Roles of Gastrodin in Improvement of Learning/ Memory Capacity with CHAT Elevation in AD Rat Model Dependant on NT-3 and IGF-1 Up Regulation Related Neuroprotective Effects
- 1. Neuroscience Institute of Kunming Medical University, Kunming, Yunnan, China
Abstract
Objective: This study attempts to explore the effects of Gastrodin on the behavioral and pathological amelioration in AD rat model induced by fimbria of hippocampus transected, and elucidate the underlying molecular mechanism of Gastrodin takes its action
Methods: AD rat model was induced by cutting off the fibmbria of hippocampus by using the self-made micro-blade according to the coordinates previously described.Two weeks later, Gastrodin was injected intraperitoneally into the AD rats, Morris Water Maze (MWM) was employed to detect the behavior of learning and memory in all three experimental groups, i.e. sham operative group, AD model group and Gastrodin injected group. Subsequently, Q-PCR and Western blot was used respectively to investigate the differential expression of various neurotrophic factors, including NGF, CNTF, BDNF, NT-3 and IGF-1 on both mRNA and protein level among three experimental groups to ascertain which up regulated
Results: The Escape latency in the Gastrodin injected groups was markedly shorter than the AD model group (p<0.05). The times for rats to pass through the quadrant containing the platform, accompanied by the time course for rats in the target quadrant in Gastrodin injected group was substantially longer than that of AD model group (p<0.05), respectively. And q-PCR and Western blot showed that the mRNA and protein level of NT-3 and IGF-1 in the Gastrodin injected group were all significantly up regulated than those of AD model and sham operative group, respectively (p<0.01), the other neurotrophic factors detected in this study, including BDNF, NT-3 and CNTF, was all found substantially elevated in Gastrodin injected group (p<0.05), and their expression order descending from Gastrodin injected group, AD model group to sham operative group. Among all the neurotrophic factors, the rise amplitude of NT-3 and IGF-1 was the maximum among all the ones detected
Conclusions: Gastrodin plays neuroprotective roles in AD rats amelioration in learning and memory capacity, which was most likely dependant on the increased secretion of some endogenous neurotrophic factors, especially NT-3 and IGF1. This will helpful to elucidate the neuroprotective roles of Gastrodin plays in AD rats, which are based on NT3 and IGF-1 raised expression. This will shed a new light on the development of optimal therapeutic strategies for clinical AD treatment in a sooner future both in theory and practice by using Gastrodin and other traditional Chinese medicines.
INTRODUCTION
Gastrodin is the main bioactive constituent of Rhizoma Gastrodiae, which is known as a famous Chinese herb. As a kind of bioactive Chinese medicine, it has been applied for the treatment of folk symptoms, such as headache, dizziness, epilepsy, stoke, amnesia since its identification from ancient times. In recent years, gastrodin has been proved to have neuroprotective roles which may be dependent on its action of scavenging ROS and reducing lipid perioxidation in neurodegenerative diseases, including Alzheimer’s Disease (AD), Parkinson’s Disease (PD) and cerebral ischemic diseases, such as cerebral ischemic infarction [1-3]. Furthermore, several studies also focused on the anti-apoptotic activity of gastrodin on particular cell types [4,5]. These characteristics ensure its applicable studies in the therapy of CNS diseases, with aim to elucidate the underlying mechanism of Gatrodin, and will deepen the insight in a better therapeutic strategy for AD by utilizing Gatrodin or other traditional Chinese Medicines. Alzheimer’s Disease (AD), characterized by a progressive decline in cognitive function from a previously established level, and is the most common cause of all the dementias. Although the exact etiology remains largely unknown, there are several theories about its causes, including possible genetic, immunological, biochemical and viral caused ones. Along with the coming of the aging the incidence rate of AD raises year by year. It has been estimated that 50-70% of those affected with dementia in these populations suffer from Alzheimer ‘s disease. In China, the incidence of senile dementia (age of onset ≥65 years) is ~6%, with Alzheimer’s Disease (AD) being the most common form, accounting for ~65% of cases [7-10]. It is clearly can be seen that nowadays, AD creates a heavy burden to both patients’ families and society and exerts a huge challenge to economic development and elderly health care. However, there are few therapeutic methods [11-16] effective to AD healing, or even ameliorating. Some methods or drugs, although have a few effects,could not exert stable and persistent therapeutic effects due to the vague mechanisms underlying different treatments. In this study, a therapeutic research that would be anticipated to play neuroprotective roles in AD rat model by using Gastrodin was carried out, combined with the exploration of the molecular mechanism underlying Gastrodin’s neuroprotective effects. These would provide a deeper insight into more preferable therapeutic strategies for AD clinical therapy by using Gastrodin or other traditional Chinese medicines, rather than existing drugs.
