeoniaceae) and Ligusticum chuanxiong Hort (Apiaceae). Astragalus membranaceus and Hirudo nipponica Whitman are the main components of NXSOL. Studies found that Astragalus membranaceus contains multiple active ingredients, such as astragalosides, which have antioxidant, anti-inflammatory, and antiapoptotic effects [6,7]. They have the ability to protect the blood-brain barrier permeability and prevent cerebral ischemia [8]. Hirudo nipponica Whitman contains the anticoagulant components, including heparin and hirudin, which exhibits anticoagulation effects, inhibits platelet aggregation, and improves blood flow, relieving acute brain injury and ameliorate outcomes of ICH [9-11]. Multiple clinical researches showed that NXSOL can effectively reduce cerebral hemorrhage, cerebral edema safely and improve the ICH patient’s quality of life [12- 15]. Whereas most of these studies were limited by small sample size, or single-center design, or no placebo-controlled group to compare the single therapeutic response with NXSOL. Therefore, it is still of great importance to verify the therapeutic value of the multitargeted NXSOL in well-designed large-scale randomized clinical trials.
Therefore, we conducted the ENSTAR trial and aimed to evaluate the efficacy, safety, medication adherence, and pharmacoeconomic evaluation of NXSOL in patients with SICH to establish evidence-based medicine.
METHODS
Design
ENSTAR is a multicenter, prospective, randomized, double- blind, placebo-controlled clinical trial. Enrolled patients will be randomly assigned in a 1:1 ratio by means of a centrally stratified block randomization method to receive NXSOL plus standard western medical treatment or placebo plus standard western medical treatment. Participants are required to be followed up for 90 days to assess the efficacy and safety outcomes. Approximately 30 study centers in China are planned to participate in ENSTAR.
Patient population
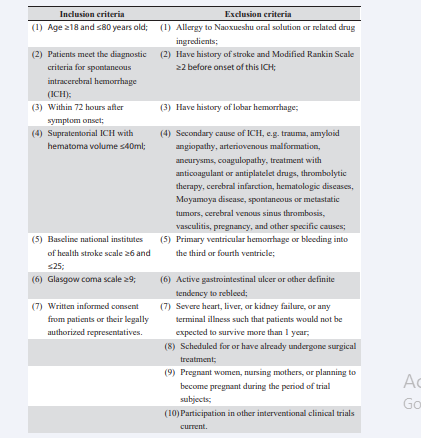
Adult supratentorial SICH patients (hematoma volume≤40ml, baseline National Institutes of Health Stroke Scale [NIHSS] ≥6 and ≤25, and Glasgow coma scale [GCS] ≥9) within 72 hours of symptom onset who meet the diagnostic criteria for SICH are consecutively enrolled into this trial. Patients who are scheduled for or have already undergone surgery treatment are excluded. The detailed inclusion and exclusion criteria are shown in Table 1
Table 1: Detailed inclusion and exclusion criteria. ICH, intracerebral hemorrhage.
Baseline assessment
Demographic information, medical history, current medications and laboratory tests will be collected. Baseline stroke severity (NIHSS and GCS) will also be assessed by certified and well-trained clinicians. Baseline non-contrast computed tomography (NCCT) scans will be performed on admission. ICH hematoma volume was measured using the ABC/2 method, in which A is the greatest diameter on the largest hemorrhage slice, B is the diameter perpendicular to A, and C is the approximate number of axial slices with hemorrhage multiplied by the slice thickness [16].
Randomization
Participants are randomly assigned, in a 1:1 ratio, to receive NXSOL or placebo. Study drugs will be packaged on the randomization sequence. The randomization sequence will be generated centrally by SAS with centrally stratified block randomization. The study drugs will be distributed to the sub- centers in groups of four. At the first visit, participants will get the drug numbers in descending order according to the order of enrollment time.
Treatment intervention
Intervention group patients receive NXSOL and control group patients receive placebo 10ml 3 times a day for 30 consecutive days. If the patient has dysphagia, the medicine or placebo can be administered through the indwelling nasogastric tube. The first dose should be given within 2 hours after randomization, and within 72 hours after symptom onset. Standard early treatment and secondary prevention management for all the enrolled patients are based on 2022 Guideline for the management of patients with spontaneous intracerebral hemorrhage: a guideline from the American Heart Association/American Stroke Association and the Chinese guidelines for diagnosis and treatment of acute intracerebral hemorrhage 2019 [5-17].
