The Role of Ghrelin in Patients with Stroke
- 1. Department of Neurology, Spital Linth, Uznach, Switzerland
- 2. Department of Medicine, Kantonsspital Winterthur, Winterthur, Switzerland
Abstract
Introduction: Ischemic stroke is the second leading cause of death and disability worldwide. Therefore, it is necessary that more neuroprotective treatments could be found. Ghrelin is a 28 amino acid peptide that is principally synthesized in the stomach mucosa but is also expressed in a variety of other tissues. Beside the well-known role in feeding and metabolism, there are some findings, that ghrelin could be neuroprotective in patients after stroke.
Methods: Patients with a first acute ischemic stroke with functionally relevant hemi- symptomatic symptoms were included until 24 hours after the event. The patient sample consisted of 14 patients with stroke (mean age = 73.6 years, range = 59 – 84 years, 6 female, 7 male, Table 1). All patients underwent the nine-hole peg test (NHPT) and a blood draw (ghrelin) on day one and three after stroke. In addition, the de Morton Mobility Index (DEMMI) was performed.
Results: The comparison of the outcome of the patients with lower and higher ghrelin blood concentrations adjusted for age and gender revealed a trend of the patients with higher levels of ghrelin and a better outcome (DEMMI).
Discussion: Ghrelin may be neuroprotective in patients with an acute stroke.
However, further studies are needed to prove it.
KEYWORDS
- Ghrelin
- Stroke
- De Morton Mobility Index
- Nine-hole peg test
CITATION
Czell D, Ballmer PE (2025) The Role of Ghrelin in Patients with Stroke. J Neurol Disord Stroke 12(1): 1232.
INTRODUCTION
Stroke is the second leading cause of death and one of the most common neurological causes of permanent disability. It is also the second leading cause of dementia worldwide [1]. It is therefore important that, in addition to optimizing the processes in the rescue chain, measures and medication are found that improve the outcome and, above all, the functional impairment. Ghrelin is known as a “hormone of hunger” as it acts as an antagonist to leptin and regulates our need for food intake. Mainly it is produced in the stomach but also in a variety of other tissues [2,3]. It is therefore also involved in the development of obesity and plays an important role of its treatment [4-7].
In recent years it has been shown that ghrelin plays a role in the processes of the central nervous system in addition to its important metabolic functions. On the one hand, it is involved in the creation and establishment of motivation and rewards [8,9], in the creation and maintenance of stress as well as in learning and the transfer of information into our memory including in reward and motivation [8,9], as well as learning and memory [9-12]. The role of ghrelin in preventing apoptosis of cells and protecting against ischemia in heart muscle cells also seems to be important regarding the protection of strokes [13-15]. Also, in neurodegenerative diseases such as Parkinson disease (PD) or Alzheimer disease a neuroprotective effect could be found [16] and also in stroke in animals [17]. In an animal study with rodents ghrelin was given for three days after an ischemic injury of the brain. It could be shown that the number of surviving neurons increased. Also the neurological deficit, infarct size, and survival of cortical neurons in rodents were improved after an artificial stroke [18,19]. In a trial with patients with a cardioembolic stroke the ghrelin blood concentrations were lower compared with healthy controls indicating that ghrelin may play a role after a stroke [20].
In this study we wanted to investigate whether ghrelin can act as a diagnostic and prognostic laboratory parameter in patients with ischemic stroke. Do patients with a higher ghrelin level at the time when the stroke take place have a better outcome.
MATERIALS AND METHODS
Patients
The prospective single-centre study was approved by the Cantonal Research Ethics Committee of Zurich (Zurich, Switzerland). Patients were recruited at the stroke unit of the Kantonsspital Winterthur (Winterthur, Switzerland) and informed written consent was obtained in accordance with the declaration of Helsinki from all study participants. Patients with a first acute ischemic stroke with functionally relevant hemi-symptomatic impairments were included until 24 hours after the event. Exclusion criteria were patients with relevant dysphagia, nutritional risk screening of 3 [21] neurodegenerative diseases, patients with previous ischemic insults, clinically relapsed polyneuropathy, patients with gait disorders due to microangiopathic changes of the brain or rheumatic diseases, patients with previous psychiatric diseases and antidepressant or neuroleptic medication, as well as patients with congenital and/or acquired substance defects of the brain and/or spinal cord.
The patient sample consisted of 14 patients with stroke (mean age = 73.6 years, range = 59 – 84 years, 28 female, 25 male, Table 1).
