Cytoarchitecture Alteration of the Cerebral cortex and Hippocampus by Cadmium Induced Neurotoxicity: The Ameliorating Effect of Launaea taraxacifolia Aqueous Extract
- 1. Department of Human Anatomy, Federal University of Technology, Nigeria
Abstract
Neurotoxicity is a major health concern due to the various mechanisms and severity of neurological damage caused by heavy metals such as cadmium. Launaea taraxacifolia has been demonstrated to be a powerful antioxidant to scavenge ROS and protect cells from oxidative damage. This study examined the effects of Launaea taraxacifolia aqueous extract (LTAE) in cadmium-induced neurotoxicity in wistar rats. A total of 32 wistar rats were randomly divided into four groups (n = 8 per group). Group I received distilled water orally for 21 days, Group II was administered 5 mg/kg body weight (bwt) of cadmium orally for 21 days, Group III received 400 mg/kg bwt of LTAE orally for 21 days, and Group IV was treated with 5 mg/kg bwt of cadmium followed by 400 mg/kg bwt of LTAE for 21 days. Cadmium exposure resulted in a significant (p < 0.05) reduction in body weight, increased MDA and decreased GSH levels, and caused alterations in the neuronal cytoarchitecture of the cerebral cortex and hippocampus. However, co-administration of LTAE with cadmium mitigated these toxic effects by reversing body weight loss, MDA and GSH levels, and restoring the microanatomy of the cerebral cortex and hippocampus. These findings suggest that LTAE has a protective effect against cadmium-induced neurotoxicity and may serve as a potential therapeutic strategy for mitigating the neurological consequences of cadmium exposure.
Keywords
• Cerebral cortex
• Hippocampus
• Cadmium
• Launaea taraxacifolia
• Neurotoxicity
Citation
OLAWUYI TS, AKINGBADE GT, ADEJOLA EO (2025) Cytoarchitecture Alteration of the Cerebral cortex and Hippocampus by CadmiumInduced Neurotoxicity: The Ameliorating Effect of Launaea taraxacifolia Aqueous Extract. J Neurol Transl Neurosci 10(1): 1102.
INTRODUCTION
Neurotoxicity refers to the adverse effects of biological, chemical, or physical agents on the structure or function of the central and peripheral nervous systems. It is primarily caused by exposure to neurotoxic substances such as lead, mercury, cadmium, and manganese [1]. Cadmium (Cd) is recognized as the seventh most hazardous industrial heavy metal, commonly found in food, water, soil, air, and tobacco. Due to its prolonged biological half-life of 20–30 years and slow elimination from the body, Cd accumulates in human tissues over time [2]. The widespread presence of cadmium and other contaminants has led to heavy metal-induced neurotoxicity becoming a significant public health concern [3]. Cadmium exposure has been shown to adversely affect multiple organs, including the brain, due to its long retention in the body [2 4]. Additionally, Cd is considered a potent neurotoxin, as it induces oxidative stress and alters neuronal membrane integrity [1]. The nervous system, both central and peripheral, is highly sensitive to cadmium toxicity [5]. The effects of Cd exposure include neurological disorders, peripheral neuropathy, olfactory dysfunction, cognitive impairments, and behavioral changes [6]. Furthermore, cadmium has been implicated in the pathogenesis of neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, and peripheral polyneuropathy [7,8]. One major pathway of cadmium-induced brain damage is its accumulation in brain tissue, where it disrupts energy metabolism, membrane integrity, and mitochondrial function by attacking thiol-containing proteins such as glutathione (GSH) [2]. This process results in oxidative damage and excessive reactive oxygen species (ROS) production [9]. Studies have shown that cadmium exposure alters the activity of key antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and GSH [2-5]. By increasing ROS production and impairing antioxidant defense mechanisms, Cd induces neuronal damage, leading to neurodegeneration and memory deficits [10]. Launaea taraxacifolia (LT), commonly known as “Wild Lettuce,” is a tall, rhizomatous annual or perennial plant that produces grayish-blue flowers [11]. In Nigeria, it is called “Yanrin” by the Yoruba, “Ugu” by the Igbo, and “Nonon Barya” by the Hausa. It is an upright perennial herb with rosette-shaped leaves at the base of the stem and is widely consumed as a vegetable in Africa. Due to its rich composition of flavonoids, vitamins, minerals, and other nutrients, L. taraxacifolia is known for its significant nutritional and therapeutic value [12]. Additionally, studies have reported its antiviral, anticancer, anti-inflammatory, and neuroprotective properties [13-16].This study aims to evaluate the neuroprotective potential of Launaea taraxacifolia aqueous extract against cadmium-induced neurotoxicity in adult male Wistar rats.