Patients for whom direct surgical treatment is planned are excluded from this study at initial assessment. Patients who are subsequently judged to require surgical treatment after NXSOL or placebo treatment in the judgement of the investigator will be included in the intention-to-treat analysis, but will be excluded from per-protocol analysis in order to avoid the effect on the outcome.
Concomitant management
All the enrolled patients are requested to be admitted into stroke units or intensive care units if necessary. Participants will be prohibited to receive acupuncture and any other kinds of TCMs (including herbal decoctions, granules, injections and patent medicines) for the treatment of ICH during the experimental drug administration period.
Using western medicines, such as diuretics, antihypertensives, antidiabetic drugs, lipid-lowering agents, and additional medications needed based on the patient’s condition, will be recorded in detail in the case report form.
Follow-up
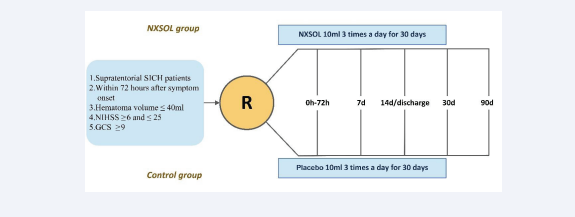
Study visits will be performed at the screening period (within 72 hours after onset), day 7, day 14 or before discharge (whichever occurs first among these two will be judged as the follow-up point), day 30 and day 90 (endpoint) following symptom onset [Figure 1].
Figure 1: EStudy flow chart. NXSOL, Naoxueshu oral liquid; SICH, spontaneous intracerebral hemorrhage; NIHSS, National Institute of Health Stroke Scale; GCS, Glasgow Coma Scale.
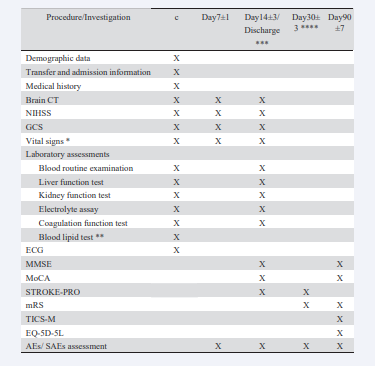
At the screening period, CT should be performed. The follow- up CT should be completed at day 7, day 14 or before discharge (whichever occurs first) to get the hematoma volume and edema volume. The patients will be given the remaining NXSOL/placebo at discharge and return their pill box and the rest of the pills to the investigator at 30 days when face-to-face follow-up. At baseline and during follow-up visits, clinical information (not limited to that) including the neurological examination (NIHSS, GCS and Modified Rankin Scale [mRS] score), vital signs, laboratory tests with recorded results, concomitant medications, European five- dimensional health scale (EQ-5D-5L), Telephone Interview for Cognitive Status-Modified (TICS-M), Minimum Mental State
Examination (MMSE), Montreal Cognitive Assessment (MoCA), Patient-reported Clinical Outcome Scale for Stroke (STROKE- PRO), adverse events (AEs) and serious AEs (SAE) will be collected [Table 2].
Table 2 Trial assessment flow chart. Abbrevations: CT, computed tomography; NIHSS, National Institute of Health Stroke Scale; GCS, Glasgow Coma Scale; ECG, Electrocardiogram; MMSE, Minimum Mental State Examination; MoCA, Montreal Cognitive Assessment; STROKE-PRO, Patient-reported Clinical Outcome Scale for Stroke; mRS, Modified Rankin Scale; TICS-M, Telephone Interview for Cognitive Status-Modified; EQ-5D-5L, five-level EuroQol five-dimensional questionnaire; AE, adverse event; SAE, serious adverse event. *Vital signs include blood pressure and heart rate; **Blood lipid test include total cholesterol, low-density lipoprotein, high-density lipoprotein and triglycerides; ***Visit information will be collected at 14±3 days or discharge (whichever occurs first). The patients will be given the remaining NaoXueShu oral liquid/placebo at discharge; ****Patients will return their pill box and the rest of the pills to the investigator at 30±3 days when face-to-face follow-up.
If SAEs occur, investigators should adhere to the protocol and Good Clinical Practice guidelines. The Endpoint Adjudication Committee (EAC) will adjudicate the report. The AEs and SAEs will be reported using standard tabulated terminology.
STUDY OUTCOMES
Primary Outcome
Proportion of patients with favorable functional outcome defined as a mRS ≤2 at 90 days.