MATERIALS AND METHODS
Animals and Grouping
A total of 28 male Sprague-Dawley (SD) rats, 90 days old, weighing 200-220g, were provided by the Experimental Animal Center of Kunming Medical University, Yunnan Province, China. The rats were bred under the laboratory conditions that the temperature of (25±2)?, and saturated humidity of 40-60% was maintained, with dark and light relay (12/12h). The rats were allowed to access food and drink ad libitum. They were randomized into 3 groups (Table 1): (1) sham operative group (only open a skull window, with fimbria-fornix intact) with Normal Saline (NS) injection (2) AD group with NS injection; (3) AD + Gastrodin injected intraperitoneally. All experiments regarding animal care and surgery were approved and guided by the National Institutional Animal Care of Kunming Medical University, China
Preparation of AD rat model and Drug treatment
AD rat model was induced by fimbria-fornix transection. Briefly, rats were immobilized on a test bench in the prone
| Group | Treatment: Behavior test: qPCR /WB | Treatment: Behavior test: IHC |
|---|---|---|
| (n) | (n) | |
| Sham (n=8) | 5 | 3 |
| AD (n=10) | 7 | 3 |
| AD+Gastrodin (50mg/kg) (n=10) | 7 | 3 |
position and anaesthetized with 2% Pentobarbital Sodium (50mg/kg). Then a midline incision over the scalp was made to expose the right parietal bone, and a 2mm×2mm bone window was made by using a manual trephine beside the midline scalp. Subsequently, a microblade of 2.0mm wide and 0.2mm thick was used to cut in along with the brain coronal plane according to the coordinate described previously (Sommer et al., 2017): 2.0 mm behind the coronal suture, 3.0 mm beside the midline scalp and 4.0 mm below the coronal. Then move the blade outward for 1 mm, then down for 1 mm, and up and down 10 times until the fimbria-fornix was transected. Finally, the microblade was moved 1 millimeters to the left and pulled out. Thereafter, Penicillin was given three days intramuscularly to prevent from infection following operation. Following the model establishment, the rats in AD+ Gastrodin group were injected intraperitoneally with a dose of 50mg/kg Gastrodin (Longjin Pharmaceutical Limited Company, Kunming, China). Meanwhile, rats in the sham operative and AD group were injected the NS intraperitoneally with identical volume. Then, the wound was sutured. The rats with dura injured were excluded from this study.
Morris Water Maze
On the 4th days after AD model establishment, the Morris water maze was used to evaluate the cognitive (learning and memory) function of three groups of rats for 6 days in order to verify the animal model. The Morris water maze (MWM) consists of a circular pool, underwater platform, high-definition camera, digital camera, display and statistical analysis software. The pool was filled with 30cm deep water (24±1?). A hidden escape platform was 15 cm from the pool’s edge and 2 centimeter below the water surface. The whole tank was divided into four quadrants. The hidden platform training session of the rats was conducted for four consecutive days. The escape latency of reaching the platform was recorded by a computer system (Shanghai, Jiliang). Then, after a week of Gastrodin treatment, another 6 days’ Morris water maze test was conducted to detect the effects of Gastrodin. On day 14 of the MWM test, the Probe test that was performed for 2 days, during which the platform was removed. The time cost in each quadrant and the time crossing the platform area was recorded within 60 s.
Sample Harvest
After the last day of MWM test, all rats in the three experimental groups were anaesthetized and sacrificed. The brain tissues were dehydrated by 30% sucrose overnight and then were cut into 10μm thick frozen section on the freezing microtome (Leica CM1900, Germany) for immunohistochemical staining. In addition, the hippocampal tissues used for Q-PCR and Western Blot was reserved in 1.5ml EP tube without RNase at -80?.