MATERIALS AND METHOD
Preparation and Concentration Launaea taraxacifolia Aqueous Extract
Fresh leaves of Launaea taraxacifolia were collected, air-dried for four days, and then ground into a fine powder. A total of 300 g of the powdered leaves was soaked in 4 liters of distilled water and heated at a constant temperature of 60°C for 24 hours. The mixture was then filtered using filter paper. The filtrate was concentrated in vacuum using a rotary evaporator and the yield (Y) was calculated by the formula below:
Y (%) = (Mass of extract) / (Mass of plant material used)×100
Experimental Animals
Thirty-two (32) adult male wistar rats, weighing 200–250 ± 15 g, were used for this study following ethical approval from the Center for Research and Development, Federal University of Technology, Akure. The rats were housed in well-ventilated cages under optimal temperature conditions and maintained on a 12-hour light/dark cycle. Before the commencement of administration, the animals were acclimatized for fourteen days. All procedures involving the rats were conducted in accordance with the National Institute of Health (NIH) Guidelines for the Care and Use of Laboratory Animals (2008) [17].
Experimental Design
The thirty-two Wistar rats were randomly assigned into four (4) different groups with eight rats in each group to ensure even distribution of mean body weight across groups. The body weight and the average weight of each group were taken and recorded every three days. All the experiments were held between 9:00 am and 3:00 pm. The animals were treated as follows Table 1.
Table 1: Animal Grouping and Drug Administration.
|
GROUPS |
TREATMENT |
ROUTE OF ADMINISTRATION |
|
Group I (Control) |
Distilled water |
Oral |
|
Group II |
5 mg/kg/bwt Cd only |
Oral |
|
Group III |
400 mg/kg/bwt LTAE only |
Oral |
|
Group IV |
5 mg/kg/bwt Cd, followed by 400 mg/kg/bwt LTAE |
Oral |
Intoxicated animal groups received daily oral administration of cadmium chloride (CdCl2 ) dissolved in distilled water at the dose of 5 mg/kg/bwt for 21 days using oral cannula. The dose of Cd was chosen based on several literature data showing a significant increase in Cd accumulation in brain tissues and alteration of the biochemical properties in the rat [9]. LTAE was dissolved in distilled water at the dose of 400 mg/kg/bwt and doses were administered every day for 3 weeks using oral cannula.
Histological Analysis
Brain samples were fixed in 10% neutral buffered formalin and processed for routine tissue processing using established protocols of haematoxylin and eosin for general neuronal architecture [18]. Stained sections were observed under a light microscope. Image J software was used to analyze and quantify photomicrographs for intact neurons.
Biochemical Analysis
Malondialdehyde Assay: Malondialdehyde (MDA), a secondary product of oxidative stress formed during lipid peroxidation (LPO), was determined as described by Manal et al. [18].
Glutathione Assay: GSH activity (to determine antioxidant status) was analyzed using the method of Manal et al. [18].
Statistical Analysis: Statistical evaluation was performed using one-way ANOVA followed by Tukey post hoc test using GraphPad Prism 8.0. All values were expressed as mean ± standard error of mean (SEM). A P-value <0.05 was considered significant.