Secondary outcomes
Efficacy outcomes
1 Rate of death from any cause within 90 days.
2. Changes in absolute and relative brain edema volume on cranial CT at 7 and 14 days (Absolute edema volume = total [hematoma + edema] volume - hematoma volume; Relative edema volume = Absolute edema volume / hematoma volume).
3. NIHSS change from baseline at 7 and 14 days.
4. Self-reported outcomes using STROKE-PRO at 14 and 30 days.
5. Ordinal distribution of mRS at 30 and 90 days.
6. Quality of life assessment using the EQ-5D-5L and cognitive function assessment using TICS-M, MMSE and MoCA scores at 90 days.
7. Economic evaluation of drugs at 90 days using EQ-5D-5L, total drug and hospitalization costs.
Safety Outcomes
1. Rate of hematoma expansion defined as a 33% increase or a 6ml increase in volume compared to baseline CT at 7 and 14 days.
2. Rate of severe systemic bleeding defined by The Global Utilisation of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO) at 90 days [18].
3. Rate of moderate bleeding defined by GUSTO at 90 days [18].
Steering committee
Steering committee will give scientific and strategic instruction on this study, and be responsible for the design, execution and publishing of the study, and will make sure of the study quality, conduction and management.
Data and safety monitoring boards
An independent data and safety monitoring boards (DSMBs) will monitor the study progress, to make sure it meets the highest standards of ethics and safety. It composes of academic members, including independent statistician who will be responsible for generating the unblinded interim analyses and have no other involvement in the trial. DSMB will give advice on safety data, terminating the study, continuing the study or revising the protocol before continuing.
Sample size estimates
According to the data from previous studies, the proportions of mRS ≤2 at 90 days were ranged from 44% to 83.8% [19-21]. So the proportion of mRS ≤2 in control group at 90 days was set at 64%. It was assumed that the proportion of mRS ≤2 in the NXSOL treatment group will be increased by 12%, that is, the proportion of mRS ≤2 in the NXSOL group at 90 days was 76%. The significance level was set to 5% and power level to 80%. The allocation ratio of NXSOL treatment group and control group was 1:1. The sample size for each group was estimated to be 226. Considering a dropout rate of 15%, a total of 520 patients was needed in this study, with 260 patients in each group.
Statistical analysis
Baseline indicators will be compared between groups using t-tests or Wilcoxon rank-sum tests for continuous data and chi- square tests, Fisher’s exact tests or Wilcoxon rank-sum tests for categorical data. The primary efficacy evaluation will be based on an intention-to-treat (ITT) analysis and the missing values can be imputed by last observation carry forward (LOCF). Differences between study groups in the proportion of 90-day mRS will be assessed with the use of logistic regression models. The OR and the 95% CI will be reported. In addition, whether the treatment effects differ in certain predefined subgroups will be assessed by testing the interaction between the treatment and the subgroup with the use of logistic regression models. Subgroup analysis includes age (<65 vs ≥65 years old; <75 vs ≥75 years old), sex (female, male), baseline severity of stroke (6 ≤NHISS score ≤15 vs 16≤ NHISS score ≤25), time from last known well to treatment time (≤24hours vs 24–48hours vs 48–72hours). For comparison between groups, all hypothesis tests will be performed with a two-sided alpha level of 0.05 (α=0.05). All statistical analyses are processed by SAS V9.4 and will be performed on a predetermined statistical analysis plan.
DISCUSSION
ENSTAR is a well-designed multicenter, prospective, double- blind, placebo-controlled clinical trial of NXSOL in SICH patients. This will allow us to exclusively answer the question of efficacy and safety of NXSOL combined with standard western medical treatment compared with standard western medical treatment alone in patients with SICH at initial assessment.
Due to the complex pathophysiological process of secondary brain injury following ICH, current clinical trials of western medications for ICH have not yielded satisfactory treatment outcomes [22-24]. NXSOL is a new variety of TCM approved by the National Medical Products Administration (NMPA) for the treatment of acute stage and early recovery stages of ICH (Drug approval number: Z20070059). NXSOL promotes Qi, activates blood, and removes blood stasis, thereby promoting hematoma absorption, reducing brain edema, modulating inflammatory factor to improve the microenvironment, and reducing free radicals. Research has validated the therapeutic efficacy of NXSOL in treating ICH, though its mechanisms of action remain
under exploration. Animal studies indicate that NXSOL effectively enhances the expression of the ZO-1 protein and suppresses AQP4 protein expression in rats with ICH. This action reduces blood- brain barrier permeability, alleviates brain edema, and protects brain tissue post-hemorrhage [25]. A recent study showed that NXSOL can accelerate the clearance and decomposition of hemoglobin in hematoma by interfering with the activation of M2 microglia via regulating the Nrf2/CD163/HO-1 after ICH [26]. NXSOL was a potential drug by suppressing the inflammatory response and may provide a novel therapeutic strategy against hematoma after ICH.