Table 1: Characteristic (sex and age) and results (ghrelin level (day 1 and 3), DEMMI (day 1-3), NHPT (day 1 and 3) of the patients
|
Patient |
Sex |
Age |
Ghrelin [pg/ml] |
DEMMI |
NHPT |
|
1 |
m |
72 |
140/124 |
76/79/81 |
24/21 |
|
2 |
m |
81 |
85/72 |
56/57/57 |
19/17 |
|
3 |
f |
79 |
132/101 |
61/67/75 |
27/24 |
|
4 |
m |
64 |
82/67 |
67/71/72 |
31/22 |
|
5 |
f |
69 |
188/163 |
83/88/92 |
24/20 |
|
6 |
f |
74 |
105/77 |
88/93/94 |
22/19 |
|
7 |
m |
79 |
67/57 |
56/57/57 |
23/20 |
|
8 |
m |
57 |
103/88 |
67/72/78 |
25/22 |
|
9 |
f |
76 |
145/123 |
68/87/89 |
24/19 |
|
10 |
f |
84 |
178/167 |
66/78/84 |
26/22 |
|
11 |
m |
65 |
163/124 |
81/85/92 |
23/19 |
|
12 |
m |
73 |
78/56 |
67/69/72 |
22/21 |
|
13 |
f |
77 |
134/112 |
73/79/86 |
25/24 |
|
14 |
m |
83 |
156/63 |
72/76/81 |
25/21 |
All patients underwent the nine hole peg test (NHPT) and the blood draw (ghrelin) on day one and three days after stroke (Table 2). In addition, the de Morton Mobility Index (DEMMI) was performed (see details below)
Nine Hole Peg Test (NHPT)
The NHPT consists of a board (wood): with 9 holes (10 mm diameter, 15 mm depth), placed apart by 32 mm and a container for the pegs: square box (100 x 100 x 10 mm) apart from the board. The participants were instructed to remove all the pegs from the holes, one by one, and place them into the container and then to take back the pegs from the container, one by one, and replace them into the holes on the board, as quickly as possible. The board should be placed at the client’s midline, with the container holding the pegs oriented towards the hand being tested. Only the hand being evaluated should perform the test, the hand not being evaluated was permitted to hold the edge of the board in order to provide stability. The scores are based on the time taken to complete the test activity, recorded in seconds. The stopwatch started from the moment the participant touches the first peg until the moment the last peg entered the last hole [21].
De Morton Mobility Index (DEMMI)
The DEMMI is a performance test with 15 items divided into five categories (mobility in bed, on the chair, standing, walking and dynamic balance). It was developed in Australia in 2008, validated in 2011 at the University of Health in Bochum for the German-speaking area on geriatric rehab patients. examination also possible in the patient’s bed/room. They were carried at day one, two and three after stroke. Values (DEMMI score) can be achieved between 0 and 100. Higher scores mean a higher degree of mobility. The lowest clinically relevant difference is given as 10 points. There is no provision for grading individual mobility levels based on scores.
Ghrelin
Ghrelin was taken in the routine blood collection on the stroke unit on day 1 and day 3. It was brought cooled into the laboratory and stored there at -80 degrees. Ghrelin concentrations were determined by ELISA analysis in the Institute of Veterinary Medicine in Zurich by Prof. Lutz.
STATISTICAL ANALYSIS AND RESULTS
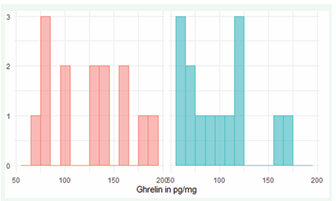
There was a slight preponderance of male patients (8 male, 6 female). The age distribution showed that the patients were predominantly older adults, with a mean age of 73.8, a median of 75, and an age range of 57 to 84 years. Peptide ghrelin concentrations vary widely between patients, with a range of 67 to 188 pg/ml on the first day and 56 to 167 pg/ml on the third day. On average, the values on the first day were significantly higher with a mean value of 125 pg/ml.4 compared to the third day with 99 pg/ml.57 (Figure 1A).
Figure 1a: Ghrelin (pg/mg) day 1 (red) and 3 (blue)
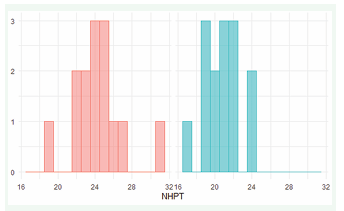
For the nine-hole peg test, there was a general decline in test scores from a mean of 24.29 on the first day to a mean of 20.79 on day 3 (Figure 1b).