RESULTS
Observatory Report on Average Body Weight of Wistar Rats
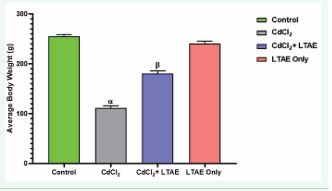
The total weight gain/loss was determined by subtracting the initial weight from the final weight. As shown in Figure 1,
Figure 1 Effects of LTAE on body weight of wistar rats across groups. All data were estimated with the help of one-way ANOVA trailed by Tukey’s multiple comparisons tests. Data were expressed as mean ± S.E.M. β: significant change with respect to control; α: significant change with respect to CdCl2. Value of p < 0.05 was considered significant.
there was a significant body weight loss (p < 0.05) in the animals treated with CdCl2 only compared to the controls. In addition, the body weight loss observed in animals in the CdCl2 +LTAE group was significantly different from the CdCl2 only group (p < 0.05). While body weight gain in LTAE group was significantly higher compared to the CdCl2 only group (p < 0.05), but not different from the control group.
Effect of LTAE on Malondialdehyde in Cd-induced Neurotoxicity
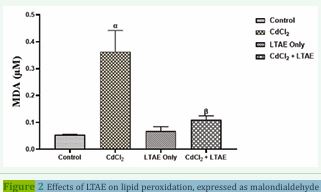
Figure 2 Effects of LTAE on lipid peroxidation, expressed as malondialdehyde (MDA). All data were estimated with the help of one-way ANOVA trailed by Tukey’s multiple comparisons tests. Data were expressed as mean ± S.E.M (n =5). α: significant change with respect to control; β: significant change with respect to CdCl2. Value of p < 0.05 was considered significant.
Figure 2 shows the MDA level in the brain homogenates of wistar rats across groups. There was a significant increase in lipid peroxidation, expressed as malondialdehyde (MDA) in CdCl2-treated group compared with the other groups (p < 0.05). In addition, rats that received CdCl2 + LTAE and LTAE only showed a significant decrease in the MDA levels as compared with CdCl2 group (p < 0.05).
Effect of LTAE on GSH in Cd-induced Neurotoxicity
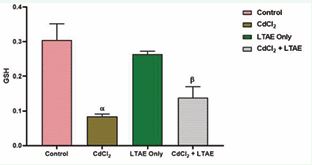
Figure 3 shows the GSH level in the brain homogenates of wistar rats across groups. There was a significant decrease in the level of glutathione in CdCl2-treated group compared with the control group (p < 0.05). In addition, rats that received CdCl2 + LTAE and LTAE only showed a significant increase in the GSH levels as compared with CdCl2 group (p < 0.05).
Figure 3 Effect of LTAE on glutathione levels in cadmium-induced neurotoxicity in rat brain across groups. All data were estimated with the help of one-way ANOVA trailed by Tukey’s multiple comparisons tests. Data were expressed as Mean ± S.E.M (n =5). α: significant change with respect to control; β: significant change with respect to CdCl2 . Value of p < 0.05 was considered significant.
Effects of LTAE on the Histology of the Brains of Wistar Rats
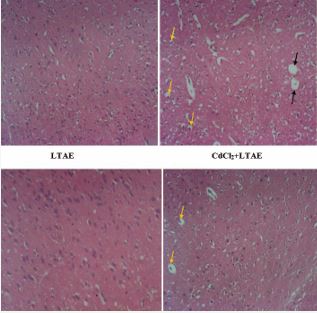
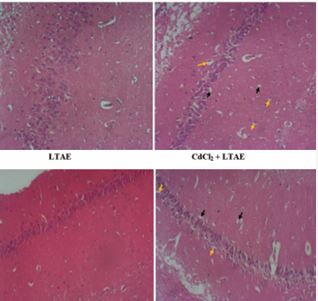
Plate 1 Representative photomicrographs of Haematoxylin and Eosin staining of the cerebral cortex of Wistar rats, magx100. The cerebral cortex of the control group presents normal neuronal morphology. In contrast, the cadmium group (CdCl2) was characterized by various degenerative changes with pyknotic nuclei (yellow arrows) and neuronal vacuolation (black arrows). LTAE alone treated group with well-organized neuronal cells. CdCl2+LTAE treated group with reduced pyknotic cells (yellow arrows) and absent of neuronal vacuolation comparable to the control. (CdCl2= Cadmium chloride; LTAE= Launaea taraxacifolia aqueous extract).