A multicenter observational study has preliminarily shown that NXSOL combined with standard western medicine treatment could relieve hematoma volume and cerebral edema safely and improve patients’ neurological function [13]. A randomized controlled trial (RCT) of 88 ICH patients has shown that, compared with control group, the white blood cell (WBC) count, hematoma volume, NIHSS score, mRS and traditional Chinese medicine syndrome score were significantly decreased in NXSOL group [12]. Additionally, NXSOL has demonstrated efficacy in minimally invasive hematoma evacuation surgery. A study has shown that NXSOL could relieve hematoma volume and cerebral edema after clot removal surgery and had the potential to improve patients’ short-term neurological function [14]. The results of a systematic review and meta-analysis of 14 eligible randomized controlled trials showed that the use of NXSOL alone or combined with other drugs or auxiliary methods had obvious function of arousal, improved nerve function after 4 weeks of treatment and improved the patient’s quality of life [8]. A network meta-analysis showed that NXSOL combined with western medicines was effective for improving neurological function, reducing both hematoma and edema volumes and proinflammatory factor expression in patients with hypertensive ICH [27]. However, high heterogeneity was found in some of their results, the reasons might be related to the number of samples, the quality of the literature, the subject of study, and the duration of treatment. More large-sample and high-quality RCTs are still needed.
As we are all aware, there are multiple factors that influence the prognosis of ICH. Considering that the effectiveness of NXSOL may be impacted by varying severity levels of patient’s condition, this study specifically selected patients with moderate ICH. Factors influencing the prognosis include hematoma volume, hematoma location, GCS score, age, and others [28]. Among these factors, infratentorial hemorrhage is recognized as an independent predictor for ICH prognosis. For this study, patients with supratentorial hemorrhage are chosen to ensure consistency in clinical characteristics among participants. Based on ICH-GS score criteria, patients with a hematoma volume less than or equal to 40ml and a GCS score greater than or equal to 9 were included [28]. Additionally, a NIHSS range of 6 to 25 was utilized to identify patients with significant neurological deficits. Following recommendations from 2022 Guideline for the Management of SICH, for patients with supratentorial ICH of >20- to 30-mL volume with GCS scores in the moderate range (5–12), minimally invasive hematoma evacuation with endoscopic or stereotactic aspiration can be useful to reduce mortality compared with medical management alone, so individuals who had undergone surgery or were scheduled for surgery were also excluded from this study [5]. Furthermore, patients aged 80 years or older were not included in order to explore the most likely population benefiting from NXSOL administration. It should be noted that additional studies need to be designed in order to further confirm the efficacy and safety of NXSOL in patients with severe ICH as well as surgical and elderly patients.
Another trial is ongoing in the world to provide more evidence on NXSOL in treatment of ICH which is an observational clinical registration study (Registration Study for the Prevention and Treatment of Cerebral Hemorrhage using Naoxueshu Oral Liquid, ChiCTR2300074892) [29]. Compared with this trial, ENSTAR is a multicenter, prospective, randomized, double-blind, placebo- controlled clinical trial in supratentorial hemorrhage but without intention to surgery at initial assessment.
CONCLUSION
In conclusion, ENSTAR is initiated to investigate the efficacy and safety of NXSOL in patients with SICH. It will help provide strong evidence for potential advantages of NXSOL use in SICH patients.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
This study is supported by the National Key R&D Program of China (2022YFC3501100, 2022YFC3501102). This study was funded by the Shandong Wohua Pharmaceutical Technology Co., Ltd with the investigation drugs provided for free.
Informed consent
The authors declare that they consent for publication.
Ethics approval
This study involves human participants and all procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and the principles of the Declaration of Helsinki. This study obtained Ethics approval from the Institutional Review Board of Beijing Tiantan Hospital, Capital Medical University with number KY2023-077-02. Participants gave informed consent to participate in the study before taking part. This trial has been registered at the Chinese Clinical Trial Registry (https://www. chictr.org.cn/searchprojEN.html). The trial registration number is ChiCTR2300074347.
Guarantor
YJ and XZ.