Figure 1b: NHPT on day 1 (red) and 3 (blue)
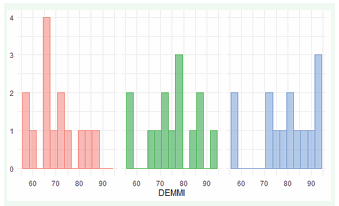
The DEMMI scales showed increasing mobility over the three days. The mean value increased from 70.07 (Day 1) to 75.57 (Day 2) to 79.29 (Day 3) (Figure 1c).
Figure 1c: DEMMI on day 1 (red), 2 (green) and 3 (blue)
In the following, four different multiple regressions were calculated to determine the influence of gender and ghrelin on the functional tests (DEMMI and NHPT) at both test points. The results of the linear regression show that one day after the stroke, the independent variables sex (sex), age (age) and ghrelin could not explain a significant proportion of the variance of the dependent variable DEMMI. The F-value was =2.667, with a corresponding p-value of 0.105, indicating a lack of significance. The coefficient of certainty R² was 0.445, which means that the model explains about 44.5% of the variance of the dependent variable. The adjusted R² was 0.278. This indicates a moderate adjustment of the model. Despite the lack of significance, the effect size (f²=0.385) showed a medium effect accordingly. The individual regression coefficients showed that no significant effect can be found at a α = 0.05 level as well (Table 2).
Table 2: Overview of the time line when NHPT and DEMMI were performed and ghrelin level were taken
|
First day |
Second day |
Third day |
|
Nine-hole-Peg-Test |
|
Nine-hole-Peg Test |
|
Modifizierte Rankin-Scale2 |
|
Modifizierte Rankin-Scale |
|
Ghrelin |
|
Ghrelin |
|
De Morton Mobility Index (DEMMI) |
DEMMI |
DEMMI |
Table 2: Regression model 1
|
Predictor |
b |
b 95% CI [LL, UL] |
sr2 |
sr2 95% CI [LL, UL] |
Fit |
|
(Intercept) |
95.34** |
[43.33, 147.35] |
|
|
|
|
Sex female |
3.80 |
[-7.94, 15.54] |
.03 |
[-.10, .16] |
|
|
Age |
-0.56 |
[-1.23, 0.12] |
.19 |
[-.13, .51] |
|
|
Ghrelin_1 |
0.11 |
[-0.03, 0.26] |
.16 |
[-.14, .46] |
|
|
|
|
|
|
|
R2 = .445 |
|
|
|
|
|
|
95% CI[.00,.63] |
The results of the linear regression showed that three days after stroke, a significant proportion of the variance of the dependent variable DEMMI could be explained by the independent variables sex (sex), age (age) and ghrelin. The F-value was (3,10) =4.173, with a corresponding p-value of 0.037, indicating a significant result of the overall model. The coefficient of certainty R² was 0.556, which means that the model explains about 55.6% of the variance of the dependent variable. The adjusted R² was 0.423. This indicates a good adaptation of the model. The effect size (f²=0.733) showed a strong effect according to Cohen (1992).
Despite the significant overall model, a consideration of the individual regression coefficients showed that no significant effect could be found at a α = 0.05 level (Table 3).
Table 3: Regression model 2
|
Predictor |
b |
b 95% CI [LL, UL] |
sr2 |
sr2 95% CI [LL, UL] |
Fit |
|
(Intercept) |
105.65** |
[44.34, 166.96] |
|
|
|
|
Sexweiblich |
11.09 |
[-3.40, 25.57] |
.13 |
[-.12, .38] |
|
|
Age |
-0.57 |
[-1.34, 0.21] |
.12 |
[-.12, .36] |
|
|
Ghrelin_3 |
0.11 |
[-0.08, 0.30] |
.07 |
[-.11, .25] |
|
|
|
|
|
|
|
R2 = .556* |
|
|
|
|
|
|
95% CI[.00,.70] |
The results of the linear regression showed that one day after the stroke, the independent variables sex (sex), age (age) and ghrelin could not explain a significant proportion of the variance of the dependent variable NHPT. The F-value was (3,10)=0.413, with a corresponding p-value of 0.747, which does not indicate a significant result of
the overall model. The coefficient of determinacy R² was 0.110, which means that the model explains about 11% of the variance of the dependent variable. The adjusted R² was negative at −0.157, indicating a very small model adjustment. The effect size (f²=-0.136) showed no effect according to Cohen (1992).
A look at the individual regression coefficients showed that no significant effect could be found at a α = 0.05 level as well (Table 4).