Plate 2 Representative photomicrographs of Haematoxylin and Eosin staining of the CA1 region of the hippocampus of Wistar rats, magx100. The CA1 region of the control group presents normal neuronal morphology. In contrast, the cadmium group (CdCl2) was characterized by various degenerative changes with pyknotic nuclei (yellow arrows) and neuronal vacuolation (black arrows). LTAE alone treated group with well-organized neuronal cells. CdCl2+LTAE treated group with reduced pyknotic cells (yellow arrows) and reduced neuronal vacuolation (black arrows) comparable to the control. (CdCl2= Cadmium chloride; LTAE= Launaea taraxacifolia aqueous extract).
Plate 1 and 2 are the photomicrographs of the haematoxylin and eosin staining of the cerebral cortex and hippocampus of Wistar rats across groups, respectively. Microscopic examination of the control and the LTAE alone treated group shows normal basic histological features of the cerebral cortex and hippocampus. However, the cadmium group (CdCl2 ), shows disorganization of neuronal cell layers, with degenerating and pyknotic nuclei, chromatolytic cells and a reduced layer of neuronal cells and neuronal vacuolation. The neuronal cells were however preserved in the cadmium treated groups (CdCl2 +LTAE). These results show that LTAE was able to protect the histo-architectural morphology of the cerebral cortex and hippocampus following cadmium administration.
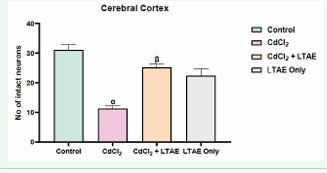
Figure 4 Morphometric assessment of intact neurons in the cerebral cortex of rat brain across groups. All data were estimated with the help of one-way ANOVA trailed by Tukey’s multiple comparisons tests. Data were expressed as Mean ± S.E.M. α: significant change with respect to control; β: significant change with respect to CdCl2. Value of p < 0.05 was considered significant.
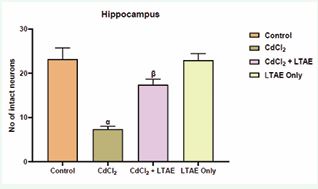
Figure 5 Morphometric assessment of intact neurons in the cerebral cortex of rat brain across groups. All data were estimated with the help of one-way ANOVA trailed by Tukey’s multiple comparisons tests. Data were expressed as Mean ± S.E.M. α: significant change with respect to control; β: significant change with respect to CdCl2. Value of p < 0.05 was considered significant.
One way ANOVA test [Figure 4 and 5] shows a significant decrease in the neuronal population of cells in the animal group administered with CdCl2 alone. But with a co-administration of CdCl2 and LTAE, there was a significant increase in the number of neuronal cells compared with neuronal counts from cadmium treated group. Maximal neuronal cell population was seen in the control group. This implied that CdCl2 induced neuronal cell degeneration while LTAE restored and increased the populations of neuronal cells.