Contributors
YJ and XZ obtained funding, concept and design the trial. YJ, XZ, WW, JJ, AX and YG contributed to the study design. WW and JJ drafted the manuscript. YJ, WW, JJ, YZ, MX, XL and YG assisted to promote the project progress. AX and WW completed the statistical work. All authors approved the submitted version.
Statistical analysis: YX, HL and YP. All authors approved the submitted version
ACKNOWLEDGEMENTS
We thank all investigators and participating hospitals of the ENSTAR trial.
Open access and author archiving
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial.
REFERENCES
- Wang Y-J, Li Z-X, Gu H-Q, Zhai Y, Jiang Y, Zhao XQ, et al. China Stroke Statistics 2019: A Report From the National Center for Healthcare Quality Management in Neurological Diseases, China National Clinical Research Center for Neurological Diseases, the Chinese Stroke Association, National Center for Chronic and Non-communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention and Institute for Global Neuroscience and Stroke Collaborations. Stroke Vasc Neurol. 2020; 5: 211-39.
- Feigin VL, Forouzanfar MH, Krishnamurthi R, George A Mensah, Myles Connor, Derrick A Bennett, et al. Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014; 383: 245-54.
- Zhu H, Wang Z, Yu J, Xiuli Yang, Feng He, Zhenchuan Liu, et al. Role and mechanisms of cytokines in the secondary brain injury after intracerebral hemorrhage. Prog Neurobiol. 2019; 178: 101610.
- Wilkinson DA, Pandey AS, Thompson BG, Richard F Keep , Ya Hua, Guohua Xi. Injury mechanisms in acute intracerebral hemorrhage. Neuropharmacol. 2018; 134: 240-248.
- Greenberg SM, Ziai WC, Cordonnier C, Dar Dowlatshahi, Brandon Francis, Joshua N Goldstein, et al. 2022 Guideline for the Management of Patients With Spontaneous Intracerebral Hemorrhage: A Guideline From the American Heart Association/American Stroke Association. Stroke. 2022; 53: e282-e361
- Cai J, Pan R, Jia X, Yue Li , Zijun Hou , Run-Yue Huang, et al. The combination of astragalus membranaceus and ligustrazine ameliorates micro-haemorrhage by maintaining blood–brain barrier integrity in cerebrally ischaemic rats. J Ethnopharmacol. 2014; 158: 301-09.
- Yi QJLM, Research MM. The Inhibition of Astragalus and Salviae Miltiorrhizae Injection on the Neuronal Apoptosis of the Penumbra Around the Intracerebral Hemorrhage in Rats. 2012
- Wu L, Li Y, Wang X, Xiaomeng Ren, Haiyan Zhu, Yikun Sun, et al. A Systematic Review and Meta-Analysis on the Treatment of Cerebral Hemorrhage with NaoXueShu Oral Liquid. BioMed Res Int. 2017; 2017: 1-11.
- 9. Zhang ZQ, Tang T, Luo JK, et al. Effect of qi-tonifying and stasis- eliminating therapy on expression of vascular endothelial growth factor and its receptors Flt-1, Flk-1 in the brain of intracerebral hemorrhagic rats. Chi j of integ med. 2007; 13: 285-90.
- Dong H, Ren J-X, Wang J-J, Li-Shuai Ding, Jian-Jun Zhao, Song-Yan Liu, et al. Chinese Medicinal Leech: Ethnopharmacology, Phytochemistry, and Pharmacological Activities. eCAM. 2016; 2016: 1-11.
- Li X, Zhu Z, Gao S, Lei Zhang, Xiaojing Cheng, Shiyao Li, et al. Inhibition of fibrin formation reduces neuroinflammation and improves long-term outcome after intracerebral hemorrhage. Int Immunopharmacol. 2019; 72: 473-478.
- Song J, Lyu Y, Wang P, Yuting Nie, Huiqiang Lu, Li Gao, et al. Treatment of Naoxueshu Promotes Improvement of Hematoma Absorption and Neurological Function in Acute Intracerebral Hemorrhage Patients. Front Physiol. 2018; 9: 933.
- Song J, Nie Y, Qin X, Pingping Wang, Huiqiang Lu, Li Gao, et al. Efficacy of Naoxueshu in acute spontaneous intracerebral hemorrhage: a multicenter observational study. Neurol Sci. 2022; 43: 1885-91.
- Song J, Nie Y, Wang P, Huiqiang Lu, Li Gao. Naoxueshu relieves hematoma after clot removal in acute spontaneous intracerebral hemorrhage. Brain and Beh. 2021; 11: e01957.