Q-PCR
Gastrodin treated for 14 days, the hippocampus tissues of all rats were harvested and homogenized. The total RNA was extracted from the hippocampal tissues by Trizol reagent (Takara, Japan). Then the RNA concentration was measured by spectrophotometer (Thermo fisher, USA) at wave length of 260/280nm RNA, a total of 3000ng, was reverse transcribed into cDNA with the Revert AidTM First Strand cDNA Synthesis kit (Thermo fisher, USA).RT cDNA synthesis was conducted in a reaction system of 20μl, containing reaction buffer (4 μl) reverse transcriptase (1μl), Oligo dT primer (1μl ), Ri block (1μl ), dNTP (2μl) and DEPC water, add up to 20μl according to manufacturer’s instructions (Takara, Japan). Subsequently, Q-PCR was performed using the specific primers (Takara, Japan), the sequences of the primers were shown in Table 2. With the cDNA as a template, the relative expression levels of NGF, BDNF, NT-3, IGF-1 and CNTF in the hippocampus of AD rats of all experimental groups were detected by Q-PCR. The sequences of the primers for Q-PCR were shown in Table 2. The reaction system comprised cDNA (1μl), former primer (0.6 μl), reverse primer (0.6 μl), nucleus free water (7.2 μl) and SYBR (10μl), 20μl in total. PCR amplification conditions were maintained as follows: predenaturation at 95? for 5 min, denaturation at 95? for 10 s and amplification at 53? for 10 s, followed by extension at 72? for 30s, 40 cycles in total. β-actin was used as an internal reference. All data were measured in triplicate. When gene expression level in QPCR was calculated, 2-??Ct method was used.
Western blot
The hippocampus tissues of the rats were collected, homogenized, and centrifuged (12000×5min, 4?). The protein was separated by a 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to the PVDF membrane. After blocked with 5% nonfat milk at room temperature for 1h, the membrane was incubated with the primary antibodies including β-actin, NGF, BDNF, NT-3, IGF-1 and CNTF and CHAT. After wash-out for 3 times, membranes were incubated with (HRP)-conjugated secondary antibodies. The information of the antibodies was shown in Tab 3. The ECL chromogenic liquid was used to capture the image information on the BIO-RAD gel imaging instrument, and the gray scales of the image was measured by Image J software.
| Group | Escape latency | Crossing times | Time stay in target zone |
|---|---|---|---|
| 1d 2d 3d 4d | |||
| AD model group* | 89.23±1.10 848.20±1.08 82.49±1.08 80.34±1.10 | 10.02±1.661 | 49.34±15.7 |
| Sham operative group | 85.62±7.05 81.30±6.95 81.52±7.01 80.56±7.03 | 9.92±1.665 | 50.16±15.6 |
| Gastradin treated group# | 44.14±2.74 41.63±2.7 39.31±2.75 37.75±2.70 | 5.91±1.663 | 59.09±15.5 |
Statistics
All data are expressed as the mean ±standard deviation (SD) and analyzed by one-way ANOVA and t-test using SPSS software (Version 19.0, SPSS Inc., Chicago, IL, USA)). A level of p<0.05 represents significant difference.
RESULTS
Gastrodin treatment leads to cognitive functional improvement in AD rats
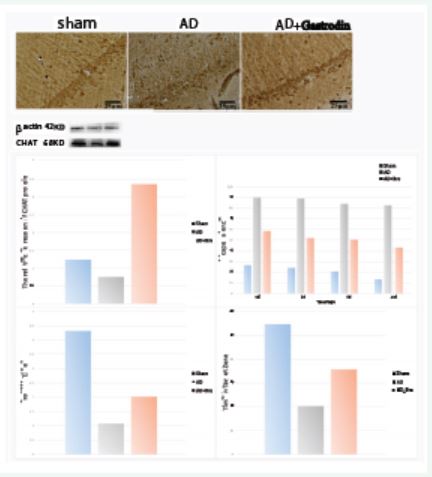
Revealed by Western blot (Figure 1b, p<0.01) and immunohistochemistry (Figure 1a), the expression level of ChAT in the hippocampus of AD rats in AD+ Gastrodin group was significantly higher than that of the AD model group. Revealed by Morris water maze test (Figure 1C-D), the rats in AD+ Gastrodin group showed a markedly shorter escape latency compared with AD model group in the four days’ training course (Figure 1C, p<0.01). In the probe test, both the number of times across the platform area and the time course stay in the target quadrant in AD+Gastrodin
group was substantially larger or longer than that of AD model group, respectively (Figure 1D-E, p<0.01). Note: Δ, # compared with * respectively, for escape latency, crossing time and times study in the target zone, p<0.01.