DISCUSSION
Cadmium, a highly toxic heavy metal, is known to induce various pathological alterations in the nervous system, primarily through mechanisms such as oxidative stress, inflammation, and disruption of neuronal function [19]. This study demonstrates that L. taraxacifolia can effectively counteract these detrimental effects, highlighting its potential as a therapeutic agent for mitigating cadmium-induced neurotoxicity due to its rich phytochemical composition.Body weight changes are commonly used as an indicator of an animal’s overall health and as a measure of the toxic effects of metals and medications [20]. In this study, exposure to cadmium resulted in significant body weight loss, as observed in the cadmium-treated group (CdCl?). This finding aligns with previous research, which has shown that cadmium exposure leads to weight reduction due to nutrient depletion and metabolic dysfunction [9]. However, rats that received L. taraxacifolia aqueous extract alongside cadmium (CdCl?+LTAE) exhibited less weight loss compared to the cadmium-only group. This observation is consistent with the findings of Idorenyin et al., who reported that the ethanolic extract and fractions of L. taraxacifolia reversed body weight loss induced by aluminum chloride [21]. The antioxidant properties of L. taraxacifolia may help mitigate cadmium-induced toxicity by enhancing metabolic processes and preventing excessive weight loss, suggesting its potential neuroprotective role in cadmium-induced neurotoxicity. MDA levels are frequently raised when ROS attack cellular membranes, resulting in structural damage and membrane integrity loss [22]. In this study, cadmium exposure resulted in a significant increase in MDA, a lipid peroxidation product that serves as a key indicator of oxidative damage, as evident in cadmium treated group. This finding is in agreement with previous research whereby a significant elevation in the MDA level in serum of cadmium-treated rats was reported indicating that cadmium exposure disrupts cellular homeostasis by inducing lipid peroxidation [23]. Furthermore, glutathione (GSH), a major intracellular antioxidant, was significantly altered in the brains of cadmium-exposed rats. Specifically, the results demonstrate a marked decrease in GSH concentrations following cadmium administration. This reduction in GSH concentration is consistent with several studies that have reported a depletion of this crucial antioxidant after exposure to cadmium, suggesting that cadmium either directly consumes GSH or induces its breakdown through mechanisms involving oxidative stress [24]. Treatment with Launaea taraxacifolia aqueous extract significantly attenuated the increase in MDA levels and restored GSH levels in the brain, as evident in the ameliorative group (CdCl2+LTAE). This finding is in agreement with previous research whereby a significant reduction in MDA level and elevation in GSH level in the brain of cadmium-treated rats was reported [25]. These findings imply that the plant extract might strengthen the antioxidant defense system in order to produce its protective effects.Histopathological analysis of brain tissue revealed significant neuronal damage in cadmium-treated rats,characterized by pyknotic nuclei, a reduced neuronal cell layer, and neuronal vacuolation, particularly in regions associated with cognitive functions such as the hippocampus and cerebral cortex. These pathological changes observed in cadmium-exposed rats (CdCl? group) are in agreement with previous studies that reported disrupted tissue cytoarchitecture and nuclear damage in the brains of cadmium-treated animals [26,27]. However, administration of L. taraxacifolia aqueous extract appeared to ameliorate these histopathological changes, as seen in the ameliorative group (CdCl?+LTAE), where there was a reduction in neuronal damage and improved preservation of tissue architecture compared to the cadmium-only group. These findings are in line with the study by Owoeye et al., which reported significant histological improvement in the brains of rats co-treated with L. taraxacifolia aqueous extract and cisplatin [28]. Similarly, Olatunde and Onwuka demonstrated that L. taraxacifolia ethanolic extract reversed alterations in CA3 and Purkinje cells induced by lead acetate [29,30]. These histological findings support the neuroprotective potential of L. taraxacifolia and suggest that it may provide cellular defense against the toxic effects of cadmium.The observed reduction in neuronal damage in the L. taraxacifolia-treated group may be attributed to the plant’s anti-inflammatoryand antioxidant properties. Additionally, its ability to modulate cell death pathways may contribute to the preservation of neuronal integrity. Hematoxylin and eosin (H&E) staining of brain tissue further confirmed these findings by assessing neuronal viability. In the cadmium- only group, a significant reduction in neuronal cell density was observed, indicating neuronal degeneration and loss. Conversely, rats treated with L. taraxacifolia exhibited higher neuronal cell density, suggesting that the extract protects against cadmium-induced neurodegeneration. Launaea taraxacifolia may have neuroprotective effects by attenuating cytoarchitectural distortion, neuronal loss and neuroinflammation. This is evident from the improved neuronal cell count and preservation of neuronal integrity observed in the treated groups.