- Duan JY, Liang X, Jia M, Xi, Min Wang, Lin Lei, et al. Systematic review and Meta-analysis on efficacy and safety of Naoxueshu Oral Liquid in treatment of hypertensive intracerebral hemorrhage. Zhongguo Zhong yao za zhi. 2021; 46: 2984-94.
- Kothari RU, Brott T, Broderick JP, W G Barsan, L R Sauerbeck, M Zuccarello, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996; 27 :1304-5.
- Chinese Society of Neurology CSS. Chinese guidelines for diagnosis and treatment of acute intracerebral hemorrhage. Chin J Neurol. 2019; 52: 12.
- Berkowitz SD, Granger CB, Pieper KS, K L Lee, J M Gore, M Simoons, et al. Incidence and predictors of bleeding after contemporary thrombolytic therapy for myocardial infarction. The Global Utilization of Streptokinase and Tissue Plasminogen activator for Occluded coronary arteries (GUSTO) I Investigators. Circulation. 1997; 95: 2508-16.
- Qureshi AI, Palesch YY, Barsan WG, Daniel F Hanley, Chung Y Hsu, Renee L Martin, et al. Intensive Blood-Pressure Lowering in Patients with Acute Cerebral Hemorrhage. N England J Med. 2016; 375: 1033- 43.
- Wang WJ, Lu JJ, Wang YJ, Chun-Xue Wang, Yi-Long Wang, Kolin Hoff, et al. Clinical characteristics, management, and functional outcomes in Chinese patients within the first year after intracerebral hemorrhage: analysis from China National Stroke Registry. CNS Neurosci Ther. 2012; 18: 773-780.
- Yan Y, Wang M, Zhang L, henwei Qiu, Wenfei Jiang, Men Xu, et al. Nao- Xue-Shu Oral Liquid Improves Aphasia of Mixed Stroke. Evid-Based Complement Alternat Med. 2015; 2015: 709568.
- Bobinger T, Manaenko A, Burkardt P, Vanessa Beuscher, Maximilian I Sprüge, Sebastian S Roeder, et al. Siponimod (BAF-312) Attenuates Perihemorrhagic Edema And Improves Survival in Experimental Intracerebral Hemorrhage. Stroke. 2019; 50: 3246-3254.
- Irvine H, Male S, Robertson J, Caitlin Bell, Oladi Bentho, Christopher Streib. Reduced Intracerebral Hemorrhage and Perihematomal Edema Volumes in Diabetics on Sulfonylureas. Stroke. 2019; 50: 995- 998.
- Silva Marques J, Ennis G, Venade G, Rita João Soares, Nuno Monteiro, Ana Gomes. Association of Statins With Functional Outcome and 30- Day Mortality in Patients With Intracerebral Hemorrhage. Cureus. 2021; 13: e14421.
- WANG Xiaofeng TC, ZHANG Yan, YUAN Mengchen, ZHANG Hanlai, WANG Liqin, AN Na, GAO Yonghong. Effect of Naoxueshu Oral Liquid on the expression of ZO-1 and AQP4 proteins in rat model of intracerebral hemorrhage. CHINA MEDICAL HERALD 2019; 16: 5.
- Li Y, Tian C, Wei Y, Haoqi Liu, Na An, Ke Song, et al. Exploring the pharmacological mechanism of Naoxueshu oral liquid in the treatment of intracerebral hemorrhage through weighted gene co-expression network analysis, network pharmacological and experimental validation. Phytomedicine. 2023; 108: 154530.
- Mei L, Fengqun M, Xiaozhuo L, Wang Qing, Fan Mingming, Zuo Zhengyao, et al. Effect Western Medicines Combined With Nao-Xue- Shu in Patients With Hypertensive Intracerebral Hemorrhage: A Network Meta-Analysis. Front Pharmacol. 2022; 13: 892904.
- Ruiz-Sandoval JL, Chiquete E, Romero-Vargas S, Juan J Padilla- Martínez, Salvador González-Cornejo, et al. Grading Scale for Prediction of Outcome in Primary Intracerebral Hemorrhages. Stroke. 2007; 38: 1641-4.
- Wang K, Chen Y, Geng Q, Jinjuan Dou, Dahe Qi, Weidong Zhu, et al. Registration Study for the Prevention and Treatment of Cerebral Hemorrhage using Naoxueshu Oral Liquid: Protocol for a Research Study. J stroke cerebrovas dis. 2024; 33: 107649.