Table 4: Regression model 3
|
Predictor |
b |
b 95% CI [LL, UL] |
sr2 |
sr2 95% CI [LL, UL] |
Fit |
|
(Intercept) |
31.14** |
[12.11, 50.17] |
|
|
|
|
Sexweiblich |
0.94 |
[-3.36, 5.23] |
.02 |
[-.12, .16] |
|
|
Age |
-0.11 |
[-0.36, 0.14] |
.09 |
[-.19, .36] |
|
|
Ghrelin_1 |
0.01 |
[-0.05, 0.06] |
.01 |
[-.07, .09] |
|
|
|
|
|
|
|
R2 = .110 |
|
|
|
|
|
|
95% CI[.00,.31] |
The results of the linear regression showed that one day after the stroke, the independent variables sex (sex), age (age) and ghrelin could not explain a significant proportion of the variance of the dependent variable NHPT. The F-value was (3,10)=0.304, with a corresponding p-value of 0.822, which does not indicate a significant result of the overall model. The coefficient of certainty R² was 0.084, which means that the model explains about 8.4% of the variance of the dependent variable. The adjusted R² was negative at −0.191, indicating a very small model adjustment. The effect size (f²=-0.16) showed no effect according to Cohen (1992).
A look at the individual regression coefficients showed that no significant effect could be found at a α = 0.05 level as well (Table 5).
Table 5: Regression model 4
|
Predictor |
b |
b 95% CI [LL, UL] |
sr2 |
sr2 95% CI [LL, UL] |
Fit |
|
(Intercept) |
23.49** |
[8.86, 38.11] |
|
|
|
|
Sexweiblich |
1.36 |
[-2.10, 4.81] |
.07 |
[-.19, .33] |
|
|
Age |
-0.04 |
[-0.22, 0.15] |
.02 |
[-.12, .16] |
|
|
Ghrelin_3 |
-0.01 |
[-0.05, 0.04] |
.01 |
[-.07, .08] |
|
|
|
|
|
|
|
R2 = .084 |
|
|
|
|
|
|
95% CI[.00,.26] |
DISCUSSION
In our study we wanted to see whether ghrelin plays a role in patients suffering from stroke. In particular, we wanted to see if stroke patients have a better outcome when they have higher ghrelin concentrations than patients with low ghrelin to demonstrate a possible neuroprotective impact of ghrelin. A neuroprotective effect of ghrelin has been demonstrated in various animal studies. These included artificially generated strokes [22], brain injury [23], paraplegic syndromes [24] and damage to the hippocampus region [25] and substantia nigra [26]. So far, however, this neuroprotective effect could only be demonstrated in animal models. It was also shown that circulating ghrelin is lower in rats with acute stroke [20]. The reason for the low ghrelin after a stroke has not yet been clarified [27,28]. It would now be exciting to find out to what extent the animal models can be transferred to humans and whether ghrelin can play a role in the treatment of strokes.
In our study, most likely due to the low number of total patients, only a trend could be demonstrated, that patients with lower ghrelin concentrations had a worse outcome and patients with higher ghrelin concentrations had a better outcome. Further studies are needed to determine the mechanism of ghrelin and its neuroprotective role in acute stroke patients.
DECLARATIONS
Ethics approval and consent to participate The prospective single-center study was approved by the Cantonal Research Ethics Committee of Zurich (Zurich, Switzerland). Patients were recruited at the stroke unit of the Cantonal Hospital Winterthur (Zurich, Switzerland) and informed written consent was obtained in accordance with the declaration of Helsinki from all study participants.
Consent for publication
There is a signed consent of all patients for publication their data of this trial.
Authors` contributing
D.C. and P.B. carried out the investigation and supervised the project. D.C. wrote the manuscript with support from P.B.
Availability of supporting data
Supporting data is available from the author.
Trial registration
Name of the registry: Clincal Trial
Trial registration number: NCT 03264742 Date of registration: 02/28/2017
URL of trial registry record: www.clinicaltrial.gov
ACKNOWLEDGEMENT
We thank the neurology team of the cantonal hospital of Winterthur Switzerland for supporting us with this project
REFERENCES
- World Health Organizition (WHO). The top 10 causes of death.
- Lago F, Gonzalez-Juanatey JR, Casanueva FF, Gomez-Reino J, Dieguez C, Gualillo O, et al. the same peptide for different functions: Player or bystander? Vitam Horm. 2005; 71: 405-432.
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999; 402: 656-660.
- Cummings DE, Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol Behav. 2006; 89: 71-84.
- Tschop M, Smiley DL, Heiman ML, Ghrelin induces adiposity in rodents. Nature. 2000; 407: 908-913.
- Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 2003; 37: 649-661.
- Briggs DI, Andrews ZB. Metabolic status regulates ghrelin function on energy homeostasis. Neuroendocrinology. 2011; 93: 48-57.
- Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006; 116: 3229-3239.
- Naleid AM, Grace MK, Cummings DE, Levine AS. Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides. 2005; 26: 2274-2279.
- Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, Horvath B, et al. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 2006; 9: 381-388.
- Spencer SJ, Xu L, Clarke MA, Lemus M, Reichenbach A, Geenen B, et al. Ghrelin regulates the hypothalamic-pituitary-adrenal axis and restricts anxietyafter acute stress. Biol Psychiatry. 2012; 72: 457-465.
- Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, Jung S, et al. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci. 2008; 11: 752- 753.
- Baldanzi G, Filigheddu N, Cutrupi S, Catapano F, Bonissoni S, Fubini A, et al. Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT. J Cell Biol. 2002; 159: 1029-1037.
- Chang L, Ren Y, Liu X, Li WG, Yang J, Geng B, et al. Protective effects of ghrelin on ischemia/reperfusion injury in the isolated rat heart. J Cardiovasc Pharmacol. 2004; 43: 165-170.
- Frascarelli S, Ghelardoni S, Ronca-Testoni S, Zucchi R. Effect of ghrelin and synthetic growth hormone secretagogues in normal and ischemic rat heart. Basic Res Cardiol. 2003; 98: 401-405.
- Andrews ZB. The extra-hypothalamic actions of ghrelin on neuronal function. Trends Neurosci. 2010; 34: 31-40.
- Liu Y, Wang PS, Xie D, Liu K, Chen L. Ghrelin reduces injury of hippocampal neurons in a rat model of cerebral ischemia/ reperfusion. Chin J Physiol. 2006; 49: 244-250.
- Miao Y, Xia Q, Hou Z, Zheng Y, Pan H, Zhu S. Ghrelin protects cortical neuron against focal ischemia/reperfusion in rats. Biochem Biophys Res Commun. 2007; 359: 795-800.
- Cheyuo C, Wu R, Zhou M, Jacob A, Coppa G, Wang P. Ghrelin suppresses inflammation and neuronal nitric oxide synthase in focal cerebral ischemia via the vagus nerve. Shock. 2011; 35: 258-265.
- Kantorova E, Chomova M, Kurca E, Sivak S, Zelenak K, Kucera P, et al. adiponectin and ghrelin, new potential mediators of ischemic stroke. Neuro Endocrinol Lett. 2011; 32: 716-721.
- Kondrup J, Hamberg O, Stanga Z. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003; 22: 321-36.
- Czell D, Neuwirth C, Weber M, Sartoretti-Schefer S, Gutzeit A, Reischauer C. Nine Hole Peg Test and Transcranial Magnetic Stimulation: Useful to Evaluate Dexterity of the Hand and Disease Progression in Amyotrophic Lateral Sclerosis. Neurol Res Int. 2019: 7397491.
- Miao Y, Xia Q, Hou Z, Zheng Y, Pan H, Zhu S. Ghrelin protects cortical neuron against focal ischemia/reperfusion in rats. Biochem. Biophys. Res Commun.2007; 359: 795-800.
- Bansal V, Ryu SY, Blow C, Costantini T, Loomis W, Eliceiri B, et al. The hormone ghrelin prevents traumatic brain injury induced intestinal dysfunction. J Neurotrauma.2010; 27: 2255-2260.
- Besecker EM, White AR, Holmes GM. Diminished gastric prokinetic response to ghrelin in a rat model of spinal cord injury. Neurogastroenterol Motil. 2018; 30: e13258.
- Liu Y, Wang PS, Xie D, Liu K, Chen L. Ghrelin reduces injury of hippocampal neurons in a rat model of cerebral ischemia/ reperfusion. Chin J Physiol. 2006; 49: 244-250.
- Moon M, Kim HG, Hwang L, Seo JH, Kim S, Hwang S, et al. Neuroprotective effect of ghrelin in the 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine mouse model of Parkinson’s disease by blocking microglial activation. Neurotox Res. 2009; 15: 332-347.
- Chung H, Seo S, Moon M, Park S. Phosphatidylinositol-3-kinase/Akt/ glycogen synthase kinase-3 beta and ERK1/2 pathways mediate protective effects of acylated and unacylated ghrelin against oxygen- glucose deprivation-induced apoptosis in primary rat cortical neuronal cells. J Endocrinol. 2008; 198: 511-521.s