CONCLUSION
The findings of this study indicate that cadmium exposure can lead to neurotoxicity, while the administration of Launaea taraxacifolia aqueous extract may mitigate these effects by reducing body weight loss, MDA level, increasing GSH level, preserving cytoarchitecture, preventing neuronal loss, and alleviating neuroinflammation. These results suggest that L. taraxacifolia aqueous extract holds potential as a therapeutic strategy for combating cadmium induced neurodegeneration.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author, EOA.
REFERENCES
- Devendra KM, Dipti S, Zeeshan F, Lucy M, Himani A. Neuroprotective Potential of Flaxseed Oil in Amelioration of Cadmium Induced Neurotoxicity. Hygia Institute of Pharmaceutical Education and Research. 2022.
- Branca JJV, Fiorillo C, Carrino D, Paternostro F, Taddei N, Gulisano M, et al. Cadmium-Induced Oxidative Stress: Focus on the Central Nervous System. Antioxidants (Basel). 2020; 9: 492.
- Saikat M, Arka JC, Abu MT, Talha BE, Firzan N, Ameer K, et al. Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. Journal of King Saud University – Science. 2022; 34: 101865.
- Charkiewicz AE, Omeljaniuk WJ, Nowak K, Garley M, Nikli?ski J. Cadmium Toxicity and Health Effects-A Brief Summary. Molecules. 2023; 28: 6620.
- Arruebarrena MA, Hawe CT, Lee YM, Branco RC. Mechanisms of Cadmium Neurotoxicity. Int J Mol Sci. 2023; 24: 16558.
- Branca JJV, Morucci G, Pacini A. Cadmium-induced neurotoxicity: still much ado. Neural Regen Res. 2018; 13: 1879-1882.
- Bia?czyk A, We?niak A, Kami?ska B, Czajkowski R. Oxidative Stress and Potential Antioxidant Therapies in Vitiligo: A Narrative Review. Mol Diagn Ther. 2023; 27: 723-739.
- Wang B, Du Y. Cadmium and its neurotoxic effects. Oxid Med Cell Longev. 2013; 2013: 898034.
- Shagirtha K, Muthumani M, Prabu SM. Melatonin abrogates cadmium induced oxidative stress related neurotoxicity in rats. Eur Rev Med Pharmacol Sci. 2011; 15: 1039-1050.
- Alese MO, Bamisi OD, Alese OO. Progesterone modulates cadmium- induced oxidative stress and inflammation in hepatic tissues of Wistar rats. Int J Clin Exp Pathol. 2021; 14: 1048-1055.
- Ayobola MS, Ejeoghene RA, Olufemi IA. Potential of Launea taraxacifolia (Willd) Amin Ex. C. Jeffrey for in Vitro Regeneration. Not Sci Biol. 2011; 3: 93-96.
- Oluwole IA, Oluwadara BM, Omotayo AE, Esthinsheen O, Olusanya A, Ayodeji A, et al. Neuroprotective Potential of the Methanolic Leaf Extract of Launaea taraxacifolilia and Oil of Dennettia Tripetala Seed in Sub-chronic Lead Toxicity in Offspring of Rats. J Heavy Met Toxicity Dis. 2023; 8: 006.
- Ololade ZS, Kuyooro SE, Ogunmola OO, and Abiona OO. Phytochemical, Antioxidant, Anti-Arthritic, Anti- Inflammatory and Bactericidal Potentials of the Leaf Extract of Lactuca teraxacifolia. Global J Med Res. 2017.
- Owoeye O, Arinola GO. A Vegetable, Launaea taraxacifolia, Mitigated Mercuric Chloride Alteration of the Microanatomy of Rat Brain. J Dietary Supplements. 2017; 14: 613-625.
- Owoeye O, Onwuka SK. Lead Toxicity: Effect of Launaea taraxacifolia on the Histological and Oxidative alterations in Rat Regio III Cornu ammonis and Cerebellum. Anatomy J Africa. 2016; 5: 783-794.
