Reconstruction of Corticospinal Tract After Basal Ganglia Infarction: Case Report
- 1. Department of Radiology, The Second Hospital of Nanjing, Affiliated to Nanjing University of Chinese Medicine, China
- 2. School of Acupuncture-Moxibustion and Tuina, School of Health Preservation and Rehabilitation, Nanjing University of Chinese Medicine, China
- 3. Department of Rehabilitation, Affiliated Zhongda Hospital of Southeast University, China
- 4. Department of Radiology, Affiliated Zhongda Hospital of Southeast University, China
- 5. Department of Neurology, Affiliated Zhongda Hospital of Southeast University, China
Abstract
To observe how the pyramidal tract changes after rehabilitation of stroke patients with hemiplegia. This article reports two patients with basal ganglia infarction, who underwent brain magnetic resonance imaging (MRI) and recorded the motor function of the hemiplegic limb. Their initial assessment of motor function showed similar degrees of injury, but the final assessment after a 3-month interval showed significant differences in rehabilitation outcomes. We used diffusion tensor tractography technology to depict the corticospinal tract and observed that two patients showed different degrees of damage on the initial scan. For one of the patients, there was a significant increase in white matter fibers after rehabilitation, corresponding to an improvement in her rehabilitation score. The fiber bundle changes in the other patient were not very obvious, but the distribution of different length of fibers were changed.
Keywords
• Motor recovery
• Corticospinal tract
• Diffusion tensor tractography
Citation
Jiang W, Cao X, Quan J, Zhou H, Liu Y, et al. (2025) Reconstruction of Corticospinal Tract After Basal Ganglia Infarction: Case Report. J Neurol Disord Stroke 12(2): 1237.
INTRODUCTION
Motor dysfunction after cerebral infarction is usually due to damage to the pyramidal tracts, especially the corticospinal tract (CST). For some patients, timely rehabilitation training can partially recover motor function, but there are many patients who are difficult to recover to the pre-onset state after rehabilitation training. In this regard, many neuroimaging studies are looking for brain markers that predict prognosis, and such predictive studies may be helpful for diagnosis [1]. There have also been longitudinal studies looking for patterns of structural and functional changes in the brain during recovery. In this paper, 2 stroke patients with hemiplegia were selected. They both had cerebral infarction in the basal ganglia and their motor function improved after 3 months of rehabilitation training. We used the method of white matter fiber tracking based on diffusion tensor imaging (DTI) to show the structural basis of functional recovery.
CASE PRESENTATION
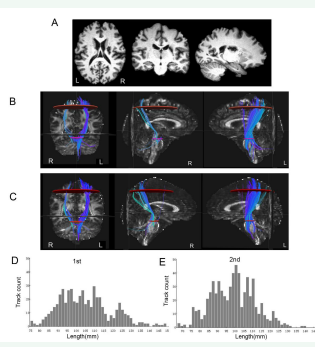
We analyzed the clinical information and the magnetic resonance imaging (MRI) data of 2 stroke patients. Motor outcome was evaluated by means of the Fugl–Meyer assessment and Action Research Arm Test (ARAT) scores to determine motor function. Patient A was a 53-year-old woman received conservative management for an infarct in the right basal ganglion [Figure 1A]. The patient presented with severe weakness of the left extremities at the onset of infarct. One month later from onset, the motor function was assessed (FMA: 28, ARAT: 0) and the MRI scan were collected as the data of the first stage. She recovered some function after 3 months, then the motor function was reassessed (FMA: 48, ARAT: 19) and the MRI were collected as the data of the second stage. Patient B was a 56-year-old man with an infarct in the left basal ganglion [Figure 2A]. He had also shown motor impairment since the onset of infarction. Also, at the first evaluation (1 month later from onset), the motor function poor (FMA: 38, ARAT: 0). He recovered well after 3 months, then the motor function was reassessed (FMA: 89, ARAT: 57).