The expression change of neurotrophic factors
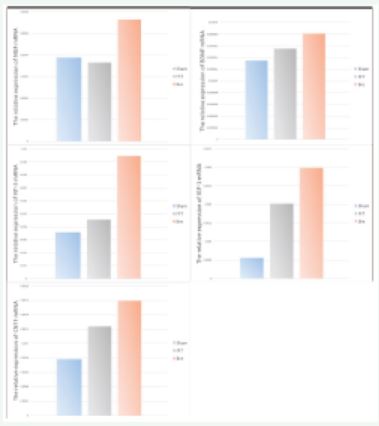
Revealed by QPCR and Western blot, the mRNA and protein expression level of NGF, BDNF, NT-3, IGF-1 and CNTF in AD+ Gastrodin group was substantially higher than that of AD model group, respectively. Among them, the elevation amplitude of NT-3 and IGF-1 lay in the most and second biggest, and NGF lay in the third (Fig2.)
Figure 1
Figure 1: Gastrodin treatment leads to improvement of cognitive function in rats subjected to AD. Immunohistochemical staining of CHAT in the hippocampus among the sham, AD and AD+Gastrodin group. Comparison of the CHAT protein levels among the sham, AD and AD+Gastrodin group. Comparison of the average escape latency to the hidden platform during the four days’ training session among these three groups. Comparison of the average number of crossings of the platform area during the probe test. Comparison of the times of the rats lingered in the target quadrant in the probe test. Data was expressed as means±SD.
Figure 2
Figure 2: Expression changes of different neurotrophic factors in three groups. Among the sham, AD model and AD+Gastrodin groups, all neurotrophic factors detected (including NGF, NT-3, ISF-1, BDNF, CNTF) showed from higher to lower level from AD+Gastrodin to Sham group, the level of neurotrophic factors of AD model group was moderate; The elevation of NGF and NT-3 was the most and the second prominent among all neurotrophic factors detected.
DISCUSSION
Gastrodin [17-21] can be used for the therapy of various diseases, including CNS diseases and Psychiatrical diseases, owing to its function as neuroprotective roles, scavenging ROS and reducing lipid perioxidation actions. And in the mean time, because of its low toxicity and remarkable pharmacological performance, we attempt to explore whether or not Gastrodin can be applied for the AD treatment, and the associated potential mechanism of Gastrodin, which is largely unknown, awaits elucidate. It is well known that Gastrodin has been already used in clinical practice for many kinds of diseases, if it could be verified to have effective actions on AD, it will greatly Facilitate Gastrodin’s usage in AD clinical medical therapy, bringing about more effective treatment and better prognosis. In this study, it has been found that Gastrodin, when intraperitoneally injected into the AD rat model, could effectively ameliorate the behavior of Morris Water Maze, i.e. significantly shortened the escape latency, and increased the times for AD rats to pass through the platform quadrant, and significantly extended the time course for AD rats to stay at the target quadrant, accompanied with ChAT expression markedly elevated, when compared to the AD model group. Furthermore, with aim to intensively elucidate the potential mechanism implicated with Gastrodin’s roles in behavior and pathological alterations above mentioned, the expression levels of some major neurotrophic factors in the hippocampus of AD rat model were detected following Gastrodin was injected. Results revealed that following Gastrodin injection, the expression level of some neurotrophic factors, especially NT-3 and IGF-1, was greatly up regulated with the most and the second largest increase amplitude, respectively, and others, including NGF, BDNF and CNTF, was also markedly elevated. It therefore clearly can be seen that Gastrodin, based on the neuroprotective molecular mechanism mainly exerted by NT-3 and IGF-1, plays a prominent role in improving the behavior and pathology in AD rats, expressed as escape latency shortening in MWM and ChAT’s expression elevation in the hippocampus. Taken together, we firstly found the potential molecular mechanism that tightly associated with Gastrodin’s roles in the amelioration of behavior and pathology in AD rat model, was most likely dependant on the neuroprotective effects, which were mainly produced by substantial up regulation of endogenous NT-3 and IGF-1 following Gastrodin intraperitoneal injection. In sooner future, a novel and promising therapeutic strategy will be applied for clinical AD treatment, by using Gastrodin, or some other bioactive substance, combined with gene interventional therapy, for example, NT-3 and/or IGF-1 gene over expression and were introduced into the hippocampus of AD patients, based on this, a new light will be shed on AD improvement, especially in behavior and pathology. It is a feasible and hopeful scheme dependant on the neuroprotective mechanism we found in the present study.
REFERENCES