- Thomford NE, Mkhize B, Dzobo K, Mpye K, Rowe A, Parker MI, et al. African Lettuce (Launaea taraxacifolia) Displays Possible Anticancer Effects and Herb-Drug Interaction Potential by CYP1A2, CYP2C9, and CYP2C19 Inhibition. OMICS. 2016; 20: 528-537.
- National Institutes of Health Guide for the Care and Use of Laboratory Animals. HEW Publication (NIH). Revised, Office of Science and Health Reports, DRR/NIH, Bethesda; USA. 2008.
- Elkhadragy MF, Kassab RB, Metwally D, Almeer RS, Abdel-Gaber R, Al- Olayan EM, et al. Protective effects of Fragaria ananassa methanolic extract in a rat model of cadmium chloride-induced neurotoxicity. Biosci Rep. 2018; 38: BSR20180861.
- Ojo OA, Rotimi DE, Ojo AB, Ogunlakin AD, Ajiboye BO. Gallic acid abates cadmium chloride toxicity via alteration of neurotransmitters and modulation of inflammatory markers in Wistar rats. Sci Rep. 2023; 13: 1577.
- Loredana E, Mustatea G. Toxicity of Heavy Metals. IntechOpen. 2022.
- Idorenyin UU, Koofreh GD, Esther OA, Helen UP, Uwemedimo FU, Nsikak AI, et al. Neuroprotective effects of Launaea taraxacifolia leaf extracts on aluminium chloride-induced motor dysfunction. Computing and Applied Sciences Impact. 2024; 1: 2-13.
- Jena AB, Samal RR, Bhol NK, Duttaroy AK. Cellular Red-Ox system in health and disease: The latest update. Biomed Pharmacother. 2023; 162: 114606.
- Ilochi O, Chuemere AN. Effect of Aqueous Extract of Hibiscus Sabdariffa on Cadmium Chloride-Induced Neurotoxicity in Male Wistar Rats. Niger J Physiol Sci. 2025; 39: 111-118.
- K?sadere ?, Karaman M, Ayd?n MF, Donmez N, Usta M. The protective effects of chitosan oligosaccharide (COS) on cadmium-induced neurotoxicity in Wistar rats. Arch Environ Occup Health. 2022; 77: 755-763.
- Oyagbemi AA, Hassan FO, Adebiyi OE, Adigun KO, Folarin OR, Ajibade TO, et al. Neuroprotective effect of Launaea taraxacifolia against neuroinflammation, memory loss and neurobehavioral deficit in a rat model of hypertension: biochemical and immunohistochemical approaches. J Herbmed Pharmacol. 2024; 13: 390-398.
- Akpan HB, Omotoso OD, Ogbonna E, Negedu MN, Adelakun SA, Oladipupo FE, et al. Histomorphological Effects of Carica papaya on Cadmium Induced Prefrontal-Cortex Damage in Adult Wistar Rats. European J Med Plants. 2018; 23: 1-10.
- Afifi OK, Embaby AS. Histological Study on the Protective Role of Ascorbic Acid on Cadmium Induced Cerebral Cortical Neurotoxicity in Adult Male Albino Rats. J Microsc Ultrastruct. 2016; 4: 36-45.
- Owoeye O, Femi-Akinlosotu OM, Adejuwon SA. Launaea taraxacifolia Aqueous Extract Attenuates Cisplatin Induced Neurotoxicity by Decreasing Oxidative Stress and Neuronal Cell Death in Rats. Arch Bas App Med. 2015; 3: 71-78.
- Olatunde O, Onwuka SK. Lead Toxicity: Effect of Launaea taraxacifolia on the Histological and Oxidative alterations in Rat Regio III Cornu ammonis and Cerebellum. Anatomy J Africa. 2016; 5: 783-794.
- Dasgupta M. Neurotoxicity, Immunotoxicity and Drug Toxicity – A Review. Adv Clin Toxicol. 2018; 3: 000S1-001.