The images were obtained 1 month and 4 months after the onset using a 3 T Philips Medical Systems (Philips, Ingenia, the Netherlands) equipped with a 16-channel head coil with a single-shot spin echo planar imaging sequence at the Affiliated ZhongDa Hospital of Southeast University. Diffusion tensor imaging (DTI) data were acquired using a single echo planar imaging (EPI) sequence; 33 diffusionweighted images (b=1000 s/mm2 ) and a reference T2- weighted image with no diffusion weighting (b=0 s/mm2 ) were obtained with the following acquisition parameters: voxel size =2×2×2 mm3 , gap = 0 mm; echo time (TE) = 107 ms; repetition time (TR) = 5835 ms; field of view (FOV) = 256 × 256 mm2 ; flip angle(FA) = 90°; matrix = 128 × 128; slices=75. High-resolution T1-weighted axial images covering the whole brain were obtained by a 3D-magnetization prepared rapid gradient-echo sequence: TR = 9.6 ms;TE = 3.7 ms; FA = 9°; acquisition matrix = 256 × 256;FOV = 256 × 256 mm2 ; thickness = 1.0 mm; gap = 0 mm,number of slices = 140.
Figure 1 The brain images and results of DTT of patient A. (A) T1 structural imaging showed infarction in the right basal ganglia of the brain. Deterministic fiber tracking at the first (B) and second (C) scans revealed corticospinal tracts. Map of fiber length and count distribution of corticospinal tract at first (D) and second (E) scans. R, right; L, left
All patients provided written, informed consent before the examination. The study protocol was approved by the Ethics Committee of Southeast University affiliated Zhongda Hospital. DTI data analysis was performed using the Pipeline for Analyzing Brain Diffusion Images (PANDA, http://www. nitrc.org/projects/panda) [2]. Deterministic fiber tracking was applied using the fiber assignment by continuous tracking (FACT) algorithm with an angle threshold of 45°. All voxels with FA≥0.2 were used as seed points [3]. Whole-brain tractography was performed for each subject in the native diffusion space. To analyse the CST, region of interests (ROIs) were drawn at the levels of the cerebral peduncle and the motor cortex on the individual axial FA map [4].
Figure 2 The brain images and results of DTT of patient B. (A) T1 structural imaging showed infarction in the left basal ganglia. Deterministic fiber tracking at the first (B) and second (C) scans revealed corticospinal tracts. Map of fiber length and count distribution of corticospinal tract at first (D) and second (E) scans. R, right; L, left
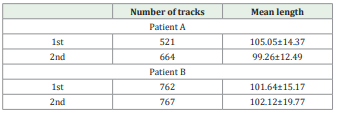
Fiber tracts passing through both ROIs were designated as the tracts of interest. The number and mean length of passing fibers (mm) were recorded for the CST using Trackvis (Version 0.6.1) (http://www.trackvis.org/). Deterministic fiber tracking of the DTI scans revealed corticospinal tract [Figure 1 and 2]. The number and mean length of the CST of each patient were calculated [Table 1]. The patient A had a right basal ganglia infarction, resulting in a significant reduction in the number of right CST. The second scan of the patient A showed a marked increase in corticospinal tract fibers compared with the first scan [Figure 1B, C]. There was no significant difference of the CST reconstructed by the two scans [Figure 2B,C], but there were changes in the number and length distribution of different fibers of patient B [Figure 2D, E].
Table 1. The number and mean length of the CST. Number of tracks.
DISCUSSION
Here, we evaluated the structural integrity in order to explain the recovery change of residual motor function in stroke patients. The pyramidal tract is the major neuronal pathway that mediates voluntary movements. The result of DTT at 4 months after onset showed that the pyramidal tract were reconstructed. In this study, there were 2 patients with basal ganglia infarction causing damage to the CST. At the 3 month follow-up, the number of fibers in the CST increased, corresponding to improvement in the patients’ motor function. In previous studies, it has been reported that white matter fibers can undergo degeneration after stroke, but there are also fibers remodeling that recover with functional recovery [5-7]. For patient A, the initial injury was more serious, so after the same time of rehabilitation training, the recovery degree was worse than that of patient B. For patient B, the initial damage of the CST is relatively mild, which may be the reason for his ideal recovery effect. In addition, the number of fibers in the CST did not change significantly after rehabilitation, but the distribution of track count with different lengths changed. It is possible that other fiber tracts in various regions of the brain were responsible for his improved motor function. In conclusion, this study validated lesion-induced white matter remodeling and the neural plasticity. It is hoped that future studies can identify the accurate and comprehensive structural networks of the brain corresponding to the improved function of patients.
CONTRIBUTORS
Each named author has substantially contributed to the patient management, conducting the underlying research, drafting and revising this manuscript. Additionally, the authors have no conflict of interest, financial or otherwise. Declaration of competing interest: The authors declare there is no conflict of interests. Acknowledgments: None. Patient’s Informed Consent The patient has signed the authorization to publish this case report.
REFERENCES